Abstract
We have analyzed human T-cell responses in parallel with serum immunoglobulin G (IgG) antibody levels after systemic vaccination with the Norwegian group B Neisseria meningitidis outer membrane vesicle (OMV) vaccine. Ten adult volunteers, with no or very low levels of serum IgG antibodies against meningococci, received three doses intramuscularly of the OMV vaccine (at weeks 0, 6, and 46). T-cell proliferation against the OMV vaccine, purified outer membrane proteins (PorA and PorB), and control antigens (Mycobacterium bovis BCG vaccine and tetanus toxoid) was measured by thymidine incorporation of peripheral blood mononuclear cells before and after vaccination. The results showed that vaccination with OMV elicits strong primary and booster T-cell responses specific to OMV as well as the PorA (class 1) protein and significant, but markedly lower, responses against the PorB (class 3) protein. The median responses to OMV and PorA were 26 and 16 times the prevaccination levels, respectively. Most of the vaccinees showed low T-cell responses against OMV and PorA before vaccination, and the maximum T-cell responses to all vaccine antigens were usually obtained after the second vaccine dose. We found a positive correlation between T-cell responses and anti-OMV IgG antibody levels (r = 0.50, P < 0.0001, for OMV and PorA). In addition, we observed a progressive increase in the percentage of CD45R0+ (memory) CD4-positive T cells (P = 0.002). In conclusion, we have shown that the Norwegian OMV vaccine against meningococcal B disease induced antigen-specific T-cell responses, kinetically accompanied by serum IgG responses, and that vaccination increased the proportion of memory T-helper cells.
Vaccination with protein antigens will usually result in both a cellular (T-cell) and humoral (B-cell) immune response. For protection against extracellular bacterial infections, like Neisseria meningitidis, bactericidal and opsonic antibodies are of crucial importance, while T cells play a more indirect role by regulating the antibody response in terms of immunoglobulin class switch, affinity maturation, and magnitude of response (1). T cells are also necessary for the induction of immunological memory and can indirectly induce killing of bacteria by activating phagocytes (24).
Although polysaccharide-based vaccines against serogroup A and C meningococci are available, this principle cannot be applied to the B serogroup (the most prevalent serogroup in Europe, America, and South Australia) due to low immunogenicity of the B polysaccharide in humans (36). Furthermore, polysaccharides fail to induce T-cell responses and give a poor and short-lived immunity in infants due to their immature immune systems (13, 26). This situation has motivated the development of a Norwegian outer membrane vesicle (OMV) vaccine against group B meningococci based on a B:15:P1.7,16 epidemic strain (44/76) in which the major antigens are outer membrane proteins (11). The porin proteins PorA (class 1) and PorB (class 3) are the most abundant neisserial outer membrane proteins (6). They are present in equal amounts and account for about 70% of the proteins (by weight) in the OMV vaccine (11). This vaccine has previously been shown to induce protection against group B meningococcal disease among teenagers in a large, double-blind, placebo-controlled study (4).
To address the question of T-cell-mediated help in vaccine-induced protective B-cell responses against meningococci, we have investigated human T-cell responses during a three-dose regimen with the Norwegian OMV vaccine. Proliferative in vitro T-cell responses against the OMV vaccine and PorA and PorB outer membrane proteins were assayed in peripheral lymphocytes from OMV vaccinees and compared with immunoglobulin G (IgG) antibody responses.
MATERIALS AND METHODS
Vaccine.
The vaccine was deoxycholate-extracted OMVs from meningococcal strain 44/76 (B:15:P1.7, 16:L3,7,9) adsorbed to aluminum hydroxide. In addition to the major outer membrane proteins (classes 1, 3, 4, and 5), the vaccine contained the Opc protein, small amounts of less-well-characterized membrane proteins, and about 8% lipopolysaccharide (LPS) (11). One dose contained 25 μg of protein, 2 μg of LPS, and 1.67 mg of Al(OH)3 and was injected intramuscularly (0.5 ml) into the deltoid muscle.
Vaccine antigens.
PorA (class 1) and PorB (class 3) outer membrane proteins were purified from mutant variants of strain 44/76, HIII5 and HI5, lacking PorB and PorA, respectively, and both lacking RmpM (class 4 protein). The porins were solubilized with Zwittergent detergent, purified by chromatography, and reconstituted as proteosomes lacking potentially lymphotoxic detergent (5, 17, 33). There was no contamination with other outer membrane proteins, as demonstrated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analyses. LPS contamination was less than 0.01% as judged by gel electrophoresis and silver staining (31).
Vaccinees, samples, and immunization schedule.
Ten healthy adult volunteers, designated 1 to 10 (three males and seven females, aged 24 to 49 years, median age = 40), with negative anamnesis for meningococcal disease and selected for low serum IgG antibody levels against group B meningococci, were given two doses of the OMV vaccine with a 6-week interval and a third dose after 10 months. Blood samples were collected, and serum and peripheral blood mononuclear cells (PBMCs) were isolated before vaccination; 2 and 6 weeks after the first and second doses; and 1, 2, and 6 weeks after the third dose. Two of the vaccinees had received one dose of an experimental group B meningococcal vaccine 10 years earlier, which contained outer membrane proteins from two meningococcal strains, 44/76 (B:15:P1.7,16) and 8047 (2b:P1.2), complexed with serogroup A, C, Y, and W-135 capsular polysaccharides (28). The others had not previously been immunized against meningococci. None of the vaccinees were found to be tonsillopharyngeal carriers of meningococci during the study period. The study was approved by the Norwegian Medicines Control Authority and the Regional Ethical Committee for Medical Sciences, Southeast Norway.
