Abstract
Background
An accurate and easily accessible method for diagnosing malignancies in local veterinary clinics has not yet been established.
Objectives
To investigate the usefulness of serum thymidine kinase 1 (TK1) protein and its autoantibody as tumor biomarkers in dogs.
Animals
Serum samples from 1702 dogs were collected from local animal hospitals and referral animal medical centers in South Korea.
Methods
TK1 protein OD value and TK1 autoantibody ratio (TK1 autoantibody OD/total IgG OD) in serum samples of dogs classified into healthy controls, group with nontumor disease, group with benign and group with malignant tumors were measured using lateral flow immunochromatographic assay methods.
Results
TK1 autoantibody levels were significantly higher in malignant tumor group (median 0.71) than in healthy controls (median 0.34), group with nontumor disease (median 0.34), and group with benign tumor (median 0.32, Welch t test, P < .0001). They were also significantly different among dogs with carcinomas (median 0.77), hematopoietic tumors (median 0.71), and sarcomas (median 0.56) than in healthy controls (median 0.34, post hoc Games‐Howell test, P < .0001). In the receiver operating characteristic curve of TK1 protein, AUC was 0.633 (95% CI: 0.592‐0.675, P < .0001). The AUC of TK1 autoantibody ratio was 0.758 (95% CI: 0.723‐0.793, P < .0001).
Conclusions and Clinical Importance
TK1 autoantibody is a potentially useful biomarker for differentiating between healthy and tumor‐bearing dogs, better than TK1 protein measurement. However, both were inadequate when used as single biomarkers for screening dogs to discover occult malignant tumors.
Keywords: autoantibody, biomarker, malignant tumors, thymidine kinase 1 protein
Abbreviations
- CLIA
competitive chemiluminescence immunoassay
- HCC
hepatocellular carcinoma
- IgY
egg yolk immunoglobulin
- LFIA
lateral flow immunochromatographic assay
- MGT
mammary gland tumor
- NI
neoplastic index
- TCC
transitional cell carcinoma
- TK1
thymidine kinase
1. INTRODUCTION
Thymidine kinase 1 (TK1), a member of deoxyribonucleoside kinase, is a key enzyme involved in the synthesis of DNA precursors through thymidine phosphorylation, and plays a role as an up‐regulator of cell proliferation in S phase. 1 There are several methods to measure the activity of TK1. The [3H]‐deoxythymidine phosphorylation assay, known as the gold standard, is a method to measure the amount of [3H]‐labeled deoxythymine converted to [3H]‐dTMP by TK1. 2 But, when TK1 presenting as a tetramer in the cell is leaked into serum, it forms a complex with different molecular weight, or is affected by an enzyme inhibitor. TK1 enzymatic assay could be affected by changes in these extracellular environments. 3 , 4 , 5 Unlike the method of measuring TK1 activity, the immunoassay method using an antibody capable of binding to a specific site of the TK1 protein can lead to more accurate results because all TK1 proteins including activated TK1 can be measured.
Tumor‐associated autoantibodies play an important role in the body's immune system by recognizing and producing antibodies against overexpressed, mutated, misfolded, or mispresented proteins released from damaged tissues in a variety of pathological conditions. 6 Unlike other proteins, tumor‐associated autoantibodies have advantages of being stably present in serum, having different target proteins, and purifying easily. 7 Since they are generated within the body's immune system, autoantibodies can be increased in large amounts within a short time, making it easy to measure using methods such as ELISA.
Although TK1 protein is significantly increased in several types of malignant tumors in dogs, methods for measuring changes in TK1 protein are mainly limited to assays that measure protein activity or ELISA. Currently, no studies have investigated changes in TK1 protein autoantibodies level in dogs with malignant tumors. In this study, we used immunoassay methods to measure TK1 protein concentration in serum samples. We purified TK1 protein through cloning and attached it to the membrane of a lateral flow immunoassay (LFIA) to measure concentrations of TK1 autoantibodies. We compared our results with those obtained from previous studies that measured TK1 protein levels using other methods.
