Abstract
An adult domestic short‐haired feline leukemia virus‐infected cat was referred for kidney failure and worsening anemia requiring transfusions. ABC blood typing was performed with an immunochromatographic strip assay at different occasions. Gel column systems were used for the major and minor crossmatching tests, and anti‐A and anti‐B titers were determined. No discrete A or B bands appeared on the immunochromatographic strips at any time point for the recipient cat. The recipient's plasma agglutinated RBCs from tested type A and B cats. The recipient's RBCs appeared compatible with plasma from 1 type A and 2 B donors, and incompatible with plasma from another type A cat. Genotyping of recipient blood revealed a single homozygous c.179G>T CMAH variant predicting a blood type B. These studies suggest an unusual weak type B or missing all ABC antigens. The latter resembles the exceedingly rare Bombay phenotype in the human ABO blood group system.
Keywords: alloantibodies, blood typing, Bombay phenotype, crossmatch, feline, H antigen
Abbreviations
- CMAH
cytidine monophosphate‐N‐acetyl‐neuraminic acid hydroxylase
- FeLV
feline leukemia virus
- FIV
feline immunodeficiency virus
- HCT
hematocrit
- ICS
immunochromatographic strip
- NeuAc
N‐acetyl‐neuraminic acid (type B antigen)
- NeuGc
N‐glycolyl‐neuraminic acid (type A antigen)
- RBC
red blood cells
- RI
reference interval
1. INTRODUCTION
The major feline blood antigen system characterizes cats as A, B, or C (also known as AB). All type B cats older than 3 months have naturally occurring anti‐A alloantibodies, triggering potentially life‐threatening acute hemolytic reactions and neonatal isoerythrolysis. 1 , 2 The prevalence of ABC blood types varies geographically and among breeds, with type A being the most common type. 3 , 4 The blood type antigenic determinants of the red blood cell (RBC) membrane are N‐glycolyl‐neuraminic acid (NeuGc) for type A and N‐acetyl‐neuraminic acid (NeuAc) for type B. 5 , 6 Blood types are determined by variants of the gene that encodes cytidine monophospho‐N‐acetylneuraminic acid hydroxylase (CMAH), the enzyme responsible for the conversion of NeuAc to NeuGc. 4 As a result, type B cats express only NeuAc and type A cats possess predominantly NeuGc, whereas type C cats have approximately equal amounts of NeuAc and NeuGc on the RBC membrane. 3
Bombay blood group phenotype, an exceptionally rare blood type in humans outside of Southeast Asia, occurs in approximately 1 in a million individuals in Europe. This blood type is characterized by the absence of the H antigen on RBCs. As the H antigen is the substrate on which the A and B antigens are formed, the individuals lacking this antigen are unable to produce A or B antigens and appear as type O on routine ABO phenotyping, regardless of their ABO genotype. 7 , 8 , 9
In this case report, blood type studies that resemble the exceedingly rare Bombay phenotype or a weak type B are described in a domestic short‐haired cat.
2. CASE HISTORY
A 4.5‐year‐old neutered male domestic short‐haired cat was referred to the Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Greece. The cat was presented with a 3‐week history of worsening anorexia, lethargy, halitosis, and vomiting. Physical examination on admission revealed poor body condition (2/5), moderate pallor, lethargy, ulcerative lesions on the dorsal surface of the tongue, and a grade IV/VI left‐sided systolic murmur.
CBC (ADVIA 120 Hematology and Cytometry System, Bayer HealthCare LLC, Diagnostics Division, Tarrytown, New York, USA) and blood smear examination demonstrated a normochromic, normocytic, non‐regenerative (reticulocytes: 5400/μL; reference intervals [RI]: <80 000/μL) anemia (hematocrit [HCT] 16%; RI: 30%‐45%), neutrophilia (neutrophils: 29 400/μL; RI: 3000‐13 400/μL), and a moderate thrombocytopenia (102 000/μL; RI: 300 000‐800 000/μL), in the presence of a few platelet aggregates on the blood smear. A serum biochemistry panel (Vitalab Flexor E Biochemistry Analyzer, Vital Scientific NV, Dieren, Netherlands) indicated increased concentration of creatinine (6.1 mg/dL [RI: 0.7‐1.6 mg/dL]), urea nitrogen (117 mg/dL [RI: 9‐32 mg/dL]), and inorganic phosphorus (10.1 mg/dL [RI: 3.5‐6.7 mg/dL]). Urinalysis showed isosthenuria (1.012; crystalloids had been given before admission), a urinary protein‐to‐creatinine ratio of 1.8 (RI < 0.4), and an inactive urinary sediment; urine culture failed to grow bacteria. The arterial blood pressure was normal. Serology was positive for feline leukemia virus (FeLV) antigen and negative for feline immunodeficiency virus (FIV) antibodies using an in‐office ELISA test (Feline SNAP Combo Plus, IDEXX Laboratories Inc., Maine, USA). Abdominal ultrasonography indicated increased cortical thickness and poorly defined corticomedullary junction of the left kidney and a normally appearing right kidney.