T-cell proliferation assay.
PBMCs were isolated from whole blood (drawn into ACD Vacutainers [Becton Dickinson, Rutherford, N.J.]) by density centrifugation (Lymphoprep; Nycomed, Oslo, Norway) (8) and assayed for in vitro proliferative responses by the thymidine incorporation method against OMV, PorA and PorB antigens, Mycobacterium bovis BCG vaccine antigen (Statens Seruminstitut, Copenhagen, Denmark), tetanus toxoid (National Institute of Public Health, Oslo, Norway), and phytohemagglutinin (PHA) (Sigma, St. Louis, Mo.). Freshly isolated PBMCs (105 cells per well) were cultured in the absence or presence of antigen in 96-well flat-bottomed microculture plates (Costar) in RPMI 1640 medium supplemented with 2 mM l-glutamine (Gibco), benzylpenicillin (100 IU/ml; Gibco), streptomycin (100 μg/ml; Gibco), and 15% heat-inactivated (30 min at 56°C), pooled human AB serum (final volume, 150 μl/well). Antigen was added in triplicate at final concentrations of 4, 0.8, 0.16, and 0.032 μg/ml for OMV and 5, 1, 0.2, and 0.04 μg/ml for PorA and PorB. These concentrations were previously shown to cover the antigen concentrations giving the maximum T-cell response in different individuals, which usually was the same within one individual at all time points tested. BCG was used at final concentrations of 20, 4, and 0.8 μg/ml; tetanus toxoid was used at 40, 8, and 1.6 μg/ml; and PHA was used at 25, 5, and 1 μg/ml. After 6 days of incubation (5% CO2, 37°C), the cultures were pulsed with [3H]thymidine (1.25 μCi/well; Amersham, Little Chalfont, United Kingdom) for 4 h, harvested on filters with a cell harvester (Skatron, Lier, Norway), and transferred to plastic vials (Maxi-vial; Packard). Scintillation liquid (Ultima Gold F; Packard) was added (10 ml/vial), and radioactivity incorporated into DNA was determined by liquid scintillation counting (TRI-CARB 1500; Packard).
To avoid exclusion of appropriate antigen-presenting cells, unfractionated PBMCs were used with the 6-day proliferation assay, which is widely accepted as a measure of T-cell activity. The T-cell-to-B-cell ratio was determined by flow cytometry in all blood samples. The T cells accounted for about 75% of the PBMCs, and the B cells varied between 5 and 15% of the PBMCs.
The proliferation results are expressed as mean disintegrations per minute of triplicate cultures for the antigen concentration giving maximum response minus the mean disintegration-per-minute values for 12 wells without antigen (medium only). Proliferative responses exceeding 2,000 dpm (disintegrations per minute for antigen − disintegrations per minute for medium) and at least threefold higher than individual background proliferation (medium only) were considered positive.
Enzyme-linked immunosorbent assay quantitation of serum IgG antibody against OMV.
To quantitate IgG antibodies against OMV in serum, vaccinee sera and reference serum were added in twofold serial dilutions (starting at 1:40 and diluted in phosphate-buffered saline [PBS]–Tween 20–1% bovine serum albumin) to OMV-coated microtiter plates (PBS, 100 μl/well, 4 μg/ml). The plates were incubated at 18°C overnight and washed in PBS-Tween 20. Thereafter, a mixture of biotinylated sheep anti-human IgG antibody (produced in our laboratory and diluted 1:8,000), alkaline phosphatase-biotin conjugate (1:6,000), and streptavidin (1:6,000) was added and incubated for 2 h at 37°C. Following washing, p-nitrophenyl phosphate (Sigma) was added and absorbance levels were recorded at 405 nm in a Thermomax microplate reader (Molecular Devices, Sunnyvale, Calif.).
The reference serum used was a postvaccination serum (MKIGG13-01) from a vaccinee in a previous vaccination trial drawn 4 weeks after the second dose, and IgG antibody concentrations were expressed in micrograms per milliliter with (5-iodo-4-hydroxy-3-nitro-phenacetyl) hapten-specific chimeric antibodies as heterologous standards as previously described for a method for IgG subclass quantitation (23).
Flow cytometric analysis of PBMC subpopulations.
Lymphocytes were analyzed for membrane markers by flow cytometry with freshly isolated whole blood. Monoclonal antibodies (labeled with fluorescein isothiocyanate or phycoerythrin) to the surface antigens; CD19 (B-cell marker), CD4 (helper T-cell marker), CD8 (cytotoxic T-cell marker), and CD45R0 (memory T-cell marker) (all from Becton Dickinson, San Jose, Calif.), were used. Two-color staining analyses were performed for CD4 and CD8 together with CD45R0. Freshly isolated whole blood from an unvaccinated control person was included in all tests at all time points. Samples were analyzed on an EPICS Profile II (Coulter Electronics, Luton, United Kingdom) flow cytometer, and matched isotype control antibodies were used to set correct regions to be analyzed. Only relative amounts of the different PBMC subsets could be determined and not the exact cell number, due to limitations of the flow cytometer.