2. MATERIALS AND METHODS
2.1. Animals and collection of samples
Serum samples from 2257 dogs were collected from local animal hospitals and referral animal medical centers that included Seoul National University Veterinary Medical Teaching Hospital and Jeonbuk University Veterinary Medical Teaching Hospital in Korea from 2016 to 2020. Among them, 1702 subjects were selected, excluding dogs whose signalments were not clear, or whose diagnoses were missing. Information such as signalment, minimum data including physical examination, serum chemistry, CBC, electrolytes, and thoracic/abdominal radiography were collected for dogs of all groups. In some dogs, abdominal ultrasonography, echocardiography, computed tomography, and magnetic resonance imaging were additionally performed diagnosis of tumor or nontumor disease. The definitive diagnosis of tumor was made by cytology and histopathology alone, or in combination. The purpose of hospital visits of the healthy control group of 343 dogs was for neutering/spaying or general health screening. No specific findings were identified. Both TK1 protein and autoantibody concentrations were measured before treatment for nontumor disease group and tumor group. All experiments were performed according to the regulations and policies of the Laboratory Animals Institutional Animal Care and Use Committee (BIC Study‐A‐202107‐220; Jeonbuk University Veterinary Medical Teaching Hospital, Iksan‐si, Jeollabuk‐do, Republic of Korea).
2.2. Cloning method for TK1 protein
To purify TK1 protein and produce TK1 antibody, TK1 protein cloning was first performed. PSK‐TK1 plasmid (=pBluescript II SK) vector digested with EcoRI and XhoI was prepared. The plasmid templates for PCR amplifications consisted of 10 ng of plasmid and 200 nM of each PCR primer, and 40 cycles were repeated at 94°C 1 minute, 94°C 1 minute, and 60°C 1 minute. E. coli strain DH5α was selected as Bacteria for transformation, incubated in 100 μL LB‐broth in a 1 mL Eppendorf tube, and shaken at 37°C for 1 hour. Each transformation was diluted and cultured in L‐agar containing ampicillin (100 μg/mL). Nine of the cloned cTK1 genes were sequenced using universal primers. The TK1 gene found in this way was requested for protein purification, and a monoclonal mouse anti TK1 antibody was devised.
2.3. LFIA methods for TK1 protein and autoantibody
The methods for TK1 autoantibody and protein were based on LFIA. Briefly, 100 μL of serum diluted 500‐fold (diluted 25‐fold in TK1 protein) in phosphate buffer was placed in the sample window and moved through a nitrocellulose membrane pad. Then, 50 nM gold nanoparticles were conjugated to canine TK1 protein/autoantibody. As the TK1 protein/autoantibody attached to antigen on the membrane pad, the results were displayed in a red line, and the results of the area in the red line were analyzed using computer vision techniques (Aniscaner, Biattic Inc., Anyang‐si, Gyeonggi‐do Republic of Korea).
2.4. Statistical analysis
Statistical analysis and graph work were processed using GraphPad Prism software (Systat Software, San Jose, California) and R studio. A box and whisker plot displayed min‐max, and median value was selected as a graph to show the difference in each protein concentration. Welch t test (parametric distribution, non‐equal variance) was applied to analyze the difference between TK1 protein and autoantibody in each different group. Games‐Howell test (parametric distribution, non‐equal variance, non‐equal sample size, n > 50) and Dunnett T3 test (parametric distribution, non‐equal variance, non‐equal sample size, n < 50) were selected for post hoc analysis for comparison between 2 or more groups. The statistically valid P value was set to .05 or less. Cut‐off value, specificity, and sensitivity were estimated using the receiver operating characteristic (ROC) curve by R studio.
3. RESULTS
Of a total of 1702 dog sera selected for the study, 568 dogs were diagnosed as tumors (benign tumors n = 67, malignant tumors n = 501), 343 dogs were included in the healthy control group. Seven hundred fifty‐two dogs visited with various diseases except for tumors. Table 1 showed the characteristics of each control and disease group by age and sex. The median age of the group with malignant tumor, benign tumor, nontumor diseases, and healthy control group were 12, 9, 10, and 5 years, respectively. According to origin, malignant tumors were classified into carcinoma, hematopoietic tumor, sarcoma, and other tumors (neuroendocrine tumors and round cell tumors) with 216, 169, 102, and 32 (14 and 18) dogs, respectively (Table 2). Carcinoma accounted for 43% of all tumors, and included hepatocellular carcinoma (HCC, n = 31), malignant mammary gland tumor (MGT, n = 50), and transitional cell carcinoma (TCC, n = 57). Hematopoietic tumors (leukemia [n = 22], lymphoma [n = 129]) accounted for 30% of total tumors. Sarcomas including hemangiosarcoma (n = 19), melanoma (n = 18), soft tissue sarcoma (n = 15), and osteosarcoma (n = 9) were found in 20% of the total. Table 3 showed the classification of the nontumor disease group that consisted of a total of 752 dogs. The most common disease group was endocrine diseases (12.9%), followed by cardiovascular diseases (11.4%), and digestive diseases (11.2%).
TABLE 1.
