Abstract
Background
5′‐adenosine monophosphate‐activated protein kinase (AMPK) agonists, particularly resveratrol (RES), have not been extensively evaluated for their effect on insulin dysregulation (ID) in horses.
Objectives
Evaluate the effects of treatment with RES (10 mg/kg PO q12h), metformin (MET; 30 mg/kg PO q12h), and aspirin (ASP; 20 mg/kg PO q24h) on experimentally induced ID.
Animals
Thirty‐three healthy, adult, light‐breed horses.
Methods
Unblinded, placebo‐controlled, experimental trial evaluating effects of AMPK agonists (RES, MET, and ASP) on experimentally induced ID. Horses were randomly assigned to a treatment group (RES, MET/ASP, RES/ASP, RES/MET/ASP, or placebo [CON]) after induction of ID with dexamethasone (0.08 mg/kg PO q24h for 7 days). Frequently sampled insulin‐modified IV glucose tolerance tests (FSIGTT) and oral sugar tests (OST) were performed at baseline, 7 days after ID, and ID plus 7 days of treatment. Minimal model and OST variables were compared between (1‐way ANOVA) and within (1‐way ANOVA for repeated measures) groups over time to determine effects of treatment on ID.
Results
Administration of dexamethasone for 14 days resulted in significantly altered insulin and glucose dynamics (SI, DI, basal [glucose], and [insulin]) and produced clinical signs of laminitis in 5 out of 33 (15%) of horses included in the study. Combination therapy with RES, MET, and ASP did not significantly improve insulin and glucose dynamics in horses with experimentally induced ID.
Conclusions and Clinical Importance
Metabolic testing before glucocorticoid administration should be considered in horses with clinical signs of metabolic syndrome.
Keywords: aspirin, equine metabolic syndrome, hyperinsulinemia‐associated laminitis, insulin dysregulation, metformin, resveratrol
Abbreviations
- AIRg
acute insulin response to glucose
- AMPK
5′‐adenosine monophosphate‐activated protein kinase
- ASP
aspirin
- AUC
area under the curve
- CON
control
- DI
disposition index
- FSIGTT
frequently sampled insulin‐modified IV glucose tolerance tests
- HAL
hyperinsulinemia‐associated laminitis
- ID
insulin dysregulation
- MET
metformin
- OST
oral sugar test
- RES
resveratrol
- Sg
glucose effectiveness
- SI
insulin sensitivity
1. INTRODUCTION
Metabolic syndrome in horses (EMS) is a collection of clinical signs including insulin dysregulation (ID), laminitis, and regional adiposity or obesity. 1 Hyperinsulinemia (a form of ID) is a risk factor for the most common form of laminitis in horses, hyperinsulinemia‐associated laminitis (HAL). 1 , 2 , 3 , 4 Medications such as metformin and aspirin are used extra‐label to improve insulin and glucose dynamics in horses with ID; however, efficacy is variable, and metformin's low oral bioavailability in this species likely contributes to this inconsistency. 5 , 6 , 7 , 8 , 9 , 10 Therefore, investigation into alternative pharmacotherapies to improve insulin and glucose dynamics and prevent HAL in horses with ID, in addition to diet and exercise interventions, is warranted.
5′‐adenosine monophosphate‐activated protein kinase (AMPK) is a well‐conserved heterotrimeric protein that is activated during low cellular energy states, resulting in activation of multiple downstream catabolic pathways including glucose transport, catabolism of lipids and proteins, regulation of cellular growth and proliferation, and regulation of tissue insulin resistance. 11 , 12 , 13 , 14 Therapeutics that target this pathway (hereafter termed AMPK agonists) are used to treat conditions such as obesity and type 2 diabetes in humans. 10 , 11 , 15 , 16 In addition to the effects of AMPK on insulin and glucose dynamics, the concentrations of phosphorylated (activated) AMPK (p‐AMPK) are lower in lamellar tissue after a high‐carbohydrate feeding in healthy ponies; given that AMPK also plays an important role in maintenance of epithelial integrity, this attenuation of AMPK activation under conditions that increase risk for HAL might be pathophysiologically relevant. 13 , 17 Therefore, AMPK agonists could be a therapeutic for treatment and prevention of EMS and associated HAL. There are several AMPK agonists that are used clinically in horses for improving insulin and glucose dynamics and preventing development of HAL, but the efficacy of these treatments has not been confirmed through large‐scale placebo‐controlled clinical trials to date.
Resveratrol (RES) is a 3,5,40‐trihydroxystilbene naturally occurring in peanuts, grapes, and red wine. 18 RES is an AMPK agonist and inhibits mechanistic target of rapamycin (mTOR) signaling, which is upregulated in lamellar tissue from experimental models of all 3 forms of laminitis (sepsis‐associated laminitis, HAL, and supporting limb laminitis). 19 , 20 RES could be an attractive therapeutic target for HAL, as it targets pathways associated with development of laminitis of diverse forms.
The purpose of this study was to evaluate the effects of a novel treatment with 3 AMPK agonists (RES [10 mg/kg PO q12h], metformin [MET; 30 mg/kg PO q12h], and aspirin [ASP; 20 mg/kg PO q24h]) on experimentally induced ID. We hypothesized that experimentally induced ID (administration of dexamethasone at 0.08 mg/kg PO every 24 hours for 7 days) would significantly alter insulin and glucose dynamics in healthy adult light‐breed horses, as in previous studies. 21 , 22 , 23 Additionally, we hypothesized that administration of AMPK agonists would improve facets of insulin and glucose dynamics and attenuate experimentally induced ID in horses.