Statistical methods.
Wilcoxon paired signed rank tests were used to test for statistical significance. The Spearman correlation coefficients were determined in the correlation analyses. PRISM software (GraphPad Software, San Diego, Calif.) was used in all statistical calculations.
RESULTS
T-cell response against OMV.
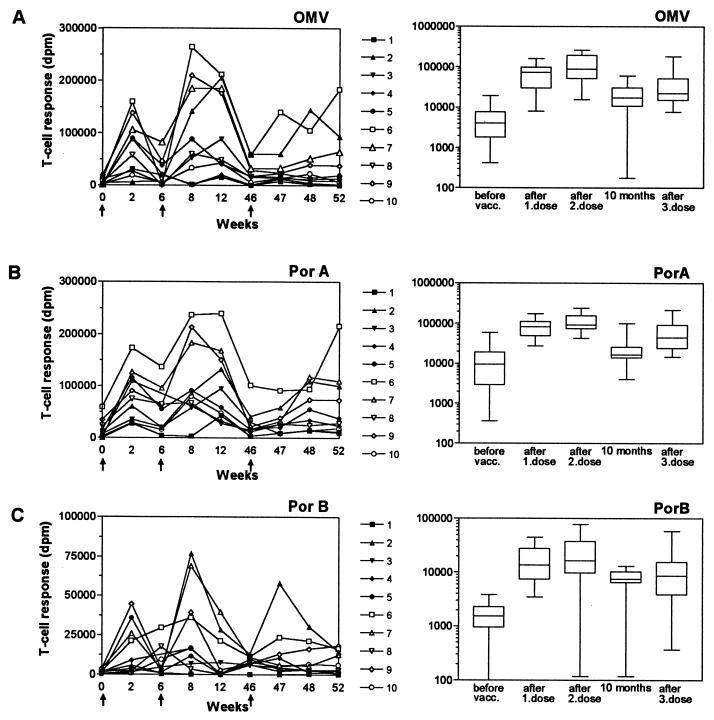
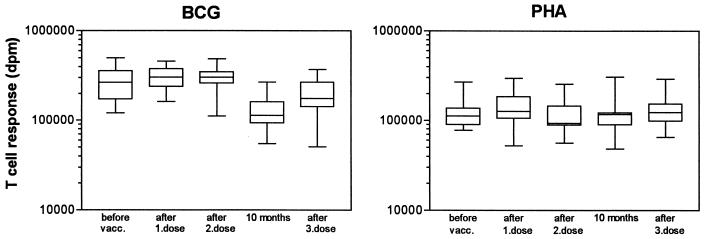
A total of 6 of 10 vaccinees showed moderate T-cell responses against OMV before vaccination (median dpm = 4,033) (Fig. 1A). After the first vaccine dose, we observed a large increase in T-cell responses against OMV in all vaccinees (median dpm = 73,200; P = 0.002), although large individual variations occurred (7- to 214-fold increase from prevaccination levels; median = 26-fold) with the maximum response observed 2 weeks after vaccination. Before the second dose was given (after 6 weeks), T-cell responses decreased in all but one vaccinee (median dpm = 13,600). The second dose induced T-cell responses which were higher than the responses obtained after the first dose in the majority of the vaccinees, with median dpm being 88,700 (6- to 209-fold increase from prevaccination levels; median = 19-fold). However, considered as a group, the differences in responses after the first and second doses were not statistically significant. Two of the vaccinees (vaccinees 5 and 8) had equal responses after the first and second doses. Vaccinees 1 and 4, who previously had been immunized against meningococci, obtained their maximum response after the first dose. Before the third dose was given (after 10 months), disintegrations per minute were higher than prevaccination levels for all vaccinees (median dpm = 17,400), except for vaccinees 1 and 4, who had responses slightly above background level. Vaccination with the third dose did result in elevation of the T-cell responses against OMV in 8 of the 10 vaccinees (median dpm = 22,250; P = 0.0098), but the level did not exceed the responses obtained after the first or second dose for any of the vaccinees (2- to 53-fold increase from prevaccination levels; median = 9-fold). The T-cell response against the control antigens, BCG and tetanus toxoid (data not shown), dropped significantly from week 46 and in the following tests, although the response to PHA was not decreased (Fig. 2). This could indicate that the responses against OMV after the third dose are underestimated but, in spite of this, will be lower than responses after the first or second dose.
FIG. 1.
T-cell responses in 10 adult vaccinees receiving three doses of OMV vaccine measured as in vitro proliferative responses in disintegrations per minute after 6 days of stimulation with vaccine antigens: OMV vaccine (A), purified PorA outer membrane protein (B), and purified PorB outer membrane protein (C). Left panels show responses for individual vaccinees, and immunizations are indicated by arrows. Right panels show maximum T-cell responses obtained before vaccination, after the first and second dose, 10 months after the first dose, and after the third dose given. Boxes extend from the 25th to the 75th percentile, with a horizontal line at the median. Vertical bars show the range of the data. Significant responses after vaccination were observed after each immunization (OMV, P = 0.002; PorA, P = 0.002; and PorB, P = 0.002 after the first dose and P = 0.0039 after the second and third doses).