Signalments of all dogs included in the study.
| Healthy control | Nontumor disease | Benign tumor | Malignant tumor | |
|---|---|---|---|---|
| Number | 343 | 752 | 67 | 501 |
| Median age (range) | 4.6 (2 mo‐17 y) | 9 (5 mo‐19 y) | 9.7 (1 y‐18 y) | 11.8 (1 y‐28 y) |
| Sex (n) |
IM (75), NM (115) IF (90), SF (63) |
IM (57), NM (354) IF (95), SF (246) |
IM (1), NM (22) IF (12), SF (32) |
IM (31), NM (210) IF (54), SF (206) |
Abbreviations: IF, intact female; IM, intact male; NM, neutered male; SF, spayed female.
TABLE 2.
Classification of dogs with malignant tumors.
| Type of tumor | Malignant tumors (n) | N |
|---|---|---|
| Carcinomas | Anal sac adenocarcinoma (3), Apocrine adenocarcinoma (5), Hepatocellular carcinoma (31), Mammary gland adenocarcinoma (50), Nasal adenocarcinoma (13), Perianal adenocarcinoma (4), Prostatic carcinoma (7), Pulmonary adenocarcinoma (8), Renal cell carcinoma (4), Sebaceous carcinoma (5), Squamous cell carcinoma (9), Transitional cell carcinoma (57), Adenocarcinoma (Thyroid, Cholangiohepatocellular, Ovarian, Unknown origin, 20) | 216 |
| Hematopoietic tumors | Acute myeloid leukemia (2), Chronic lymphocytic leukemia (20), B cell lymphoma (66), T cell lymphoma (27), Unclassified lymphoma (36) | 151 |
| Sarcomas | Chondrosarcoma (3), Hemangiosarcoma (19), Hepatic/splenic sarcoma (9), Histiocytic sarcoma (3), Leiomyosarcoma (5), Liposarcoma (3), Melanoma (18), Osteosarcoma (9), Soft tissue sarcoma (15), Synovial sarcoma (2), Sarcoma (Seminoma, Unknown origin, 15) | 102 |
| Other tumors | Heart base tumor (1), Pheochromocytoma (11), Insulinoma (2), Mast cell tumor (18) | 32 |
TABLE 3.
Classification of dogs with nontumor diseases.
| Type of nontumor disease | N |
|---|---|
| Cardiovascular | 86 |
| Dental | 19 |
| Dermatologic | 48 |
| Endocrinal | 97 |
| Digestive | 84 |
| Hematopoietic | 14 |
| Hepatobiliary | 46 |
| Immune‐mediated | 22 |
| Infectious | 11 |
| Intoxication | 7 |
| Musculoskeletal | 37 |
| Neurologic | 81 |
| Ocular | 35 |
| Reproductive | 10 |
| Respiratory | 23 |
| Surgery and trauma | 26 |
| Urinary | 70 |
| One more concurrent | 36 |
Figure 1 showed differences in TK1 protein and its autoantibody among groups. TK1 protein exhibited a significant difference between the group with malignant tumors and the healthy control group as well as the group with non‐tumor diseases (Welch's t test, post hoc Games‐Howell test, P < .0001; Figure 1A). There was no statistically significant difference in TK1 protein or its autoantibody between the group with benign tumors and the group with malignant tumors (Welch's t test, post hoc Games‐Howell test, P = .27; Figure 1A). The median of the TK1 protein OD was 194 038, range (70 151‐635 603); 164 371, range (66 642‐640 361); 169 280, range (18 142‐1 207 944); and 157 903, range (18 142‐469 390), in the order: malignant tumor, benign tumor, nontumor disease, and control group, respectively. To exclude the effect of the humoral immune status on autoantibody concentration in each sample, total IgG OD was measured simultaneously with TK1 autoantibody, and the ratio of TK1 autoantibody OD to total IgG OD (TK1 autoantibody OD/total IgG OD) was calculated. As a result, the TK1 autoantibody ratio the in the malignant tumor group was significantly different from those in the healthy control group, nontumor disease group, and dogs with benign tumor (Welch t test, post hoc Games‐Howell test, P < .0001; Figure 1C). The median of the TK1 autoantibody ratio was 0.71, range (0.06‐28.51); 0.32, range (0.07‐3.86); 0.37, range (0.07‐25.84); and 0.34, range (0.07‐3.43), in the order: malignant tumor, benign tumor, nontumor disease, and healthy control group, respectively.
FIGURE 1.