2. MATERIALS AND METHODS
2.1. Experimental animals
Thirty‐five healthy, university‐owned, light‐breed horses were enrolled. All horses included in the study were 4‐18 years of age and were screened for pituitary pars intermedia dysfunction through measurement of basal endogenous adrenocorticotropic hormone (ACTH) concentration before the study. Three horses that were initially included in the study were subsequently removed because of colic, respiratory disease, and laminitis (Obel grade 2), leaving 33 horses whose complete data sets were evaluated in this study. All horses received ad libitum grass hay and water during the study period except when feed was withheld before ID testing. Additionally, all horses received twice daily physical examinations and were monitored daily by a veterinarian. All horses were treated according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC) protocol 2020A00000026 in accordance with the guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Sample collection
The study was an unblinded, placebo‐controlled, experimental trial. Horses arrived at The Ohio State University Veterinary Medical Center on Day 1 and were acclimated for 96 hours before testing. Feed was withheld from all horses for 6 hours before testing time points by application of a muzzle. On Days 5 and 6, an oral sugar test (OST; OST1) and frequently sampled insulin‐modified intravenous (IV) glucose tolerance test (FSIGTT; FSIGTT1) were performed, respectively, to determine baseline values. On Day 5, a 14 ga 5.25″ catheter (Abbocath‐T 14 G x 5.5", Abbott Animal Health, Chicago IL) was placed in the left external jugular vein following clipping and aseptic preparation for blood collection. The OST was performed as previously described. 24 All blood samples were collected at the described time points in ethylenediaminetetraacetic acid (EDTA) and silicone‐coated tubes (K2‐EDTA BD Vacutainer tubes, Franklin Lakes, NJ). Blood was collected at Time 0 before administration of an enteral carbohydrate (Karo Light Corn Syrup, ACH Food Companies, Inc., Oakbrook, IL), which was then administered by mouth once. Plasma and serum samples were then collected from the IV catheter after a minimum collection of 10 mL of waste blood at time points: 30, 60, 90, 120, 180, 240, 300, and 360 minutes. Blood glucose values were measured stall‐side at each time point (including baseline [Time 0]) using a handheld glucometer previously evaluated with equine samples (AlphaTrak2, Zoetis Services LLC, Parsippany, NJ) immediately after collection of the blood sample. 25 , 26 Muzzles that had been placed for withholding feed pre‐testing were removed at the 120‐minute time point. The IV catheter in the left jugular vein remained in place overnight, with heparinized saline flushes administered every 6 hours to maintain patency. The FSIGTT was performed the following day after a 6‐hour period of withholding feed, implemented in the same fashion as for the OST. A second 14 ga 2″ IV catheter was placed in the right external jugular vein on the morning of the FSIGTT after clipping and aseptic preparation of the site for administration of dextrose and insulin. The FSIGTT was performed as previously described. 27 Baseline blood samples (collected as described for the OST) were obtained at 10, 5, and 1 minute(s) before the administration of dextrose. One dose of 50% dextrose (VetOne, MWI Animal Health, Boise, ID); 150 mg/kg) was administered IV in the right jugular vein at Time 0, followed by 0.1 IU/kg regular insulin IV (Humulin‐R U‐100, Eli Lilly and Company, Indianapolis, IN) 20 minutes later. The IV catheters were assigned to be used for either blood collection or administration of insulin and dextrose and were not used interchangeably; if the IV catheters were inadvertently removed during the study period, they were replaced aseptically as described above. Blood was collected at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 150, and 180 minutes after dextrose administration. Plasma and serum samples were collected at each time point following collection of 10 mL of waste blood, and blood glucose was measured stall‐side using a glucometer as described for the OST. Plasma and serum samples were processed within 3 hours after testing for the OST and FSIGTT at all study time points. All blood samples were centrifuged at 3000 rpm for 15 minutes and stored at −80°C until further testing. On Days 7‐24, all horses received 0.08 mg/kg dexamethasone (VetOne, MWI Animal Health, Boise, ID) by mouth every 24 hours to induce and maintain systemic ID. On Days 15 and 16, OST (OST2) and FSIGTT (FSIGTT2) testing was performed again as described previously. Horses were then randomly assigned to 1 of 5 treatment groups: control (CON; n = 5), resveratrol (RES; n = 7), resveratrol and aspirin (RES/ASP; n = 7), metformin and aspirin (MET/ASP; n = 7), and resveratrol, metformin, and aspirin (RES/MET/ASP; n = 7). MET (Metformin; Cardinal Health, Inc., Dublin, OH) was administered at 30 mg/kg PO every 12 hours, ASP (Aspirin)j was administered at 20 mg/kg by mouth every 24 hours, RES (Metabarol®; Equithrive, Thrive Animal Health, Lexington, KY) was administered at 10 mg/kg by mouth every 12 hours, and water (CON) was administered at 20 mLs by mouth every 12 hours, as indicated for each respective treatment group listed above. Each horse additionally received 0.08 mg/kg dexamethasone by mouth every 24 hours as described previously to maintain ID during the treatment phase of the study. On Days 23‐24, OST (OST3) and FSIGTT (FSIGTT3) testing was performed as described. Figure 1 describes the experimental design and testing time points. Horses were monitored daily for complications, including signs of laminitis and thrombophlebitis, throughout the entire study period; all horses received physical examinations twice daily.
FIGURE 1.