FIG. 2.
T-cell responses against control antigens in the OMV-vaccinated individuals after 6 days of stimulation with BCG vaccine and PHA. Maximum responses (in disintegrations per minute) obtained before vaccination, after the first and second doses, 10 months after the first dose, and after the third dose are shown. Boxes extend from the 25th to the 75th percentile, with a horizontal line at the median. Vertical bars show the range of the data.
T-cell response against the PorA protein.
The prevaccination responses against PorA antigen were generally higher than those for OMV (median dpm = 9,500) (Fig. 1B). The vaccine response against PorA antigen showed the same overall pattern as that for OMV in all vaccinees. The responses after the first and second vaccine doses were of the same magnitude as the OMV responses but generally higher after the third dose. After the first vaccine dose, we observed a large increase in T-cell responses against the PorA antigen in all vaccinees (median dpm = 82,900; P = 0.002) and that maximum values were obtained after 2 weeks (3- to 350-fold increase from prevaccination levels; median = 7-fold). Before the second dose was given (after 6 weeks), T-cell responses decreased in all vaccinees (median dpm = 55,300), and after the second dose, we observed an increased response in 7 of the 10 vaccinees (median dpm = 93,100) with a 3- to 500-fold increase from prevaccination levels (median = 13-fold). Considered as a group, the differences between the responses after the first and second doses were statistically significant (P < 0.05). Before the third dose (at 10 months), T-cell responses were usually higher than prevaccination levels (median dpm = 16,600). The third dose induced an increase in the T-cell responses in 9 of the 10 vaccinees (median dpm = 44,700; P = 0.014), but the level of response was lower than that of the responses induced by the preceding doses.
T-cell response against the PorB protein.
A total of 4 of the 10 vaccinees showed weak T-cell responses against the PorB antigen before vaccination (<4,000 dpm), whereas the others showed no response. The vaccine-induced proliferation response against the PorB antigen was markedly lower compared to those for OMV and the PorA antigen with respect to all three doses given (Fig. 1C). Vaccinee 1 showed no response to the PorB antigen, whereas the others showed significant responses ranging from a 4- to a 360-fold increase from prevaccination levels (median = 9-fold) after the first dose (median dpm = 17,700; P = 0.002). T-cell responses after the second dose varied between a 2- and a 116-fold increase from prevaccination levels (median = 16-fold). Six of the vaccinees showed an increased response after the second dose compared to the first dose, while two vaccinees showed a decreased response (median dpm = 16,600). However, considered as a group the increases were not statistically significant. The response against PorB showed different kinetics than those for the OMV and PorA antigens when individual responses were examined (Fig. 1C). The response to the first dose evolved more slowly, and half of the vaccinees did not reach their maximum response until 6 weeks after vaccination (and not after 2 weeks, as for the OMV and PorA antigen). After the second dose, all vaccinees showed maximum responses after 2 weeks and no further increase after 6 weeks, as was observed for half of the vaccinees with the OMV and the PorA antigen. As for the OMV and PorA antigen, the PorB T-cell responses after 10 months were also higher than prevaccination levels (median dpm = 7,400), and T-cell responses against the PorB antigen obtained after the third dose did not exceed the responses induced by the first and second doses.
IgG antibodies against OMV.
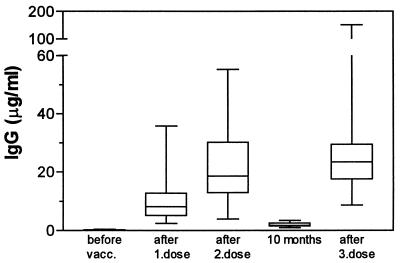
IgG antibodies were not detectable or present at concentrations below 0.5 μg/ml in prevaccination sera. All vaccinees showed significant IgG responses after each immunization (P = 0.002) (Fig. 3), with a maximum response 2 weeks after vaccination, except for vaccinee 4, who reached maximum response 6 weeks after the second and third doses. Three of the vaccinees obtained their maximum IgG responses after the first dose, one obtained it after the second dose, and six obtained them after the third dose. The IgG response after the first dose varied between 2 and 36 μg/ml (median = 8 μg/ml). We generally observed an increase in IgG antibody levels after the second dose, ranging from 4 to 55 μg/ml (median = 19 μg/ml); however, considered as a group the increases were not statistically significant. After 10 months, IgG antibodies were still above prevaccination levels for all vaccinees (median = 2 μg/ml). The third vaccine dose generally induced a further increase compared to the second dose, with IgG antibody levels between 9 and more than 150 μg/ml (median = 24 μg/ml), and the increase after the third dose was significantly higher than that for the IgG response after the first dose (P < 0.005).
FIG. 3.
OMV-specific IgG antibody responses in serum measured by enzyme-linked immunosorbent assay for 10 adult vaccinees given three doses of OMV vaccine at weeks 0, 6, and 46. Data are expressed as maximum responses obtained 1, 2, or 6 weeks after each dose given. Boxes extend from the 25th to the 75th percentile, with a horizontal line at the median. Vertical bars show the range of the data. Significant responses after vaccination were observed after each immunization (P = 0.002).