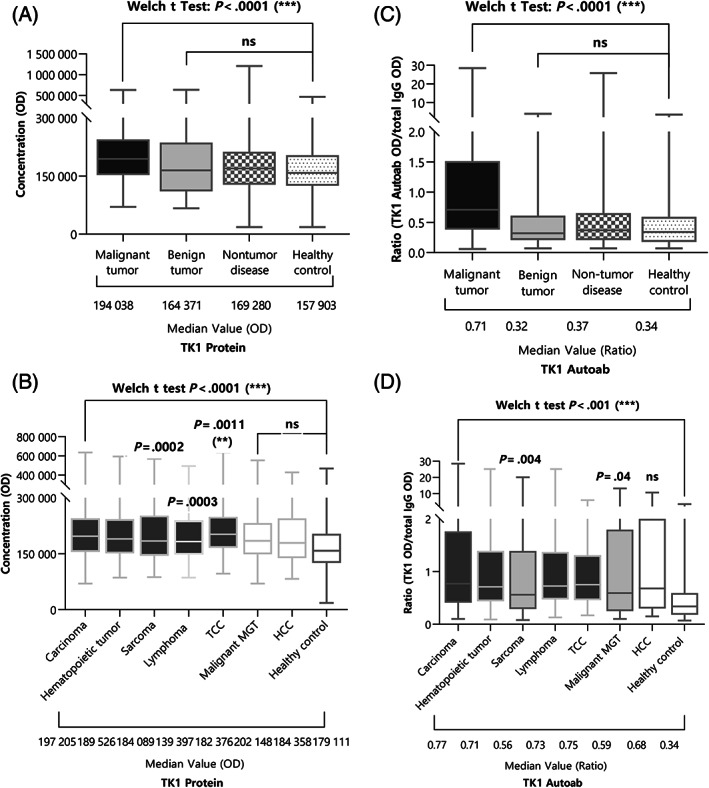
Results of (A) and (B) TK1 protein OD, and (C) and (D) TK1 autoantibody ratio (TK1 Autoantibody OD/total IgG OD) in each group and several malignant tumor groups. The TK1 autoantibody ratio showed a significant difference in the malignant tumor group compared to that in the healthy control group, nontumor disease group, or dogs with benign tumor. TK1 protein could not discriminate between group with benign tumors and group with the malignant tumors. TK1 protein was significantly higher in dogs with carcinoma including TCC, hematopoietic tumor including lymphoma, and sarcoma. TK1 autoantibody ratio was significantly higher in dogs with carcinoma including TCC and malignant MGT, hematopoietic tumor including lymphoma, and sarcoma.
Figure 1B,D showed how TK1 protein value and TK1 autoantibody ratio appeared in each malignant tumor groups classified by origin. TK1 protein was significantly higher in carcinoma including TCC, hematopoietic tumor including lymphoma, and sarcoma, than that of the control group (Welch t test, post hoc Dunnett's T3 test, carcinoma, hematopoietic tumor P < .0001, sarcoma P = .0002, lymphoma P = .0003, TCC P = .0011; Figure 1B). Median of TK1 protein OD was 197 205, range (70 151‐645 603); 189 526, range (86 065‐593 683); 184 089, range (87 411‐570 374); 182 376, range (86 065‐493 199); 202 148, range (96 645‐635 603); 184 358, range (70 151‐553 688); 179 111, range (82 736‐427 279); and 139 397, range (57 405‐234 229), in the order: carcinoma, hematopoietic tumor, sarcoma, lymphoma, TCC, malignant MGT, HCC, and healthy control group, respectively. Compared with the healthy control group, TK1 autoantibody ratio was significantly higher in carcinoma, hematopoietic tumor, sarcoma, lymphoma, TCC, and malignant MGT (Welch t test, post hoc Dunnett's T3 test, carcinoma, hematopoietic tumor, lymphoma, TCC P < .0001, sarcoma P = .004, malignant MGT P = .04; Figure 1D). Median of TK1 autoantibody ratio was 0.77, range (0.10‐28.51); 0.71, range (0.09‐25.18); 0.56, range (0.08‐20.15); 0.73, range (0.17‐5.90); 0.75, range (0.17‐5.90); 0.59, range (0.10‐13.15); 0.68, range (0.15‐10.61); and 0.34, range (0.07‐3.43), in the order: carcinoma, hematopoietic tumor, sarcoma, lymphoma, TCC, malignant MGT, HCC, and healthy control group, respectively. In addition, although results were not included because of the small number of dogs collected, hemangiosarcoma, osteosarcoma, and soft tissue sarcoma showed statistically significant differences, similar to those in the sarcoma group, when compared to the healthy control group.