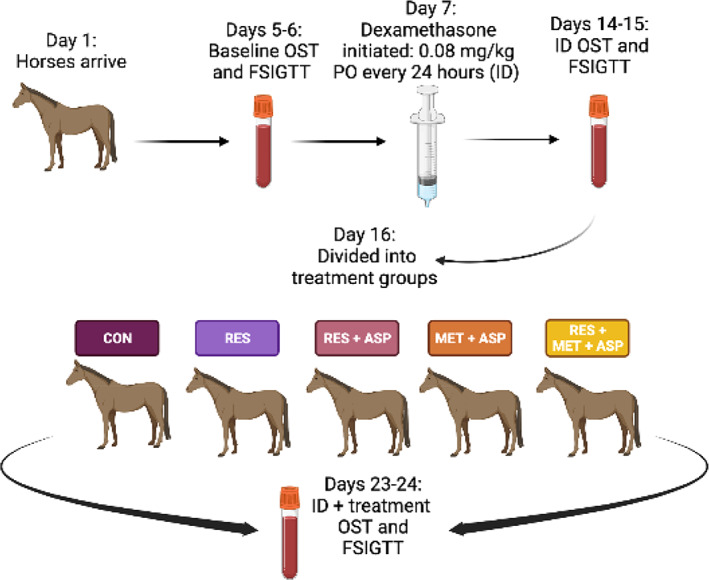
Experimental design from Day 1 to 24; control (CON), resveratrol (RES), resveratrol/aspirin (RES/ASP), metformin/aspirin (MET/ASP), resveratrol/metformin/aspirin (RES/MET/ASP).
2.3. Insulin and glucose measurements
Blood glucose concentrations were measured and recorded at every blood collection time point during FSIGTT and OST testing. Plasma insulin concentrations were measured using a commercially available Insulin ELISA (MP Biomedicals, Solon, OH) previously validated for use with equine samples 28 ; plasma [insulin] was measured at baseline, at every time point during the FSIGTT, and times 0‐240 minute time points for the OST. Minimal Model parameters (MINMOD MILLENIUM Minimal Model Software, MINMOD, Inc., Los Angeles, CA) were calculated from the FSIGTT data. These parameters included the acute insulin response to glucose (AIRg), glucose effectiveness (Sg), insulin sensitivity (SI), and disposition index (DI; Table 1). Area under the curve (AUC) of insulin and glucose concentrations were calculated by the trapezoidal method from the OST data.
TABLE 1.
Description of parameters derived from Minimal Model analysis of FSIGTT data.
| Minimal Model parameters | Units | Definition | Reference values (95% CI) |
|---|---|---|---|
| Insulin sensitivity (SI) | (L•min−1•mU−1) | Ability of insulin to promote glucose disposal and prevent glucose production endogenously | 0.16‐5.88 (SI × 104) |
| Glucose effectiveness (Sg) | (min−1) | Ability of glucose to control its own disposal | 0.12‐2.95 (Sg × 102) |
| Disposition index (DI) | … | Ability of beta islet cell to secrete insulin normalized to the degree of insulin resistance | 39.3‐1675 (DI × 104) |
| Acute insulin response to glucose (AIRg) | [μL−1.min] | First phase of insulin response to glucose | 67‐805 |
Note: Minimal Model parameters evaluated after frequently sampled intravenous glucose tolerance test (FSIGTT), definitions, and normal equine values. 40
2.4. Statistical analysis
Statistical analyses were performed using a commercially available software program (GraphPad Prism v 9.4, GraphPad Software, La Jolla, CA). Normality was determined for all data using the Shapiro‐Wilk, D'Agostino, and Pearson omnibus normality tests. Area under the curve (time 0‐360 minutes) of [glucose] (AUCglu) and area under the curve (time 0‐240 minutes) of [insulin] (AUCins) were calculated from OST data. A 1‐way ANOVA for repeated measures or paired t tests were performed to compare the calculated OST and FSIGTT variables within treatment groups or between baseline and ID values, respectively. A 1‐way ANOVA (or Friedman test for non‐normal data sets) was also used to compare variables derived from the OST and FSIGTT tests between treatment groups. Statistical significance was accepted at P < .05.
3. RESULTS
A total of 33 horses were included (17 geldings and 16 mares). Various breeds were represented, including American Quarter Horse (n = 12), Warmblood (n = 7), Thoroughbred (n = 7), Standardbred (n = 3), mixed breed/grade (n = 2), Arabian (n = 1), and Appaloosa (n = 1). The endogenous plasma [ACTH] was within normal seasonal reference ranges for all horses, with a median concentration of 19.2 with a range of 10.7‐35.0 pg/mL. The median age was 15 with a range of 5‐18 years, and median body weight was 539.8 with a range of 387.8‐675.4 kg.
3.1. Dexamethasone significantly alters insulin and glucose dynamics
There was a significant decrease in SI and DI from FSIGTT2 (ID) compared with those values derived from FSIGTT1 (baseline; P < .0001; Figure 2). Additionally, there was a significant increase in basal plasma [glucose] and [insulin] between baseline and ID (P < .001; Figure 2). There were no significant differences in AIRg or Sg between the baseline and ID time points.
FIGURE 2.