Due to the severe, likely chronic kidney disease (international renal society‐based stage IV, with proteinuria), the cat was hospitalized. Serial CBC results indicated worsening of anemia (HCT: 13% on third day post‐admission) requiring a transfusion. Blood typing was performed by an in‐clinic immunochromatographic strip (ICS) assay (Feline Quick Test BT, Alvedia, Limonest, France). Unexpectedly, no discrete A or B bands appeared on the strip, whereas the control band was clearly visible (Figure 1). Blood typing was attempted 2 more times with the same ICS assay, including 1 with concentrated RBCs after whole blood centrifugation and decanting of the plasma showing the same results (Figure 1). In addition, a glass slide back typing was performed by admixing 2 drops of the patient's serum and 1 drop of reference type A packed RBCs (BSA Animal Blood Bank, Porto, Portugal). 3 Gross agglutination was noticed (Figure 2), implying that recipient's blood was more likely type B. Since a type B blood unit was not accessible at the time, no blood was transfused and the cat was treated with recombinant human erythropoietin A, along with isotonic fluids and other supportive treatments. The cat's clinical condition and clinicopathologic parameters exacerbated, which prompted the owner's decision for humane euthanasia 1‐month after admission. No permission for necropsy was granted.
FIGURE 1.
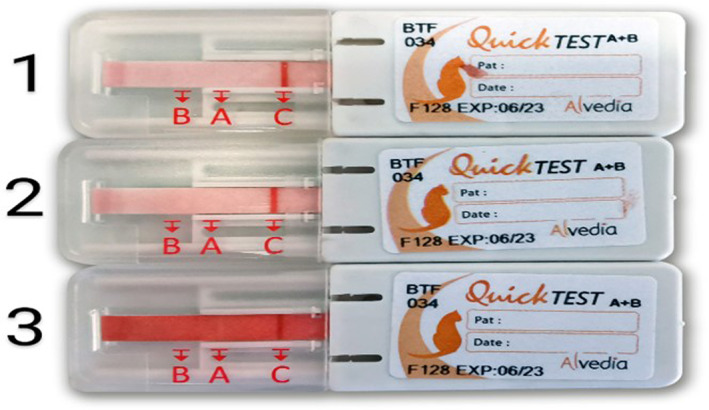
In‐clinic blood typing with the immunochromatographic strip using the cat's whole blood (1, 2) and concentrated red blood cells after decanting the plasma (3). The control band is clearly seen, as opposed to no discrete A or B bands. Type O in the feline ABC system has not previously been described in cats.
FIGURE 2.

Glass slide back typing by mixing 2 drops of recipient serum and 1 drop of reference A red blood cells. Gross agglutination is seen.
Due to the unexpected blood typing results, 2 left‐over EDTA‐anticoagulated blood samples were sent to the manufacturer of the ICS feline typing assay for further assessment. Cartridges from a different ICS lot failed anew to type as A or B. Based upon major and minor crossmatching test performed using a gel column test (Feline Gel Test XM, Alvedia, Limonest, France), the recipient's plasma was crossmatched against RBC samples from 2 type A and 2 type B donor cats, resulting in agglutination in all instances (Figure 3). Titration of anti‐A and anti‐B alloantibodies, mixing the recipient's plasma against RBCs from 2 A and 2 B donor cats, different from those used in the major crossmatching, indicated a positive reaction up to a dilution of 1/32 against both A donor RBCs, and up to a dilution 1/4 against both B donor RBCs (Figure 4). A negative (compatible) crossmatch was found when the plasma samples from 2 type B cats were tested against RBCs from the recipient; however, whereas a negative (compatible) crossmatch was observed with plasma from 1 type A cat, a positive (incompatible) crossmatch was noticed with the plasma of the second type A cat (no titration available; Figure 5).