The nontumor disease group was subdivided into 14 categories according to the characteristics of the disease, and the differences between TK1 protein and its autoantibody were compared. In the case of TK1 protein OD, hepatobiliary disease (median: 198 626, range [98 849‐441 720]) only was higher than that of the healthy control group (median: 139 397, range (57 405‐234 229), Dunnett's T3 test, P = .03; Figure 2A), in TK1 autoantibody ratio, only immune‐mediated disease (median: 0.19, range [0.08‐0.89]) was significantly different from the control group (median: 0.34, range (0.07‐3.43), Dunnett's T3 test, P < .005; Figure 2B).
FIGURE 2.
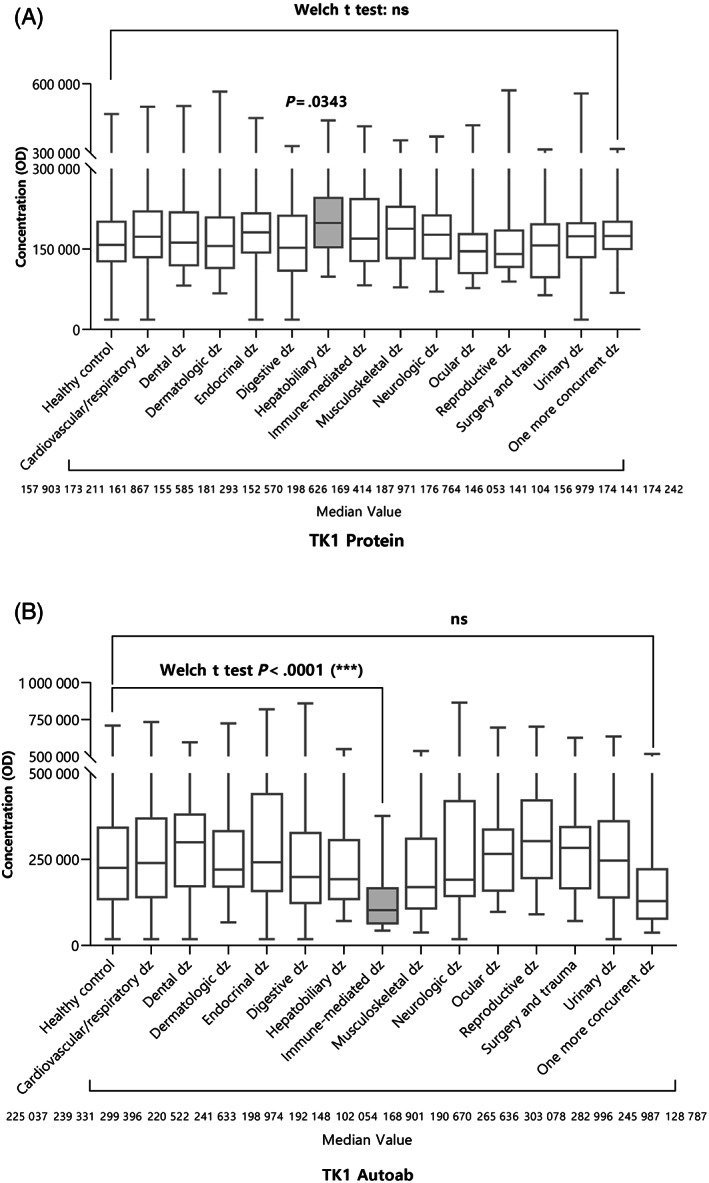
Measurement of (A) TK1 protein OD, and (B) TK1 autoantibody for each type of nontumor disease. In the case of TK1 protein, hepatobiliary disease was higher than that of the healthy control group. Only immune‐mediated disease was significantly different from the healthy control group in the TK1 autoantibody ratio.
For the purpose of discriminating between the presence or absence of malignant tumors, ROC curves were constructed to optimize the sensitivity and specificity of using TK1 protein and autoantibody ratio for discriminating healthy controls and malignant tumor group. Results were shown in Figure 3. TK1 protein OD and autoantibody ratio were corrected through normalization, before conducting the ROC curve. The AUC of TK1 autoantibody ratio was 0.758 (P < .001, 95% CI [0.723‐0.793]), and when the cut‐off value was 0.55, the specificity was 72.3% and sensitivity 69.1% (Figure 3). In the ROC curve of TK1 protein, AUC was 0.633 (P < .001, 95% CI [0.592‐0.675]), and when the cut off value was 0.584, the specificity was 56.1% and the sensitivity was 67.5% (Figure 3).
FIGURE 3.