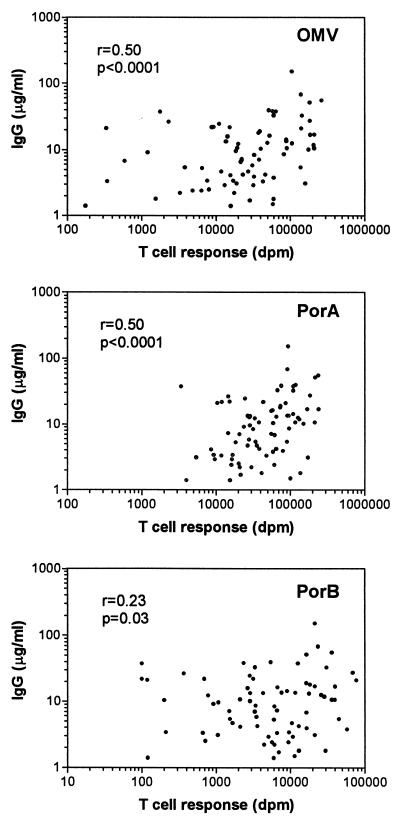
Correlation between T-cell proliferation and IgG antibody levels.
Nonparametric correlation analysis was applied to establish a relationship between the vaccine-induced T-cell and antibody responses. A positive correlation between T-cell responses against the vaccine antigens and the corresponding levels of IgG antibodies against OMV in serum was observed (Fig. 4). The correlation was the same for the PorA antigen and OMV (r = 0.50; P < 0.0001), whereas the correlation for the PorB antigen was weaker (r = 0.23; P = 0.03).
FIG. 4.
Correlation between OMV-specific IgG antibody responses in vaccinee sera and T-cell responses against OMV vaccine and PorA and PorB outer membrane proteins. Spearman correlation coefficients are shown.
Changes in cell membrane markers during vaccination.
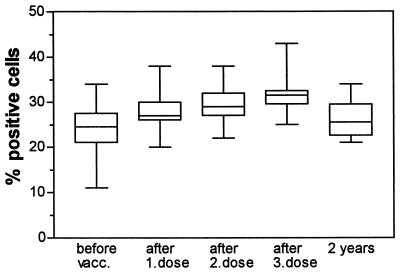
We analyzed the proportion of CD4+, CD8+, CD19+, and CD45R0+ cells of PBMCs from the vaccinees by flow cytometry throughout the vaccination trial. The percentage of CD4+ cells in peripheral blood was found to fluctuate in a vaccine-dependent manner throughout the study period and to correlate with T-cell proliferation against the vaccine antigens: OMV (r = 0.38; P = 0.0002), PorA (r = 0.43; P < 0.0001), and PorB (r = 0.4; P = 0.0001) (data not shown). This was not the case for CD4+ cells from a nonvaccinated control person who was included at all time points throughout the study. We found a significant increase in the percentage of CD4+ cells after the first vaccine dose (median = 44% before vaccination and 49% after vaccination; P = 0.0039) and also after the third dose (median = 46% before vaccination and 54% after vaccination; P = 0.002) (data not shown). In addition, we observed a progressive increase in the number of activated-memory T-cells (CD45R0+ cells) after vaccination within the CD4+ cell population, even after a single dose of OMV vaccine (P = 0.0020) (Fig. 5). Two years after the onset of vaccination, the proportion of CD45R0+ cells was slightly above prevaccination levels, but the difference was not statistically significant. We found no increase in the percentage of CD8-positive CD45R0+ T cells and no significant changes in the proportion of B cells (CD19+ cells) after vaccination (results not shown).
FIG. 5.
Percentages of CD4+-CD45R0+ memory T cells measured by flow cytometry in PBMCs from 10 vaccinees before OMV vaccination, after one dose, after a second dose given 6 weeks after the first, after a third dose given after 10 months, and 2 years after the first dose was given. Boxes extend from the 25th to the 75th percentile, with a horizontal line at the median. Vertical bars show the range of the data. P is 0.0039 for the increase after one dose, and P is 0.002 for the increase after the third dose.
DISCUSSION
We have investigated antigen-specific human T-cell responses after vaccination with the Norwegian group B meningococcal (OMV) vaccine, measured as in vitro proliferation against the vaccine itself as well as purified vaccine proteins. We compared these T-cell responses with the corresponding OMV-specific antibody concentrations in serum and in addition studied changes in PBMC subsets during the vaccination schedule. This is, to our knowledge, the first study in which human T-cell responses after vaccination against meningococci have been systematically measured.