FIGURE 3.

Major crossmatching test with the cat's plasma and type A (2 cats) or type B (2 cats) red blood cells. Identical agglutination pattern as indicated here was noticed in all instances.
FIGURE 4.

Upper part: Titration of anti‐A alloantibodies using crossmatching tests with consecutive dilutions of the cat's plasma and red blood cells (RBCs) from 2 type A cats. A positive crossmatch was noticed until dilution 1/32 in both cats. Lower part: Titration of anti‐B alloantibodies using crossmatching test with consecutive dilutions of the cat's plasma and RBCs from 2 type B cats. A positive crossmatch was noticed until dilution 1/4 in both cats.
FIGURE 5.
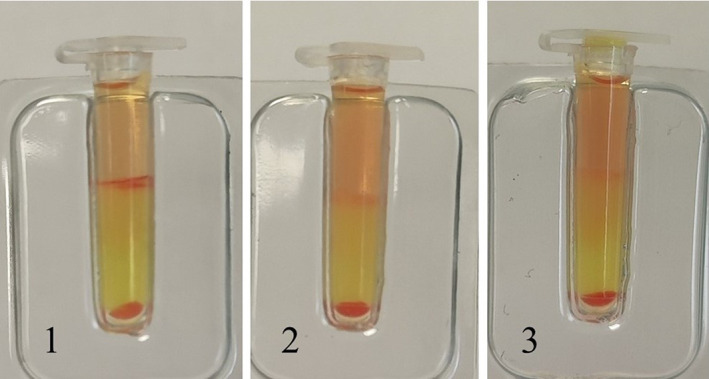
Minor crossmatching test using the cat's red blood cells and plasma from a type A cat, indicating a positive (incompatible) crossmatch (1). A negative (compatible) crossmatch was seen with another A cat (2) and the plasma from 2 type B cats (3).
A genotyping panel of 4 CMAH variants was assessed using frozen EDTA‐anticoagulated blood, which revealed a single homozygous c.179G>T CMAH variant predicting a type B. 10
3. DISCUSSION
This is a report of a cat without appreciable A or B antigen on erythrocytes assessed by an in‐clinic typing kit. The cat described herein was confirmed as of bb genotype, bearing the CMAH variant c.179G>T, which results in blood type B in Turkish domestic cats and other breeds. 10 , 11 As the home of this domestic short‐haired cat is Greece and neighboring Turkey, it is not surprising to find the same CMAH variant. However, this does not explain the cat's phenotype, as an ICS assay failed multiple times to identify the cat's blood type as A, B, or C (AB), including 1 time using concentrated RBCs, suggesting a “null” phenotype (ie, both NeuAc and NeuGc were missing), in the context of detectable anti‐A (titer 1/32) and anti‐B (titer 1/4) alloantibodies. The fact that NeuAc appears not absorbed or synthesized and at the same time the CMAH enzyme is also mutated, might indicate that CMAH is further changed to a precursor antigen different than NeuAc or NeuGc. Overall, these blood type studies bear similarities with an unusual “weak B” phenotype or the exceedingly rare Bombay‐like phenotype of the human ABO blood group system, although a precursor substrate analogous to the H antigen in the ABO system has yet to be identified in cats. 8 , 9
Humans with the Bombay phenotype appear to type as group O by the ABO testing, regardless of their ABO genotype, as they lack the H‐antigen scaffold on RBCs to form the A and B antigen. 7 Therefore, when anti‐A and anti‐B reagents are matched against their RBCs, no agglutination is seen, but they produce antibodies against the A and B RBC antigens, as well as express a clinically important anti‐H titer. Individuals with the Bombay phenotype must only receive H antigen‐negative blood. 9 In this cat, testing against the precursor substrate was technically not possible.