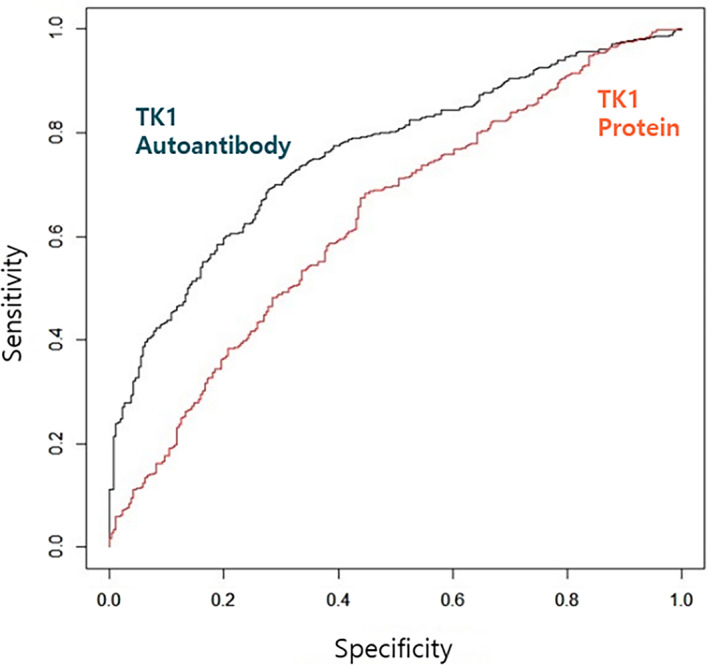
ROC curve of TK1 protein OD and TK1 autoantibody ratio. In the ROC curve of TK1 protein, AUC was 0.633 (P < .001, 95% CI [0.592‐0.675]), and the AUC of TK1 autoantibody ratio was 0.758 (P < .001, 95% CI [0.723‐0.793]).
4. DISCUSSION
In this study, TK1 protein and its autoantibody concentration were measured to investigate their potentials as tumor biomarkers using serum samples of dogs. TK1 protein showed a statistically significant increase in group with malignant tumors compared to that in healthy control group or nontumor disease group. However, it showed no significant difference between group with malignant tumors and those with benign tumors. Concentrations of TK1 autoantibodies appeared to be significant different from those of dogs with malignant tumors. However, when AUC was calculated to assess the sensitivity and specificity of using TK1 protein and autoantibody, it was found that other biomarkers might be needed rather than relying on them alone.
Even though various methods are applied to measure TK1 activity or protein concentration, TK1 is consistently increased significantly compared to control group in human patients with lymphoma and leukemia, and is proportional to staging, and could be used to monitor the therapeutic effect. 8 , 9 In solid tumors, TK1 activity has an ambiguous increase, compared to hematopoietic tumors. But since the development of the TK1 protein assay method, significant increases are also confirmed in gastric cancer, ovarian cancer, lung cancer, prostate cancer, and breast cancer. 10 , 11 Most studies that adopts enzymatic assay to measure changes of TK1 in specific tumors, such as lymphoma, have reported that TK1 can be a diagnostic marker for malignant tumors in dogs. 12 , 13 TK1 protein concentration measured by sandwich TK1 ELISA is also found that it is stably increased not only in hematopoietic tumors, but also in solid tumors, compared with the results of TK1 activity assay. 14 They also measure TK1 activity and immunoaffinity in the serum of CLL and MGT dogs, and speculate that the higher TK1 activity in CLL than in MGT could be because of the presence of more inactive TK1 protein in the mass of MGT. 15 There have been no reports using TK1 autoantibody for the screening of malignant tumors in both human and veterinary medicine. We evaluated the change of TK1 by measuring TK1 autoantibody and compared with the TK1 protein assay.
TK1 protein concentration in the serum was statistically significantly higher than that of the control group in all individual tumors. Compared with the results of Jagarlamudi et al. who performed TK1 protein assay, lymphoma and malignant MGT showed a similar increase, 15 but there were no reports on TCC and HCC in dogs, so further evaluation is needed. The TK1 autoantibody was significantly higher in carcinoma, hematopoietic tumor, and sarcoma than in the healthy control group (Figure 1D). We were able to demonstrate that the TK1 autoantibody is useful both in the screening and diagnosis of hematopoietic tumors, and various types of carcinomas and sarcomas. TK1 autoantibody ratio was also significantly high in lymphoma and transitional cell carcinoma (Figure 1D), consistent with several papers reporting a significant increase in TK1 activity in dogs with lymphoma. 12 , 13 In the box and whisker plots of each group, there was an overlap in error bars, indicating the need to supplement it with a multi‐biomarker approach to develop a novel NI.