It is well known that most adults have antibodies against meningococci in serum, probably caused by repeated immune stimulation by nasopharyngeal carriage (14). Our study group was selected on the basis of low IgG antibody levels against OMV in order to obtain vaccinees as naive as possible. By using this criterion, we could be at risk for selecting for poor vaccine responders. However, this was not the case since all the vaccinees showed both humoral and cellular immune responses after vaccination. The second and third vaccine doses induced typical booster IgG antibody responses in most of the vaccinees, as expected for a nonimmune population. However, low T-cell responses against the OMV and the PorA protein were observed before vaccination for most of the vaccinees, indicating some degree of cellular immunity against meningococci. This could be due to cross-reactive T-cell epitopes or previous carriage of meningococci, not reflected in the antibody response, or a mitogenic effect of OMV proteins, as will be discussed below. T-cell responses in nonimmunized individuals have also been reported in a previous study by Wiertz and coworkers, in which the majority of the volunteers tested showed T-cell reactivity against meningococcal proteins, but the participants in this group also had specific antibodies in serum (34).
We observed strong T-cell responses against the whole OMV vaccine and the PorA protein in all vaccinees after vaccination. All vaccinees, except one, also responded to the PorB protein, but the responses were considerably lower. In general, the vaccinees showed a further increase in T-cell responses after the second vaccine dose. However, considered as a group the increases were not statistically significant, except for the response against the PorA component. On the other hand, we do not know the size of booster T-cell responses that would be expected after meningococcal vaccination, as this has not been previously studied. If we exclude the two vaccinees (vaccinees 1 and 4) who had received one dose of an experimental protein-polysaccharide meningococcal vaccine 10 years earlier (28), the increase in T-cell responses against OMV after the second dose is statistically significant (P = 0.02). Vaccinees 1 and 4 showed a significantly decreased T-cell response against OMV after the second dose compared to the first dose, probably due to immunological memory established 10 years earlier, even though no specific IgG antibodies were detectable in serum at the time of recruitment for this study. We observed an effect of the third dose for OMV and the PorA protein in nearly all vaccinees, but no statistically significant effect for the PorB protein. There was no further increase in the T-cell responses after the third dose compared to the second dose. However, care should be taken in interpreting the results after the third dose, because the responses against the control antigens were markedly lower at the last four time points compared to the previous samples. This must be due to an unknown systematic change in the experimental conditions which was not identified. Antigen-specific T-cell responses after the third dose are thus probably underestimated compared to responses after the first and second doses. In contrast, the third dose of vaccine generally induced a further increase in OMV-specific IgG antibody levels in serum compared to the second dose, as also demonstrated in a previous study by Rosenqvist et al. (27).
The positive correlation observed between T-cell responses and anti-OMV antibody levels (r = 0.5, P < 0.0001, for the OMV and PorA protein) is consistent with the assumption that the antibody response is T cell dependent. If we use only the results obtained after the first and second doses, we observe an even better correlation (r = 0.7 and 0.6 for OMV and PorA, respectively; P < 0.0001). Furthermore, the functional correlation could be even higher since the kinetics of the T-cell response and of the antibody response are most likely different, leading to maximum responses at different time points.
Expression of the costimulatory molecule B7-2 is essential for B-lymphocyte-dependent T-cell stimulation (7). Neisseria porins have the ability to activate naive, resting mouse splenic B cells and induce expression of B7-2 but have not been shown to directly stimulate T cells in a mitogenic manner (21, 32). The porins may, however, increase T-cell activation indirectly by inducing B7-2 on B cells (19, 32). In our experimental system, the contribution from B cells proliferating in a mitogenic response to porins is small compared to the antigen-specific proliferation of T cells and, importantly, represents a constant contribution throughout the study, since the percentage of B cells for each vaccinee did not change significantly. An eventual mitogenic effect of the porins as well as LPS present in the OMV vaccine is nevertheless very small, since we observed very low responses against the porins as well as OMV in the vaccinees before vaccination compared to the responses obtained after vaccination.
The PorA protein shows limited heterogeneity among meningococci (3, 20), and a vaccine based solely on PorA proteins is now being tested with humans (9, 25). Bactericidal antibodies are considered to be important for protection against meningococcal disease, and bactericidal activity in sera from OMV vaccinees correlates with the presence of antibodies directed against the PorA protein (27). We observed the strongest T-cell responses against the PorA protein, indicating that this protein could be the most immunogenic T-cell antigen that is recognized in the context of major histocompatibility complex molecules expressed by the vaccinees in our study. This could be caused by the presence of more stimulatory T-cell epitopes, thereby inducing a higher frequency of circulating PorA-specific T cells in peripheral blood, but also more efficient uptake, processing, and presentation by the antigen-presenting cells. Our findings of a dominant PorA antigen response compared to the PorB response are in accordance with the results of a T-cell epitope study based on synthetic peptides from the class 1 protein (35) and emphasize the importance of PorA in the OMV vaccine. Although the participants in our study were selected on the basis of low preimmunity against meningococci, they were adults (24 to 49 years), whereas the main target group for the vaccine is teenagers, who possibly could respond differently.
The importance of the PorB protein in meningococcal vaccines is still a matter of debate. Monoclonal antibodies to PorB seem to have low bactericidal activity (29), but patients surviving systemic meningococcal disease have been reported to have a higher IgG level against the PorB protein than against the PorA protein (16, 17). Furthermore, half of the vaccinees in our study showed significant T-cell responses against PorB epitopes, and the possibility that PorB could play a role in providing T-cell help for the antibody response against OMV could not be excluded.