Uncommon human blood type variants in the ABO system, are weak A and B subgroups. Individuals with these phenotypes possess decreased A or B antigens on their RBCs and show variable degrees of agglutination with anti‐A or anti‐B antibodies, respectively. It is suggested that weak blood group B phenotypes might be caused by sequence variations in the CBF/NF‐Y regulatory region of the ABO gene. 12 A low percentage of individuals with weak A or B subgroups contain anti‐A or anti‐B antibodies in their plasmas as observed in the cat of this report. 13
Blood type genotype‐phenotype discordance are rarely reported in cats, although earlier reports were not identical with this case. 10 , 11 , 14 Recently, 5 distinct RBC antigens beyond the ABC blood group system were hypothesized to be present and 1 of these was thought to correspond to the previously described Mik antigen. 15 , 16 Importantly, the results of blood typing might uncommonly be affected by disease states in humans and animals. For example, loss of A and B antigens from the RBCs of human patients with hematological malignancies is a fairly frequent occurrence and DNA methylation of the ABO promoter was shown to account for the loss of ABO allelic expression in leukemic patients. 17 In a study of cats, 4 blood type A domestic short‐haired cats with FeLV‐related anemia were mistyped as AB type, even after eliminating by washing the weak autoagglutination in 3 of these cats. 18 Some likely form of antigenic mimicry with the B antigen on the surface of RBCs or a reduction in CMAH activity converting NeuAc to NeuGc in type A cats might explain these results. The cat of this report was FeLV positive; it remains unknown if this affected the antigen expression and alloantibody titers. The advanced chronic kidney disease prompted the owner's decision for euthanasia 1‐month post‐admission, not allowing to retype the cat in the long term to check for possible phenotype reversal. In a recent multicenter study, no association between overall retroviral status (FeLV or FIV positive/negative) and blood type, between FeLV status and blood type or between FIV status and blood type was established and no difference was found in the distribution of blood types between healthy or sick cats. 19 In the latter study, the number of FeLV‐antigenemic cats was relatively small (n = 47), which might have adversely affected the validity of the results.
Historically, the ICS assay used in this cat has reliably detected the ABC blood types in anemic and non‐anemic cats. In 1 study, its diagnostic sensitivity was found to be 97.7% and 95.7% for blood type A and B, respectively, whereas its specificity was 100% for A and 97.1% for B blood type, respectively, whereas a second study found a sensitivity and specificity of 100% for all blood types. 20 Unfortunately, because of the owner's decision for euthanasia, it was not technically feasible in this case to collect more fresh blood and repeat the blood typing by alternative methods, such as the historically considered gold‐standard tube method, gel column or flow cytometry, or other reagents (ie, Triticum vulgaris lectin). 3 , 18 , 20 , 21 , 22
In conclusion, in this domestic short‐haired cat, the constellation of the inability to establish the cat's ABC blood group by an ICS assay, the back typing result, the major/minor cross matching results, along with the genotyping results, resemble the exceedingly rare Bombay phenotype in the human ABO blood group, although a substrate antigen analogous to the H antigen has not been yet identified in cats. The possibility of a weak type B similar to human medicine, with anti‐A and anti‐B antibodies and low B antigen site density on the cat's RBCs, which makes them undetectable with the common typing kits cannot be excluded.
CONFLICT OF INTEREST DECLARATION
B. Canard works for Alvedia, A. Kehl works for Laboklin GmbH & Co. and U. Giger has been a scientific advisor to Alvedia and Laboklin. No other authors declare a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. The authors are grateful to Dominique Rigal, MD, for his invaluable scientific advice.