In the results of the nontumor disease group, TK1 autoantibody ratio was not statistically significant compared to the healthy control group, except for dogs diagnosed with immune‐mediated disease (before treatment) (Figure 2B). The median value of the TK1 autoantibody ratio in the healthy control group was 0.34, whereas the mean in the dogs with immune‐mediated disease was as low as 0.19. Considering that immunosuppressants are often administered for therapeutic purposes in dogs with immune‐mediated disease, and that TK1 activity was reduced when a chemotherapy protocol including prednisolone was administered in dogs with lymphoma and leukemia, 16 , 17 it was highly likely that immunosuppressants had an effect on lowering TK1 autoantibody concentrations.
Most of the studies investigating TK1 changes in various tumors adopt the assay of measuring TK1 activity. Jagarlamudi et al. compare the differences between TK1 activity and TK1 protein assay between lymphoma and solid tumors, in which the AUC of TK1 protein assay is 0.96, and AUC of TK1 activity is 0.84, confirming that the ELISA method using antibody is more accurate. The TK1 protein assay shows higher accuracy as AUC 0.88, the AUC of TK1 activity was 0.59 in solid tumor. 15 Based on measurements of TK1 activity and CRP in dogs with malignant tumors, Selting et al. have designed a neoplastic index (NI) through statistical analysis for the purpose of screening malignant tumors. 18 As results, the AUCs of TK1 and CRP are 0.873 and 0.818, respectively, and NI's AUC is calculated as an improvement of 0.844. 18 In this study, we found that the AUC of TK1 autoantibody was 0.758 (sensitivity 69.1%, specificity 72.3%), and the AUC of TK1 protein was 0.63 (sensitivity, 67.5%, specificity 56.1%) in malignancy (Figure 3). It was found that TK1 autoantibody could be more useful for tumor biomarker than TK1 protein. The method using the autoantibody adopted in this study was difficult to compare, because it has not previously been reported in either human or veterinary medicine, but the sensitivity and specificity of the TK1 protein assay in this study were somewhat lower than those of Jagarlamudi's research. It was speculated that Jagarlamudi's study separated hematopoietic tumor from solid tumor and targeted a small number of samples compared to this study. The following causes were speculated for the low sensitivity and specificity of the TK1 autoantibody ratio: missing diagnosis because of insufficient signalment or minimum data base in some dogs, possibility of the dog's immune status affecting the TK1 autoantibody even though it was corrected for the total IgG concentration, no discrimination of staging the tumors, an increase in the number of samples belonged to outliers as the number of samples collected increased compared to previous research, and the unknown mechanisms of action of TK1 autoantibody. Subsequent re‐evaluation is necessary for a larger number of subdivided samples sorted by tumor stage. The correlation between TK1 protein assay and autoantibody assay had no statistical significance. To explain this difference between the TK1 protein released into the serum and the autoantibody produced in response thereto, further studies are needed to elucidate the mechanism of the autoantibody and the interaction between TK1 autoantibody and protein.
While the TK1 activity assay is difficult to apply to solid tumors, the method using autoantibody can be a powerful tool for screening almost all malignant tumors, except neuroendocrine tumors. Protein assay is known to be an improved method for measuring serum TK1 concentration compared to TK1 activity assay, but the method using TK1 autoantibody proved superior in sensitivity and specificity compared to that protein assay method. To improve the accuracy of NI for screening malignant tumors, it would be necessary to develop a multi‐biomarker model that includes TK1 autoantibody and TK1 protein.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All experiments were performed according to the regulations and policies of the Laboratory Animals IACUC (BIC Study‐A‐202107‐220), Jeon Buk University Veterinary Medical Teaching Hospital, Iksan, Jeon Buk, Korea.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This research was supported by Biattic Inc. (Anyang, Korea).