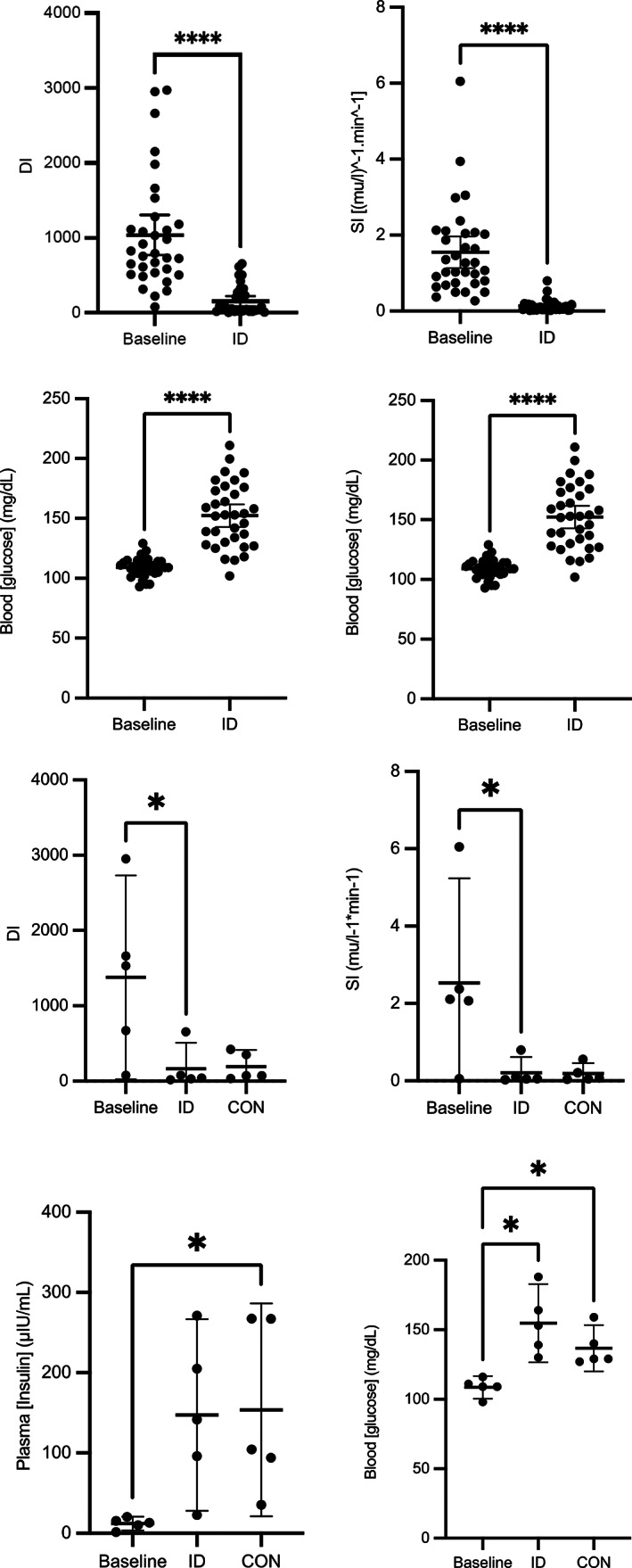
After 7 days of dexamethasone administration (0.08 mg/kg PO q24h), significant decreases in insulin sensitivity (SI; P < .001) and disposition index (DI; P < .001), as well as significant increases in basal [insulin] and [glucose] (P < .001) were observed when comparing baseline and ID time point values for all horses. After 14 days of dexamethasone, there were significant increases in basal [insulin] and basal [glucose] in CON group compared to baseline (P = .01). Asterisk (*) indicates P < .05; error bars represent mean plus 95% confidence interval.
3.2. Minimal Model parameters
Minimal Model parameters (defined in Table 1) were calculated from the FSIGTT and compared among treatment groups. There were no significant effects of treatment on any parameter (AIRg, SI, DI, or Sg) between treatment groups compared to CON (Figures 3 and 4).
FIGURE 3.
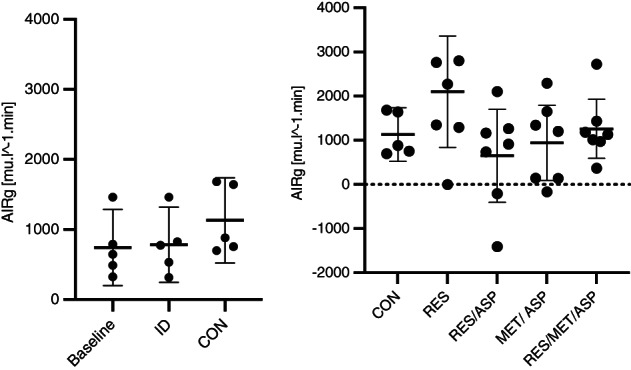
Minimal Model parameters AIRg (acute insulin response to glucose) for each treatment group with mean plus 95% confidence intervals; resveratrol/metformin/aspirin (RES/MET/ASP), resveratrol (RES), resveratrol/aspirin (RES/ASP), metformin/aspirin (MET/ASP), and control (CON). There was no significant effect of treatment on this parameter.
FIGURE 4.
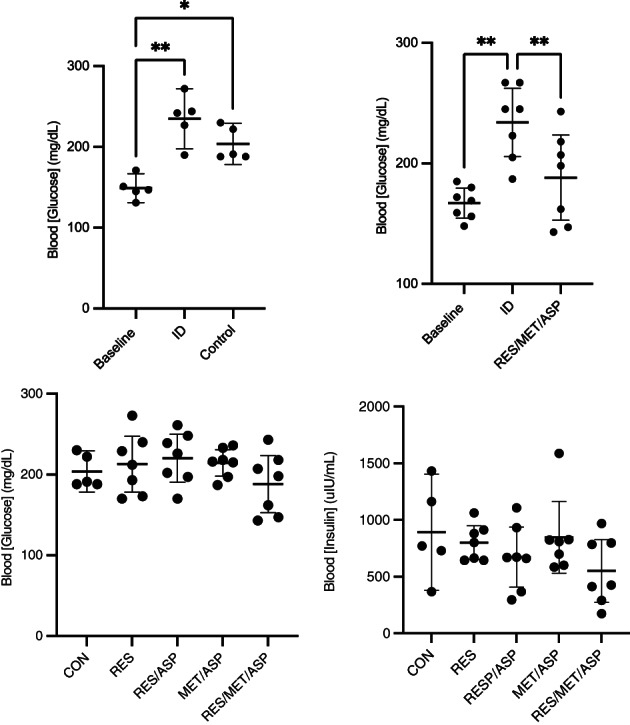
There is a significant increase in post‐prandial peak glucose within control (CON) group from baseline (oral sugar test [OST]; OST1) to ID (OST2) and ID plus treatment (OST3). There is a significant decrease in post‐prandial peak [glucose] evaluated by the OST 3 in the resveratrol/metformin/aspirin (RES/MET/ASP) from insulin dysregulation (ID; 7 days of oral dexamethasone treatment) compared to ID plus 7 days of treatment with mean plus 95% confidence intervals; asterisks (*) indicate significant differences between time points (P < .05). There were no significant differences in post‐prandial peak [glucose] in response to treatment in any group [resveratrol (RES), resveratrol/aspirin (RES/ASP), metformin/aspirin (MET/ASP), control (CON), RES/MET/ASP] when compared between treatment groups at OST3. Additionally, there was no significant decrease in post‐prandial peak [insulin] after treatment measured at OST3.