Ginoudis A, Canard B, Kehl A, et al. Discordance between ABC blood phenotype and genotype in a domestic short‐haired cat. J Vet Intern Med. 2024;38(1):358‐362. doi: 10.1111/jvim.16927
REFERENCES
- 1. Auer L, Bell K. Transfusion reactions in cats due to AB blood group incompatibility. Res Vet Sci. 1983;35:145‐152. [PubMed] [Google Scholar]
- 2. Bücheler J, Giger U. Alloantibodies against A and B blood types in cats. Vet Immunol Immunopathol. 1993;38:283‐295. [DOI] [PubMed] [Google Scholar]
- 3. Griot‐Wenk ME, Giger U. Feline transfusion medicine. Blood types and their clinical importance. Vet Clin North Am Small Anim Pract. 1995;25:1305‐1322. [DOI] [PubMed] [Google Scholar]
- 4. Gavazza A, Rossi G, Antognoni MT, Cerquetella M, Miglio A, Mangiaterra S. Feline blood groups: a systematic review of phylogenetic and geographical origin. Animals. 2021;11:3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrews GA, Chavey PS, Smith JE, Rich L. N‐glycolyneuraminic acid and N‐acetylneuraminic acid define feline blood group A and group B antigens. Blood. 1992;79:2485‐2491. [PubMed] [Google Scholar]
- 6. Griot‐Wenk L, Pahlsson P, Chisholm‐Chait A, et al. Biochemical characterization of the feline AB blood group system. Anim Genet. 1993;24:401‐407. [DOI] [PubMed] [Google Scholar]
- 7. Dean L. The Hh blood group. In: Dean L, ed. Blood Groups and Red Cell Antigens. 1st ed. Bethesda (MD): National Center for Biotechnology Information (US); 2005:33‐38. [Google Scholar]
- 8. Talukder B, Datta SS, Mukherjee S, Mukherjee K. Prevalence of Bombay group blood in southern Bengal population. Indian J Hematol Blood Transfus. 2014;30:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobs JW, Horstman E, Gisriel SD, Tormey CA, Sostin N. Incidental discovery of a patient with the Bombay phenotype. Lab Med. 2023;54:e14‐e17. [DOI] [PubMed] [Google Scholar]
- 10. Kehl A, Heimberger K, Langbein‐Detsch I, et al. Molecular characterization of blood type A, B, and C (AB) in domestic cats and a CMAH genotyping scheme. PloS One. 2018;13:e0204287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kehl A, Mueller E, Giger U. CMAH genotyping survey for blood types A, B and C (AB) in purpose‐bred cats. Anim Genet. 2019;50:303‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seltsam A, Wagner FF, Gruger D, et al. Weak blood group B phenotypes may be caused by variations in the CCAAT‐binding factor/NF‐Y enhancer region of the ABO gene. Transfusion. 2007;47:2330‐2335. [DOI] [PubMed] [Google Scholar]
- 13. Harmening DM. The ABO blood group system. In: Harmening DM, ed. Modern Blood Banking and Transfusion Practices. 7th ed. Philadelphia, PA: FA Davis Company Publications; 2019:119‐148. [Google Scholar]
- 14. Kehl A, Truchet L, Langbein‐Detsch I, Müller E, Giger U. Updates on practical ABC blood compatibility testing in cats. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2019;47:425‐438. [DOI] [PubMed] [Google Scholar]
- 15. Binvel M, Arsenault J, Depré B, Blais MC. Identification of 5 novel feline erythrocyte antigens based on the presence of naturally occurring alloantibodies. J Vet Intern Med. 2021;35:234‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinstein NM, Blais MC, Harris K, Oakley DA, Aronson LR, Giger U. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen. J Vet Intern Med. 2007;21:287‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bianco‐Miotto T, Hussey DJ, Day TK, O'Keefe DS, Dobrovic A. DNA methylation of the ABO promoter underlies loss of ABO allelic expression in a significant proportion of leukemic patients. PloS One. 2009;4:e4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seth M, Jackson KV, Giger U. Comparison of five blood‐typing methods for the feline AB blood group system. Am J Vet Res. 2011;72:203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spada E, Jung H, Proverbio D, et al. Lack of association between feline AB blood groups and retroviral status: a multicenter, multicountry study. J Feline Med Surg. 2022;24:e194‐e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spada E, Proverbio D, Baggiani L, Bagnagatti de Giorgi G, Perego R, Ferro E. Evaluation of an immunochromatographic test for feline AB system blood typing. J Vet Emerg Crit Care. 2016;26:137‐141. [DOI] [PubMed] [Google Scholar]
- 21. Goy‐Thollot I, Nectoux A, Guidetti M, et al. Detection of naturally occurring alloantibody by an in‐clinic antiglobulin‐enhanced and standard crossmatch gel column test in non‐transfused domestic shorthair cats. J Vet Intern Med. 2019;33:588‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stieger K, Palos H, Giger U. Comparison of various blood‐typing methods for the feline AB blood group system. Am J Vet Res. 2005;66:1393‐1399. [DOI] [PubMed] [Google Scholar]