Interestingly, we detected a significant increase in the percentage of helper T cells that expressed the CD45R0 marker after vaccination. CD45R0 is up-regulated on activated (2, 18) and memory (15, 22) T cells. There is a general increase in CD45R0+ T cells with age (10, 12, 30), probably reflecting accumulation of T cells responding against various antigens throughout life. A significant increase in the CD45R0+ T-cell population was observed already after the first vaccine dose and increased after the second and third doses. This suggests that the OMV vaccine induces T-cell activation and hence memory T-cells, which is reflected in the PBMC population. Two years after the onset of vaccination, the CD45R0+ T-cell level was back to slightly above prevaccination level, although the difference from prevaccination level was not statistically significant. Consistent with our results, an increase of CD4+ T cells that express CD45R0 after in vitro stimulation with pertussis antigens has recently also been described for PBMCs isolated from vaccinated infants (37).
In conclusion, we have shown that the Norwegian OMV vaccine against meningococcal B disease induced strong proliferative human T-cell responses against OMV and the PorA protein, whereas responses against the PorB protein were considerably lower. The antigen-specific T-cell responses correlated with the level of OMV-specific IgG antibodies in serum of the vaccinees. The vaccine also induced an increase in the proportion of peripheral CD45R0+ memory cells within the CD4+ T-cell population.
REFERENCES
- 1.Ada G L. The immunological principles of vaccination. Lancet. 1990;335:523–526. doi: 10.1016/0140-6736(90)90748-t. [DOI] [PubMed] [Google Scholar]
- 2.Akbar A N, Terry L, Timms A, Beverly P C L, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T-cells. J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- 3.Barlow A K, Heckels J E, Clarcke I N. The class 1 outer membrane protein of Neisseria meningitidis: gene sequence and structural and immunological similarities to gonococcal porins. Mol Microbiol. 1989;3:131–139. doi: 10.1111/j.1365-2958.1989.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 4.Bjune G, Høiby E A, Grønnesby J K, Arnesen O, Fredriksen J H, Halstensen A, Holten E, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Frøholm L O, Lystad A, Bakketeig L S, Hareide B. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 5.Blake M S, Gotschlich E C. Purification and partial characterization of the major outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1982;36:277–283. doi: 10.1128/iai.36.1.277-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake M S, Gotschlich E C. Functional and immunological properties of pathogenic Neisseria surface proteins. In: Inouye M, editor. Bacterial outer membranes as model systems. New York, N.Y: John Wiley & Sons, Inc.; 1986. pp. 377–400. [Google Scholar]
- 7.Bluestone J A. New perspectives of CD28-B7-mediated T-cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 8.Bøyum, A. 1968. Separation of leukocytes from blood and bone marrow. Scand. J. Clin. Lab. Invest. 21(Suppl. 97):108. [PubMed]
- 9.Claassen I, Meylis J, van der Ley P, Peeters C, Bronst H, Robert J, Borsboom D, van der Ark A, van Straaten I, Roholl P, Kuipers B, Poolman J. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–1008. doi: 10.1016/0264-410x(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 10.Cossarizza A, Ortolani C, Paganelli R, Barbieri D, Monti D, Sansoni P, Fagiolo U, Castellani G, Bersani F, Londei M, Franceschi C. CD45 isoforms expression on CD4+ and CD8+ T-cells throughout life, from newborns to centenarians: implications for T-cell memory. Mech Ageing Dev. 1996;86:173–195. doi: 10.1016/0047-6374(95)01691-0. [DOI] [PubMed] [Google Scholar]
- 11.Fredriksen J H, Rosenqvist E, Wedege E, Bryn K, Bjune G, Frøholm L O, Lindbak A-K, Møgster B, Namork E, Rye U, Stabbetorp G, Winsnes R, Aase B, Closs O. Production, characterization and control of MenB-Vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–80. [PubMed] [Google Scholar]
- 12.Gabriel H, Schmitt B, Kindermann W. Age-related increase of CD45R0+ lymphocytes in physically active adults. Eur J Immunol. 1993;23:2704–2706. doi: 10.1002/eji.1830231049. [DOI] [PubMed] [Google Scholar]
- 13.Goldschneider I, Lepow M L, Gotschlich E C, Mauck F T, Bachl F, Randolph M. Immunogenicity of group A and group C meningococcal polysaccharides in human infants. J Infect Dis. 1973;128:769–776. doi: 10.1093/infdis/128.6.769. [DOI] [PubMed] [Google Scholar]
- 14.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1307–1349. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray D. Immunological memory. Annu Rev Immunol. 1993;11:49–77. doi: 10.1146/annurev.iy.11.040193.000405. [DOI] [PubMed] [Google Scholar]