3.3. Peak post‐prandial [glucose] and [insulin]
Peak plasma [glucose] was extracted from the OST data comparing OST1 (baseline), OST2 (ID), and OST3 (ID plus treatment) within treatment groups. Post‐prandial peak [glucose] during OST was significantly decreased in response to treatment within the RES/MET/ASP treatment group ([95% CI: 152.9‐223.6 mg/dL] compared to ID [95% CI: 205.9‐262.4 mg/dL]; P = .002; Figure 4). There was a significant increase in [glucose] when comparing OST1 (baseline) to OST2 (ID) in all treatment groups, and a significant increase in [glucose] when comparing OST1 to OST3 in the control and MET/ASP treatment groups (Figure 4). There were no significant differences in peak post‐prandial [insulin] or [glucose] measured between treatment groups (Figure 4).
3.4. Basal [insulin] and [glucose]
Basal plasma [insulin] and whole blood [glucose] were determined at FSIGTT3 (ID plus treatment). There were no significant differences between treatment groups observed after 7 days of treatment (Figure 5).
FIGURE 5.
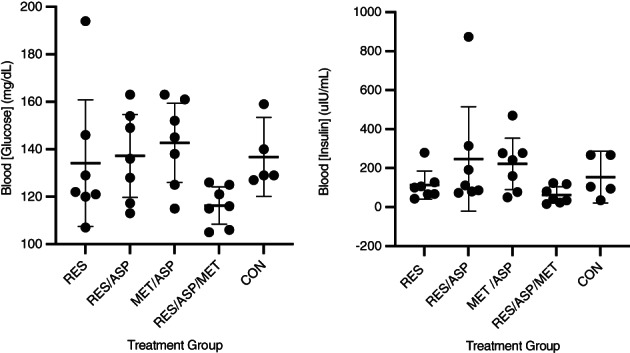
Basal glucose and insulin concentrations evaluated before frequently sampled intravenous glucose tolerance test at timepoint 3 (FSIGTT 3) after 7 days of dexamethasone (experimentally induced insulin dysregulation) plus 7 days of treatment: resveratrol (RES), resveratrol/aspirin (RES/ASP), metformin/aspirin (MET/ASP), resveratrol/metformin/aspirin (RES/MET/ASP), and control (CON). There were no significant differences in basal insulin or glucose concentrations. Error bars represent mean plus 95% confidence interval.
3.5. AUC [insulin] and AUC [glucose]
AUC[insulin] was significantly increased comparing OST2 (ID) and OST3 (ID plus treatment) to OST1 (baseline) (P < .05). There was no significant effect of treatment (OST3) on ID (OST2) AUC[insulin] (Figure 6). AUC[insulin] was significantly increased comparing OST1 to OST2 (ID) and OST3 for all treatment groups, except for RES/MET/ASP and CON, in which there was no significant increase in [glucose].
FIGURE 6.
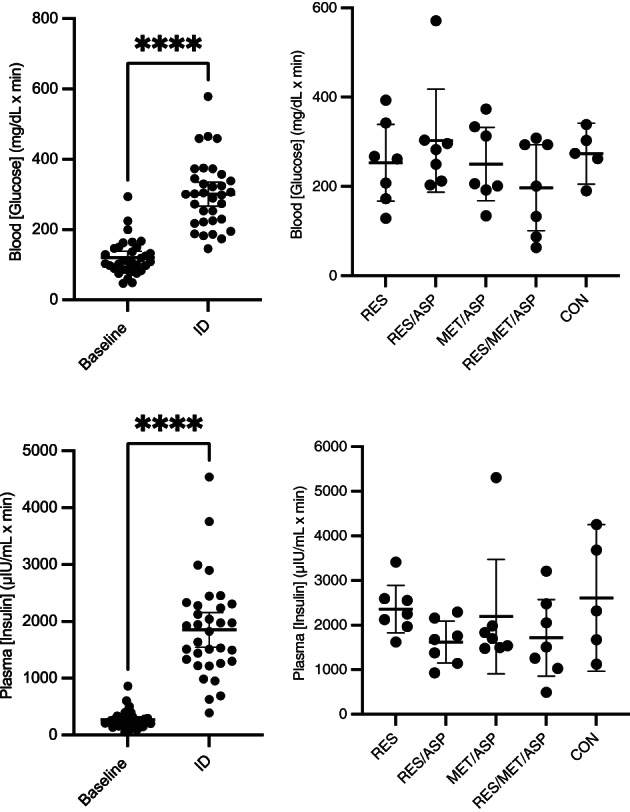
Area under the curve (AUC) of glucose (AUC[glucose] measured during the OST) and insulin concentrations (AUC[insulin] measured during the FSIGTT) at baseline for all horses and after 1 week of insulin dysregulation (ID; administration of dexamethasone by mouth for 7 days) and the effect of 7 days of treatment on AUC[glucose] and AUC[insulin] measured at OST3 and FSIGTT3, respectively. Error bars represent mean plus 95% confidence interval; resveratrol (RES), resveratrol/aspirin (RES/ASP), metformin/aspirin (MET/ASP), resveratrol/metformin/aspirin (RES/MET/ASP), and control (CON). There was a significant increase in AUC[glucose] and AUC[insulin] after 7 days of dexamethasone compared to baseline measured by the OST and FSIGTT, respectively. There were no significant differences in AUC[glucose] or AUC[insulin] between treatment groups. Asterisks (*) indicates significant difference (P < .05).