Kim Y, Park J, Kim B, et al. Serum thymidine kinase 1 protein concentrations and presence of its autoantibody as biomarkers for screening dogs with malignant tumors. J Vet Intern Med. 2024;38(1):300‐307. doi: 10.1111/jvim.16946
REFERENCES
- 1. Bello LJ. Regulation of thymidine kinase synthesis in human cells. Exp Cell Res. 1974;89:263‐274. [DOI] [PubMed] [Google Scholar]
- 2. Munch‐Petersen B. Differences in the kinetic properties of thymidine kinase isoenzymes in unstimulated and phytohemagglutinin‐stimulated human lymphocytes. Mol Cell Biochem. 1984;64(2):173‐185. doi: 10.1007/BF00224774 [DOI] [PubMed] [Google Scholar]
- 3. Jagarlamudi KK, Shaw M. Thymidine kinase 1 as a tumor biomarker: technical advances offer new potential to an old biomarker. Biomark Med. 2018;12(9):1035‐1048. doi: 10.2217/bmm-2018-0157 [DOI] [PubMed] [Google Scholar]
- 4. Bitter EE, Townsend MH, Erickson R, Allen C, O'Neill KL. Thymidine kinase 1 through the ages: a comprehensive review. Cell Biosci. 2020;10(1):1‐16. doi: 10.1186/s13578-020-00493-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou J, He E, Skog S. The proliferation marker thymidine kinase 1 in clinical use. Mol Clin Oncol. 2013;1(1):18‐28. doi: 10.3892/mco.2012.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hussain S, Saxena S, Shrivastava S, et al. Multiplexed autoantibody signature for serological detection of canine mammary tumours. Sci Rep. 2018;8(1):1‐14. doi: 10.1038/s41598-018-34097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gnjatic S, Wheeler C, Ebner M, et al. Seromic analysis of antibody responses in non‐small cell lung cancer patients and healthy donors using conformational protein arrays. J Immunol Methods. 2009;341(1–2):50‐58. doi: 10.1016/j.jim.2008.10.016 [DOI] [PubMed] [Google Scholar]
- 8. Pan ZL, Ji XY, Shi YM, Zhou J, He E, Skog S. Serum thymidine kinase 1 concentration as a prognostic factor of chemotherapy‐treated non‐Hodgkin's lymphoma patients. J Cancer Res Clin Oncol. 2010;136(8):1193‐1199. doi: 10.1007/s00432-010-0769-z [DOI] [PubMed] [Google Scholar]
- 9. Xu W, Cao X, Miao KR, et al. Serum thymidine kinase 1 concentration in Chinese patients with chronic lymphocytic leukemia and its correlation with other prognostic factors. Int J Hematol. 2009;90(2):205‐211. doi: 10.1007/s12185-009-0380-8 [DOI] [PubMed] [Google Scholar]
- 10. Liu Y, Ling Y, Qi Q, et al. Changes in serum thymidine kinase 1 levels during chemotherapy correlate with objective response in patients with advanced gastric cancer. Exp Ther Med. 2011;2(6):1177‐1181. doi: 10.3892/etm.2011.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He Q, Fornander T, Johansson H, et al. Thymidine kinase 1 in serum predicts increased risk of distant or loco‐regional recurrence following surgery in patients with early breast cancer. Anticancer Res. 2006;26(6 C):4753‐4759. [PubMed] [Google Scholar]
- 12. Nakamura N, Momoi Y, Watari T, Yoshino T, Tsujimoto H, Hasegawa A. Plasma thymidine kinase activity in dogs with lymphoma and leukemia. J Vet Med Sci. 1997;59(10):957‐960. doi: 10.1292/jvms.59.957 [DOI] [PubMed] [Google Scholar]
- 13. von Euler HP, Öhrvik AB, Eriksson SK. A non‐radiometric method for measuring serum thymidine kinase activity in malignant lymphoma in dogs. Res Vet Sci. 2006;80(1):17‐24. doi: 10.1016/j.rvsc.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 14. Jagarlamudi KK, Moreau L, Westberg S, Rönnberg H, Eriksson S. A new sandwich ELISA for quantification of thymidine kinase 1 protein levels in sera from dogs with different malignancies can aid in disease management. PloS One. 2015;10(9):1‐15. doi: 10.1371/journal.pone.0137871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jagarlamudi KK, Westberg S, Rönnberg H, Eriksson S. Properties of cellular and serum forms of thymidine kinase 1 (TK1) in dogs with acute lymphocytic leukemia (ALL) and canine mammary tumors (CMTs): implications for TK1 as a proliferation biomarker. BMC Vet Res. 2014;10(1):1‐12. doi: 10.1186/s12917-014-0228-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elliott JW, Cripps P, Blackwood L. Thymidine kinase assay in canine lymphoma. Vet Comp Oncol. 2013;11(1):1‐13. doi: 10.1111/j.1476-5829.2011.00296.x [DOI] [PubMed] [Google Scholar]
- 17. von Euler H, Einarsson R, Olsson U, Lagerstedt A‐S, Eriksson S. Serum thymidine kinase activity in dogs with malignant lymphoma, a potent marker for prognosis and monitoring the disease. Scand J Rheumatol Suppl. 2004;30(115):23‐26. doi: 10.1080/030097401300232600 [DOI] [PubMed] [Google Scholar]
- 18. Selting KA, Ringold R, Husbands B, Pithua PO. Thymidine kinase type 1 and C‐reactive protein concentrations in dogs with spontaneously occurring cancer. J Vet Intern Med. 2016;30(4):1159‐1166. doi: 10.1111/jvim.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]