- 16.Guttormsen H-K, Wetzler L M, Solberg C O. Humoral immune response to the class 1 outer membrane protein during the course of meningococcal disease. Infect Immun. 1994;62:1437–1443. doi: 10.1128/iai.62.4.1437-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guttormsen H-K, Wetzler L M, Næss A. Humoral immune response to the class 3 outer membrane protein during the course of meningococcal disease. Infect Immun. 1993;61:4734–4742. doi: 10.1128/iai.61.11.4734-4742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johannisson A, Festin R. Phenotype transition of CD4+ T-cells from CD45Ra to CD45R0 is accompanied by cell activation and proliferation. Cytometry. 1995;19:343–352. doi: 10.1002/cyto.990190409. [DOI] [PubMed] [Google Scholar]
- 19.Klaus S J, Pinchuk L M, Ochs H D, Law C L, Fanslow W C, Armitage R J, Clark E A. Costimulation through CD28 enhances T cell-dependent B cell activation via CD40-CD40L interaction. J Immunol. 1994;152:5643–5652. [PubMed] [Google Scholar]
- 20.McGuinness B, Barlow A K, Clarke I N, Farley J E, Anilionis A, Poolman J T, Heckels J E. Deduced amino acid sequences of class 1 protein (PorA) from three strains of Neisseria meningitidis. J Exp Med. 1990;171:1871–1882. doi: 10.1084/jem.171.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melancon J, Murgita R A, Devoe I W. Activation of murine B lymphocytes by Neisseria meningitidis and isolated meningococcal surface antigens. Infect Immun. 1983;42:471–479. doi: 10.1128/iai.42.2.471-479.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkensclager M, Terry L, Edwards R, Beverley P C L. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for the differential CD45 expression in T cell memory formation. Eur J Immunol. 1988;18:1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- 23.Meyer Næss L, Rosenqvist E, Høiby E A, Michaelsen T E. Quantitation of IgG subclass antibody responses after immunization with a group B meningococcal outer membrane vesicle vaccine, using monoclonal mouse-human chimeric antibodies as standards. J Immunol Methods. 1996;196:41–49. doi: 10.1016/0022-1759(96)00108-1. [DOI] [PubMed] [Google Scholar]
- 24.Paulnock D M. Macrophage activation by T cells. Curr Opin Immunol. 1992;4:344–349. doi: 10.1016/0952-7915(92)90087-u. [DOI] [PubMed] [Google Scholar]
- 25.Peeters C C A M, Rümke H C, Sundermann L C, Rouppe van der Voort E M, Meulenbelt J, Schuller M, Kuipers A J, van der Ley P, Poolman J. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine. 1996;14:1009–1015. doi: 10.1016/0264-410x(96)00001-1. [DOI] [PubMed] [Google Scholar]
- 26.Peltola H, Mäkelä P H, Käythy H, Jousimies H, Herva E, Hällström K, Sivonen A, Renkonen O-V, Pettay O, Karanko V, Ahvonen P, Sarna S. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977;297:686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- 27.Rosenqvist E, Høiby E A, Wedege E, Bryn K, Kolberg J, Klem A, Rønnild E, Bjune G, Nøkleby H. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642–4652. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenqvist E, Harthug S, Frøholm L O, Høiby E A, Bøvre K, Zollinger W D. Antibody responses to serogroup B meningococcal outer membrane antigens after vaccination and infection. J Clin Microbiol. 1988;26:1543–1548. doi: 10.1128/jcm.26.8.1543-1548.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saukkonen K, Leinonen M, Abdillahi H, Poolman J T. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine. 1989;7:325–328. doi: 10.1016/0264-410x(89)90194-1. [DOI] [PubMed] [Google Scholar]
- 30.Stulnig T, Maczek C, Böck G, Majdic O, Wick G. Reference intervals for human peripheral blood lymphocyte subpopulations from ′healthy′ young and aged subjects. Int Arch Allergy Immunol. 1995;108:205–210. doi: 10.1159/000237155. [DOI] [PubMed] [Google Scholar]
- 31.Tsai C-M. The analysis of lipopolysaccharide vaccines by silver staining following SDS-polyacrylamide gel electrophoresis. J Biol Stand. 1986;14:25–33. doi: 10.1016/s0092-1157(86)80006-3. [DOI] [PubMed] [Google Scholar]
- 32.Wetzler L M, Ho Y, Reiser H. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J Exp Med. 1996;183:1151–1159. doi: 10.1084/jem.183.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetzler L M, Blake M S, Barry K, Gotschlich E C. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes and blebs isolated from rmp deletion mutants. J Infect Dis. 1992;166:551–555. doi: 10.1093/infdis/166.3.551. [DOI] [PubMed] [Google Scholar]
- 34.Wiertz E J H J, Delvig A, Donders E M L M, Brugghe H F, van Unen L M A, Timmermans H A M, Achtman M, Hoogerhout P, Poolman J T. T-cell responses to outer membrane proteins of Neisseria meningitidis: comparative study of the Opa, Opc, and PorA proteins. Infect Immun. 1996;64:298–304. doi: 10.1128/iai.64.1.298-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiertz E J H J, van Gaans-van den Brink J A M, Schreuder G M T H, Termijtelen A A M, Hoogerhout P, Poolman J. T cell recognition of Neisseria meningitidis class 1 outer membrane proteins. J Immunol. 1991;147:2012–2018. [PubMed] [Google Scholar]
- 36.Wyle F E, Artenstein M S, Brandt B L, Tramont E C, Kasper D L, Altieri P L, Berman S L, Lowenthal J P. Immunological response in man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–522. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 37.Zepp F, Knuf M, Habermehl P, Schmitt H J, Rebsch C, Schmidtke P, Clemens R, Slaoui M. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun. 1996;64:4078–4084. doi: 10.1128/iai.64.10.4078-4084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]