3.6. Glucocorticoid‐induced laminitis
A total of 5/33 (15%) of the horses included in the study developed clinical signs of laminitis during the study or in close temporal association with the conclusion of the study (RES n = 1, RES/ASP n = 2, RES/MET/ASP n = 1, CON n = 1). One horse that was excluded from the study because of severe laminitis is not included in this total; however, this animal became laminitic after 12 days of dexamethasone administration (which was considered a complication of the study protocol) and was subsequently euthanized because of progressive disease. Clinical signs of laminitis of the horses that remained in this study included increased digital pulses (n = 2) and Obel grade 1‐2 in the forelimbs in all (n = 5). Additionally, 1/5 of the affected horses were euthanized because of progressive clinical signs after the conclusion of the study. The other 4/5 affected horses showed clinical improvement within 3‐14 days of detection of initial signs and responded well to medical management (Figure S3). The dose and length of treatment of dexamethasone administered is commonly used in equine medicine, and therefore, the high percentage of laminitis as a complication was not expected. This finding warrants further investigation into the dose of dexamethasone administered in horses for the purpose of experimentally induced ID and for clinical use.
4. DISCUSSION
The results of this study confirm, in support of previous work, that dexamethasone administration significantly alters many facets of insulin and glucose dynamics in light‐breed horses, and a large percentage (15%) of horses exposed to this treatment developed clinical signs of laminitis after 14 days of dexamethasone administration PO. 21 Although treatment with RES/MET/ASP significantly decreased post‐prandial [glucose] in horses with experimentally induced ID, it did not significantly or consistently improve other insulin and glucose variables. Similarly, insulin and glucose dynamics were not significantly altered by the other treatment groups indicating that treatment with AMPK agonists in combination did not significantly improve experimentally induced ID in this model.
Insulin and glucose dynamics were significantly altered, indicating evidence of ID in all treatment groups when comparing baseline to ID values. 21 Both SI and DI calculated from the FSIGTT (which evaluate systemic insulin sensitivity and the ability of the pancreatic β‐cells to secrete insulin, respectively) were significantly lower after 7 days of dexamethasone administration. Additionally, basal plasma [insulin] and blood [glucose] were significantly higher after 7 days of dexamethasone administration. Although other variables related to insulin and glucose dynamics were significantly affected, there was no difference in AIRg when comparing baseline to ID. When comparing insulin and glucose dynamics after 14 days of dexamethasone administration in the CON group, there were no statistically significant differences in some parameters (AIRg, basal [insulin], basal [glucose], AUC[insulin], or AUC[glucose]) between baseline, ID, or ID and treatment, indicating the model of dexamethasone may not have been sufficient to induce 14 days of ID. However, all horses in the CON group were considered ID after 14 days of dexamethasone according to the OST ([insulin] greater than 60 μIU/mL at 60 minutes; Figure S1). These results confirm that the dose of dexamethasone administered in this study effectively induces many facets of ID after 7 days and maintains ID after 14 days according to the OST. Of all the horses in the study, 15% showed clinical signs of laminitis; additionally, 1 excluded horse was removed from the study because of progressive laminitis. This horse was a 12‐year‐old large pony mare with an increased body condition score (7/9) and therefore could have been predisposed to laminitis and ID before dexamethasone administration. Other studies demonstrate that dexamethasone induces equine ID and HAL in horses. 21 , 23 , 29 Careful consideration of these effects on systemic insulin and glucose dynamics associated with dexamethasone administration should be given before use of this drug in horses, particularly in breeds with known or suspected predisposition to ID, such as Arabians, American Saddlebreds, Tennessee Walking Horses, Welsh ponies, and Morgans (among others). 30 , 31 Additionally, evaluation of insulin and glucose dynamics before, during, and after the administration of glucocorticoids is warranted in horses at risk of HAL. Based on the magnitude of alteration of insulin and glucose dynamics noted in this study and the development of laminitis in some horses, the induced severity of ID in this case might have influenced some results of the AMPK agonists; further investigation with naturally occurring‐ID or experimental controls inducing less severe ID (ie, with a lower dexamethasone dose) might be warranted.
Combination treatment with the AMPK agonists RES/MET/ASP significantly increased the AIRg in treated horses between the FSIGTT2 and FSIGTT3 time points. This variable is a measure of the acute pancreatic response to glucose, and ideally, should be minimized with treatment in horses with ID, as hyperinsulinemia is a well‐known and consistent risk factor for laminitis. 1 , 32 , 33 This finding indicates that this combination treatment might not be sufficient in reducing the risk of HAL in horses with ID. However, since there was no significant difference in the AIRg when comparing baseline to ID after administration of dexamethasone, this result is difficult to interpret. Although there were no significant effects of any treatment on insulin and glucose dynamics, these treatments might be worth evaluating in larger groups of horses, those with less severe ID (ie, naturally occurring disease), and after a longer course of treatment because significant improvement in insulin and glucose dynamics in humans with metabolic syndrome occurs after 4 weeks to 3 months of resveratrol therapy. 34 , 35 , 36 Resveratrol improves systemic insulin resistance in other models, enhances glucose uptake and metabolism, preserves β‐cells within the pancreatic islets, and prevents diabetic complications in humans and other species when administered for 30 days or longer. 37 , 38 Resveratrol has also been evaluated in horses; there was significantly lower serum insulin concentrations after an OST in horses treated with resveratrol and leucine for 6 weeks. 39
The RES/MET/ASP group was the only treatment group that showed significantly decreased post‐prandial peak [glucose] measured during the OST. Metformin significantly decreases peak post‐prandial glucose concentration, area under the glucose curve, and insulin concentration in both healthy and horses with experimentally induced ID when administered at 30 mg/kg 1 hour before enteral dextrose administration. 7 Metformin was not evaluated as monotherapy in this study; however, metformin in combination with aspirin did not significantly alter post‐prandial glucose concentration in this study when compared to placebo. Resveratrol lowers basal [glucose] in humans with type‐2 diabetes mellitus. 35 The combination of RES/MET/ASP significantly decreases post‐prandial peak glucose concentrations after enteral carbohydrate challenge. However, when the treatment groups were compared to the CON group following 7 days of treatment, there was no significant decrease in glucose or insulin concentrations, indicating this result might have limited clinical relevance.
Limitations of this study included the small number of horses per treatment group; originally, 35 horses were to be included and because of development of laminitis and other complications (colic, respiratory disease) we were limited to inclusion of 33 total horses. Due to the limited number of horses, evaluation of significant differences between breeds was not able to be performed. Various breeds were included, and therefore, risk of pre‐existing ID was possible in some horses. Further consideration of breeds and evaluation of metabolic status before inclusion in the study should be performed in future studies. Additionally, these treatments were not evaluated in naturally occurring ID, and so extrapolation of these results to animals with EMS may not be appropriate. However, the administration of dexamethasone did consistently induce ID; given that this drug is frequently administered for therapeutic purposes in equine veterinary practice, these results could be useful for mitigating risks associated with that treatment particularly in horses with breed or other pre‐existing predispositions to ID. An additional limitation was the limited duration of treatment (7 days) that was used to evaluate the effects of AMPK combination therapy on dexamethasone‐induced ID. In studies in other species, resveratrol can have significant beneficial effects on insulin and glucose dynamics and prevent complications when administered for a longer period of time (6‐10 weeks). 37 Therefore, a longer duration of treatment with this novel combination therapy (RES/MET/ASP) should be evaluated in horses with naturally occurring ID.
In conclusion, dexamethasone significantly altered insulin and glucose dynamics in horses after treatment for 7 days, and ID was maintained for the duration of administration (14 days). Additionally, administration of dexamethasone at the dose used in this study resulted in clinical signs of laminitis in 15% of horses. Novel combination therapy with RES/MET/ASP might be useful to decrease plasma glucose concentrations after an oral carbohydrate load; however, there was limited evidence that this combination therapy significantly improved insulin and glucose dynamics in experimentally induced ID. There was a lack of significant improvement in ID after various treatments with RES, MET, and ASP in combination therapy. A more robust evaluation of combination AMPK agonist therapy in future studies should ideally be performed using cases of naturally occurring equine ID for a time course greater than 7 days.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by The Ohio State University IACUC, protocol 2020A00000026.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1:
Figure S2:
Figure S3:
ACKNOWLEDGMENT
Funding provided by the Grayson Jockey Club Research Foundation.
Pinnell EF, Hostnik LD, Watts MR, et al. Effect of 5′‐adenosine monophosphate‐activated protein kinase agonists on insulin and glucose dynamics in experimentally induced insulin dysregulation in horses. J Vet Intern Med. 2024;38(1):102‐110. doi: 10.1111/jvim.16970
REFERENCES
- 1. Durham AE, Frank N, McGowan CM, et al. ECEIM consensus statement on equine metabolic syndrome. J Vet Intern Med. 2019;33:335‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46:103‐112. [DOI] [PubMed] [Google Scholar]
- 3. Asplin KE, Sillence MN, Pollitt CC, McGowan CM. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J. 2007;174:530‐535. [DOI] [PubMed] [Google Scholar]
- 4. de Laat MA, McGowan CM, Sillence MN, et al. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42:129‐135. [DOI] [PubMed] [Google Scholar]
- 5. Hustace JL, Firshman AM, Mata JE. Pharmacokinetics and bioavailability of metformin in horses. Am J Vet Res. 2009;70:665‐668. [DOI] [PubMed] [Google Scholar]
- 6. Tinworth KD, Edwards S, Noble GK, et al. Pharmacokinetics of metformin after enteral administration in insulin‐resistant ponies. Am J Vet Res. 2010;71:1201‐1206. [DOI] [PubMed] [Google Scholar]
- 7. Rendle DI, Rutledge F, Hughes KJ, Heller J, Durham AE. Effects of metformin hydrochloride on blood glucose and insulin responses to oral dextrose in horses. Equine Vet J. 2013;45:751‐754. [DOI] [PubMed] [Google Scholar]
- 8. Durham AE, Rendle DI, Newton JE. The effect of metformin on measurements of insulin sensitivity and beta cell response in 18 horses and ponies with insulin resistance. Equine Vet J. 2008;40:493‐500. [DOI] [PubMed] [Google Scholar]
- 9. Tinworth KD, Boston RC, Harris PA, Sillence MN, Raidal SL, Noble GK. The effect of oral metformin on insulin sensitivity in insulin‐resistant ponies. Vet J. 2012;191:79‐84. [DOI] [PubMed] [Google Scholar]
- 10. Ford RJ, Fullerton MD, Pinkosky SL, et al. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem J. 2015;468:125‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764‐2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cork GK, Thompson J, Slawson C. Real talk: the inter‐play between the mTOR, AMPK, and hexosamine biosynthetic pathways in cell signaling. Front Endocrinol (Lausanne). 2018;9:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burns TA, Watts MR, Weber PS, McCutcheon LJ, Geor RJ, Belknap JK. Effect of dietary nonstructural carbohydrate content on activation of 5′‐adenosine monophosphate‐activated protein kinase in liver, skeletal muscle, and digital laminae of lean and obese ponies. J Vet Intern Med. 2014;28:1280‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunn CJ, Peters DH. Metformin. A review of its pharmacological properties and therapeutic use in non‐insulin‐dependent diabetes mellitus. Drugs. 1995;49:721‐749. [DOI] [PubMed] [Google Scholar]
- 15. Mannucci E, Ognibene A, Cremasco F, et al. Effect of metformin on glucagon‐like peptide 1 (GLP‐1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24:489‐494. [DOI] [PubMed] [Google Scholar]
- 16. Xiao C, Giacca A, Lewis GF. The effect of high‐dose sodium salicylate on chronically elevated plasma nonesterified fatty acid‐induced insulin resistance and beta‐cell dysfunction in overweight and obese nondiabetic men. Am J Physiol Endocrinol Metab. 2009;297:E1205‐E1211. [DOI] [PubMed] [Google Scholar]
- 17. Zhang L, Li J, Young LH, Caplan MJ. AMP‐activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A. 2006;103:17272‐17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hou CY, Tain YL, Yu HR, Huang LT. The effects of resveratrol in the treatment of metabolic syndrome. Int J Mol Sci. 2019;20(3):535‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alayev A, Berger SM, Holz MK. Resveratrol as a novel treatment for diseases with mTOR pathway hyperactivation. Ann N Y Acad Sci. 2015;1348:116‐123. [DOI] [PubMed] [Google Scholar]
- 20. Lane HE, Burns TA, Hegedus OC, et al. Lamellar events related to insulin‐like growth factor‐1 receptor signalling in two models relevant to endocrinopathic laminitis. Equine Vet J. 2017;49:643‐654. [DOI] [PubMed] [Google Scholar]
- 21. Timko KJ, Hostnik LD, Watts MR, et al. Diagnostic evaluation of insulin and glucose dynamics in light‐breed horses receiving dexamethasone. Can Vet J. 2022;63:617‐626. [PMC free article] [PubMed] [Google Scholar]
- 22. Brennan KM, Urschel KL. Recovery of insulin sensitivity in mature horses after a 3 week course of dexamethasone therapy. Equine Vet J. 2014;46:718‐721. [DOI] [PubMed] [Google Scholar]
- 23. Tiley HA, Geor RJ, McCutcheon LJ. Effects of dexamethasone on glucose dynamics and insulin sensitivity in healthy horses. Am J Vet Res. 2007;68:753‐759. [DOI] [PubMed] [Google Scholar]
- 24. Lindase S, Nostell K, Brojer J. A modified oral sugar test for evaluation of insulin and glucose dynamics in horses. Acta Vet Scand. 2016;58:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hackett ES, McCue PM. Evaluation of a veterinary glucometer for use in horses. J Vet Intern Med. 2010;24:617‐621. [DOI] [PubMed] [Google Scholar]
- 26. Cunneen A, Wood KA, Mathison K, et al. Comparison of a continuous indwelling glucometer with a point‐of‐care device in healthy adult horses. Vet Rec. 2020;187:e21. [DOI] [PubMed] [Google Scholar]
- 27. Toth F, Frank N, Elliott SB, et al. Optimisation of the frequently sampled intravenous glucose tolerance test to reduce urinary glucose spilling in horses. Equine Vet J. 2009;41:844‐851. [DOI] [PubMed] [Google Scholar]
- 28. Rings LM, Swink JM, Dunbar LK, Burns TA, Toribio RE. Enteroinsular axis response to carbohydrates and fasting in healthy newborn foals. J Vet Intern Med. 2019;33:2752‐2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson PJ, Slight SH, Ganjam VK, Kreeger JM. Glucocorticoids and laminitis in the horse. Vet Clin North Am Equine Pract. 2002;18:219‐236. [DOI] [PubMed] [Google Scholar]
- 30. Bamford NJ, Potter SJ, Harris PA, Bailey SR. Breed differences in insulin sensitivity and insulinemic responses to oral glucose in horses and ponies of moderate body condition score. Domest Anim Endocrinol. 2014;47:101‐107. [DOI] [PubMed] [Google Scholar]
- 31. Norton EM, Schultz NE, Rendahl AK, et al. Heritability of metabolic traits associated with equine metabolic syndrome in welsh ponies and Morgan horses. Equine Vet J. 2019;51:475‐480. [DOI] [PubMed] [Google Scholar]
- 32. de Laat MA, Reiche DB, Sillence MN, McGree JM. Incidence and risk factors for recurrence of endocrinopathic laminitis in horses. J Vet Intern Med. 2019;33:1473‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Laat MA, Sillence MN, Reiche DB. Phenotypic, hormonal, and clinical characteristics of equine endocrinopathic laminitis. J Vet Intern Med. 2019;33:1456‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Most J, Timmers S, Warnke I, et al. Combined epigallocatechin‐3‐gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: a randomized controlled trial. Am J Clin Nutr. 2016;104:215‐227. [DOI] [PubMed] [Google Scholar]
- 35. Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537‐541. [DOI] [PubMed] [Google Scholar]
- 36. Zare Javid A, Hormoznejad R, Yousefimanesh HA, et al. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother Res. 2017;31:108‐114. [DOI] [PubMed] [Google Scholar]
- 37. Gong L, Guo S, Zou Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high‐fat diet‐fed mice. Life Sci. 2020;242:117212. [DOI] [PubMed] [Google Scholar]
- 38. Huang DD, Shi G, Jiang Y, Yao C, Zhu C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed Pharmacother. 2020;125:109767. [DOI] [PubMed] [Google Scholar]
- 39. Manfredi JM, Stapley ED, Nadeau JA, Nash D. Investigation of the effects of a dietary supplement on insulin and adipokine concentrations in equine metabolic syndrome/insulin dysregulation. J Equine Vet. 2020;88:102930. [DOI] [PubMed] [Google Scholar]
- 40. Treiber KH, Kronfield S, Hess TM, et al. Use of proxies and quintiles obtained from minimal model analysis for determination of insulin sensitivity and pancreatic beta‐cell responsiveness in horses. Am J Vet Res. 2005;66(12):2114‐21121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1:
Figure S2:
Figure S3:


