Abstract
We cloned the MP1 gene, which encodes an abundant antigenic cell wall mannoprotein from the dimorphic pathogenic fungus Penicillium marneffei. MP1 is a unique gene without homologs in sequence databases. It codes for a protein, Mp1p, of 462 amino acid residues, with a few sequence features that are present in several cell wall proteins of Saccharomyces cerevisiae and Candida albicans. It contains two putative N glycosylation sites, a serine- and threonine-rich region for O glycosylation, a signal peptide, and a putative glycosylphosphatidylinositol attachment signal sequence. Specific anti-Mp1p antibody was generated with recombinant Mp1p protein purified from Escherichia coli to allow further characterization of Mp1p. Western blot analysis with anti-Mp1p antibody revealed that Mp1p has predominant bands with molecular masses of 58 and 90 kDa and that it belongs to a group of cell wall proteins that can be readily removed from yeast cell surfaces by glucanase digestion. In addition, Mp1p is an abundant yeast glycoprotein and has high affinity for concanavalin A, a characteristic indicative of a mannoprotein. Furthermore, ultrastructural analysis with immunogold staining indicated that Mp1p is present in the cell walls of the yeast, hyphae, and conidia of P. marneffei. Finally, it was observed that infected patients develop a specific antibody response against Mp1p, suggesting that this protein represents a good cell surface target for host humoral immunity.
Penicillium marneffei is a dimorphic pathogenic fungus that is endemic in Southeast Asia and southern parts of China (7, 10, 39). It is the cause of one of the most common infections of human immunodeficiency virus (HIV)-infected patients in Southeast Asia (17, 39). In northern Thailand, disseminated infection by P. marneffei is the third most common opportunistic infection of HIV-positive patients, after extrapulmonary tuberculosis and cryptococcal meningitis (39). Infections by P. marneffei have also been reported for visitors who have traveled to the regions of endemicity (7).
Disseminated penicilliosis has been frequently misdiagnosed as tuberculosis, which is epidemic in regions where the fungal disease is prevalent (10, 49). Penicilliosis patients present with nonspecific symptoms such as low-grade fever, anemia, and weight loss. Diagnosis is frequently based on identification of the fungal cells in bone marrow, spleen, or lymph node biopsy samples and, therefore, is often delayed (49). We showed previously that patients develop specific antibodies against P. marneffei cells, particularly to cell wall components (49). An immunofluorescence test based on this finding was established for the diagnosis of penicilliosis. This test, however, is relatively crude and lacks sufficient specificity because whole P. marneffei cells were used as the antigen for antibody detection. Such an immunofluorescence assay might also be insufficient because many studies have indicated that penicilliosis marneffei in HIV-infected patients can be easily misdiagnosed as another fungal infection, such as histoplasmosis or cryptococcosis (10). Therefore, identification of antigenic proteins and cloning of their genes should allow the development of a more specific diagnostic test for penicilliosis.
Little is known about the fungal pathogenesis of and host immunity to P. marneffei. Although the route of infection by P. marneffei has not been established, a respiratory portal of entry would be consistent with infections caused by other fungal pathogens that produce conidia. Inhalation of conidia produces pulmonary diseases that can then be disseminated to other body sites. Recently, several studies have reported on anti-P. marneffei immunity. One study suggested the importance of cell-mediated immunity in host resistance to P. marneffei infection in a mouse model (19). Another study indicated that activated macrophages might have a role in damaging endocytosed P. marneffei conidia via a nitric oxide-dependent pathway and that such a killing process might be stimulated by gamma interferon (5). It is possible that specific antibodies recognizing cell surface components, especially that of the conidia, stimulate the phagocytic pathway to protect against infection by P. marneffei. However, neither the humoral response nor its role in protection against P. marneffei infection has been carefully addressed. Since sera from penicilliosis patients contain high levels of specific antibodies against fungal cell surface components (49), we reasoned that such an antigenic component could be a cell wall protein and that we might be able to isolate the gene that encodes this protein.
In this study, we report the cloning of the MP1 gene, which encodes an antigenic protein of P. marneffei. DNA sequence analysis reveals that the MP1 gene has an open reading frame encoding 462 amino acid residues. To elucidate its potential biological structure and function, we show that the sequence contains features similar to several yeast cell wall proteins. Our results further suggest that it is an abundant cell wall mannoprotein. In addition, immunoelectron microscopic study indicates that Mp1p is specifically located in the cell walls of yeast, hyphae, and conidia found in mold form. Finally, our results show that P. marneffei patients develop high levels of specific antibody against Mp1p, suggesting that Mp1p may represent a good cell surface target of host humoral immunity.
MATERIALS AND METHODS
Strains and growth conditions.
A P. marneffei PM4 strain isolated from a patient was used throughout the study. The cells were grown on blood agar plates at 37°C to obtain single yeast colonies. P. marneffei cells were grown in RPMI medium (Gibco BRL, Gaithersburg, Md.) at 37°C to give a yeast culture and in YPD (1% yeast extract, 2% Bacto Peptone, 2% glucose) at 30°C to give a mold culture. Escherichia coli XL-1 Blue and SOLR, from Stratagene (La Jolla, Calif.), were used for screening of the cDNA library and for phage-to-plasmid conversion.
Generation of antibodies.
To produce a polyclonal guinea pig antibody for screening of the P. marneffei expression library, P. marneffei yeast cells were washed in PBS (13.7 mM sodium chloride, 0.27 mM potassium chloride, 1 mM phosphate buffer [pH 7.4]) and suspended in PBS with 0.05% phenol at a turbidity of McFarland standard 3. An equal volume of complete Freund’s adjuvant was mixed with 500 μl of yeast suspension and injected intramuscularly into a guinea pig’s thigh. Incomplete Freund’s adjuvant was used in subsequent immunizations, and a total of four inoculations were completed in 2 months.
To prepare antibodies against Mp1p, 250 μg of purified glutathione S-transferase (GST)-Mp1p recombinant protein was mixed with an equal part of complete Freund’s adjuvant and injected subcutaneously into rabbits and guinea pigs. Incomplete Freund’s adjuvant was used in subsequent injections. Serum samples were taken 2 weeks after the third injection.
Cloning of the MP1 gene.
Total yeast RNA was isolated from 100 ml of yeast culture of P. marneffei cells with TRIzol reagent (Gibco BRL). Poly(A)+ RNA was obtained with a QuickPrep Micro mRNA purification kit (Pharmacia, Uppsala, Sweden) based on the conventional oligo(dT) cellulose method. The mRNA was then used to construct a Lambda ZAP cDNA expression library (Stratagene). The library had at least 1,000,000 independent phage plaques, with more than 95% containing inserts of an average size of 1 kb. Approximately 50,000 plaques of this library were screened with guinea pig anti-P. marneffei antiserum at a 1:500 dilution (32). Ten positive phage clones were isolated, and their cDNA inserts were excised with ExAssist helper phage in SOLR cells (Stratagene), yielding pBluescript SK (pBSK) plasmids containing the inserts.
DNA sequencing.
DNA sequencing was carried out by using vector primers of pBSK (T3 and T7) and synthetic primers. Bidirectional DNA sequencing was performed with an ABI automatic sequencer. The DNA sequence was analyzed by the Genetics Computer Group program, version 8.0 (Madison, Wis.). BLAST analysis was performed with the National Center for Biotechnology Information server at the National Library of Medicine (Bethesda, Md.) and a server at Stanford University containing the complete Saccharomyces cerevisiae genome database. The searches were done at both the protein and DNA levels.
Expression and purification of recombinant Mp1p protein from E. coli.
To produce a fusion plasmid for protein purification, primers were used to amplify the MP1 gene from the pBSK-MP1 plasmid. The sequence coding for amino acid residues 35 to 462 of Mp1p was amplified and cloned into the BamHI and XhoI sites of expression vector pGEX30 in frame and downstream of the GST coding sequence. The GST-Mp1p fusion protein was expressed and purified with the GST Gene Fusion System (Pharmacia) as described by the manufacturer. Approximately 10 mg of purified protein was routinely obtained from 1 liter of E. coli cells carrying the fusion plasmid.
In vitro translation and immunoprecipitation.
Mp1p was in vitro translated with the TNT coupled reticulocyte lysate system (Promega, Madison, Wis.). The protein was then immunoprecipitated, and the immune complex was separated on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel followed by fluorography (15).
Preparation of cell lysate and cell wall extract.
P. marneffei yeast cells were harvested and resuspended in Lyticase buffer (1 M sorbitol, 10 mM magnesium chloride, 30 mM sodium phosphate) with 100 U of Lyticase (glucanase; Sigma, St. Louis, Mo.) per ml and 1% β-mercaptoethanol. After incubation at 30°C for 30 min, the cells were pelleted by centrifugation at 2,000 × g for 5 min. The supernatant was then collected as the glucanase extract. The pellet, containing yeast spheroplasts, was resuspended in lysis buffer (1% Nonidet P-40, 150 mM sodium chloride, 50 mM Tris-HCl [pH 8], 5 mM EDTA) with protease inhibitors and incubated on ice for 1 h. After centrifugation of the above lysed cells at 12,000 × g at 4°C for 5 min, the supernatant was recovered as the cell lysate.
Immunoblot analysis and glycoprotein and ConA detection assays.
For immunoblot analysis, protein samples were run on an SDS–10% polyacrylamide gel and subsequently electroblotted onto a nitrocellulose membrane (Bio-Rad, Hercules, Calif.). The blot was incubated with a 1:1,000 dilution of anti-Mp1p antibody, and proteins were detected with the ECL fluorescence kit (Amersham Life Science, Buckinghamshire, United Kingdom).
Sugar residues in glycoconjugates were detected by the DIG glycan kit (Boehringer, Mannheim, Germany). Briefly, the glycoproteins were blotted onto a filter. The sugar residues were then oxidized to aldehyde groups and covalently attached to digoxigenin (DIG). The resulting DIG-labeled glycoconjugates were subsequently visualized with anti-DIG specific antibody conjugated with alkaline phosphatase and the chromogenic substrates BCIP-NBT (5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium).
For the concanavalin A (ConA) assay, the filter with glycoproteins was incubated with a DIG-labeled lectin (ConA; Boehringer) that binds to mannose residues. The resulting conjugates were detected as described above.
Immunogold staining and electron microscopy.
To prepare the specimens for immunogold staining and electron microscopy, P. marneffei yeast and mold cells were harvested and washed twice in PBS. Cells were fixed in filtered PBS containing 4% (wt/vol) paraformaldehyde and 2% (vol/vol) glutaraldehyde for 30 min at room temperature followed by dehydration in a graded series of ethanol and embedding in LR White (Sigma). Ultrathin sections were cut and mounted onto 200 mesh gold grids for immunostaining.
For immunostaining, sections were first blocked for 20 min in 3% (wt/vol) bovine serum albumin fraction V (BSA) (Sigma). Rabbit anti-Mp1p serum (diluted 1:80 in PBS with 3% BSA) was added and incubated with the cell sections for 2 h. A preimmune rabbit serum was used as the negative control. After a wash in PBS containing 0.1% Tween 20, the sections were incubated in 1% TBSA (20 mM Tris [pH 8.2], 1% BSA) containing 1:20-diluted goat anti-rabbit immunoglobulin G conjugated with gold particles of 10 nm in diameter (Amersham). Following a wash with 1% TBSA, the sections were counterstained with uranyl acetate and lead citrate. Electron microscopy work was done with a JEOL 100SX transmission electron microscope at 80 kV.
Nucleotide sequence accession number.
The nucleotide sequence of the MP1 gene has been deposited with GenBank under accession no. bankit 122964.
RESULTS
Cloning of MP1.
A Lambda ZAP cDNA expression library of P. marneffei was constructed and screened with human serum obtained from a penicilliosis patient who had a high antibody titer against P. marneffei cells, as indicated by immunofluorescence (49). This approach proved to be difficult because the human serum produced a high background in the filter screening assay. Therefore, an animal hyperimmune serum was generated by immunizing guinea pigs with killed P. marneffei yeast cells. The guinea pig hyperimmune serum was used for immunofluorescence staining of P. marneffei cells. The staining pattern was similar to that of patients’ sera, as both recognized the fungal cell wall (reference 49 and data not shown). About 50,000 independent phage plaques were screened with this guinea pig hyperimmune serum. Ten positive plaques were selected, purified, and converted into plasmids. When induced with isopropyl-β-d-thiogalactopyranoside, 7 of the 10 isolates produced protein bands of 50 kDa that were recognized by the guinea pig hyperimmune serum on a Western blot (data not shown). PCR and partial sequence analysis of the seven clones revealed a single gene of about 1.5 kb which was named MP1 (for mannoprotein 1). (A United States patent application [serial no. 08/655,730] was filed on 30 May 1996.)
Southern blot analysis of P. marneffei genomic DNA digested with EcoRI or PstI, with the 1.5-kb full-length MP1 gene as the probe, showed single bands for each of the two digests (data not shown), indicating that MP1 was a unique gene.
Sequence analysis of MP1.
Bidirectional DNA sequencing of MP1 revealed that the cDNA contained a single open reading frame encoding 462 amino acid residues with a predicted molecular mass of 47.8 kDa. The DNA and predicted protein sequences are shown in Fig. 1.
FIG. 1.
DNA and amino acid sequences of Mp1p. MP1 cDNA contains a single open reading frame encoding 462 amino acid residues with a predicted molecular mass of 47.8 kDa. The N-terminal cleavable signal peptide of 18 amino acids and the C-terminal cleavable GPI signal peptide of 33 amino acids are underlined. A 67-amino-acid serine- and threonine-rich region is in italics. Two potential N glycosylation sites, at positions 209 and 429, are indicated by boldface type.
Protein sequence analysis suggests that Mp1p is likely to be a yeast cell wall protein. BLAST analysis was performed to search for homologs that might suggest the potential biological functions of MP1. The BLAST results indicate that MP1 is a novel gene without a homolog in any public gene database. However, careful examination of the Mp1p sequence revealed that it has several features that are common for several fungal cell wall proteins of both S. cerevisiae (20, 27, 29, 31, 40, 44) and Candida albicans (1, 33). Mp1p has a putative N-terminal signal peptide found in most secretory proteins (47). It also has a putative C-terminal glycosylphosphatidylinositol (GPI) membrane attachment signal sequence that is commonly used for cytoplasmic membrane attachment (2, 42) and has been implicated in yeast cell wall assembly (8). After processing, the mature Mp1p should have 411 amino acid residues with a predicted molecular mass of 43 kDa as a polypeptide.
Mp1p is expected to be a glycosylated protein, since it has potential N glycosylation and O glycosylation sites. There are two N glycosylation sites at asparagine residues 209 and 429, as are also present in other GPI proteins (13). In addition, Mp1p contains a 77-amino-acid serine- and threonine-rich region in its C-terminal half as a site for O glycosylation, similar to other yeast cell wall proteins of both S. cerevisiae and C. albicans (1).
Expression and purification of Mp1p and production of Mp1p-specific antibodies.
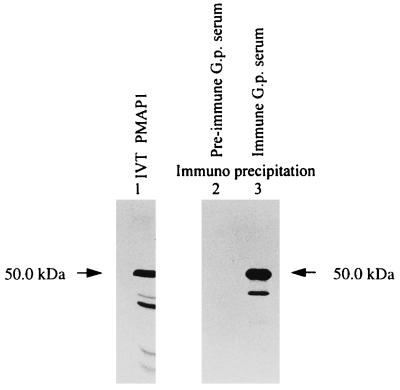
To identify Mp1p protein, the MP1 cDNA was translated in vitro with the TNT coupled reticulocyte lysate system in the presence of [35S]methionine. The resulting protein was run on an SDS-polyacrylamide gel, and a protein band of about 50 kDa was visualized (Fig. 2, lane 1). This 50-kDa protein was specifically immunoprecipitated with guinea pig anti-P. marneffei serum, but not with preimmune serum, confirming that Mp1p is an immunogenic protein of P. marneffei in guinea pig serum.
FIG. 2.
In vitro translation of MP1. MP1 was in vitro translated with Promega’s rabbit reticulocyte lysate, and a major band of 50 kDa can be detected (lane 1). Mp1p was immunoprecipitated with guinea pig (G.p.) immune serum against P. marneffei cells (lane 3) but not with preimmune serum (lane 2).
To produce recombinant Mp1p protein, the MP1 sequence minus the signal peptide (codons 35 to 462) was amplified by PCR and cloned in frame with GST in expression plasmid pGEX30. The GST-Mp1p fusion protein was expressed in E. coli and subsequently purified. The purified protein was separated on an SDS gel followed by Coomassie blue staining. After purification, several bands were visible on the gel, with the largest at 75 kDa (data not shown), consistent with the expected molecular mass of 73 kDa for the fusion protein. GST-Mp1p was probably very unstable in E. coli, since multiple protein bands were detectable even with minimal induction time (1 h) and in a protease-deficient strain, BL21. The identity of the GST-Mp1p protein was further confirmed by Western blotting with guinea pig anti-P. marneffei serum. The purified proteins were highly reactive with the guinea pig immune serum (results not shown).
To investigate the biochemical properties, cellular localization, and biological function of Mp1p, as well as its potential role in fungal pathogenesis and antifungal immunity, specific antibodies against this protein were produced. The GST-Mp1p protein purified from E. coli was used to immunize guinea pigs and rabbits to generate specific antibodies. The antibodies were then examined on enzyme-linked immunosorbent assay plates coated with Mp1p recombinant protein. Titers of greater than 100,000 were obtained, and the antibodies were used for later studies as anti-Mp1p antibodies.
Identification of Mp1p from P. marneffei cells and from glucanase extract of the cell surface fraction.
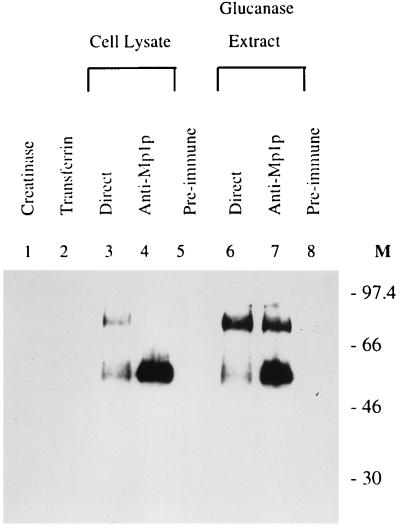
Western blot analysis with rabbit anti-Mp1p antibody was carried out to identify cellular Mp1p from total cell lysate of P. marneffei yeast cells. Two bands, of 58 and 90 kDa, were detected (Fig. 3, lane 3). Their molecular masses are higher than the predicted molecular mass of 43 kDa for the mature Mp1p of 411 amino acids. This result was expected, since Mp1p is likely to be glycosylated in P. marneffei cells, as predicted from its sequence.
FIG. 3.
Mp1p is present in the glucanase extract fraction of the P. marneffei yeast cell wall. Both the cell lysate and glucanase extract of P. marneffei yeast cells were made from the same cell pellet. They were resuspended in the same final volume so that equivalent amounts of lysate or extract could be loaded onto the gel. Western blot analysis was carried out with rabbit anti-Mp1p antibody. Mp1p was detected in both the total yeast cell lysate (lane 3) and the cell wall glucanase extract (lane 6). Two protein bands, of 58 and 90 kDa, were seen on the Western blot. They were specifically immunoprecipitated with guinea pig anti-Mp1p antibody (lanes 4 and 7) but not with preimmune serum (lanes 5 and 8). Lanes 1 and 2 show two control proteins, creatinase as a nonglycosylated protein and transferrin as a glycosylated protein. Neither reacts with rabbit anti-Mp1p antibody. M, molecular mass (in kilodaltons).
Since protein sequence information revealed that Mp1p was likely to be a cell wall protein, we asked whether Mp1p was on the surfaces of P. marneffei cells and whether it could be removed from the cell walls through glucanase extraction. Although little was known about P. marneffei cell walls and cell wall proteins, earlier studies with S. cerevisiae indicated that cell wall mannoproteins could be divided into two groups, the glucanase-extractable and SDS-extractable mannoproteins. It was also proposed that the glucanase-extractable mannoproteins were covalently linked to glucan (37, 45). To test the possibility that Mp1p was a cell surface glucanase-extractable protein, P. marneffei yeast cells were collected and treated with Lyticase in the presence of 1 M sorbitol to stabilize the cells. After digestion, the cells were pelleted and the supernatant was removed as the surface glucanase extract for further studies. The pellet was subsequently lysed in an equal volume of lysis buffer to give the cell lysate. Western blot analysis of both the glucanase extract and the cell lysate with rabbit anti-Mp1p antibody indicated that Mp1p could effectively be extracted from cell walls by glucanase treatment (Fig. 3, lanes 3 and 6).
To confirm that the immunoreactive band was Mp1p, both the cell lysate and the glucanase extract were immunoprecipitated with guinea pig preimmune and anti-Mp1p immune sera, followed by Western blot analysis with the rabbit anti-Mp1p antibody. Only the anti-Mp1p antiserum specifically precipitated Mp1p from both the cell lysate and glucanase extract preparations (Fig. 3, lanes 4 and 7). The preimmune serum failed to precipitate any Mp1p (Fig. 3, lanes 5 and 8). When we compared the relative intensities of the 90- and 58-kDa bands before and after immunoprecipitation (Fig. 3, lanes 3 and 6 and lanes 4 and 7), we noticed that the 58-kDa band was preferentially immunoprecipitated. It was not known what contributed to such disparity. Based on the specific recognition by two different antibodies raised against the recombinant Mp1p protein, we suggest that both bands are products of MP1.
Mp1p is an abundant mannoprotein in P. marneffei cells.
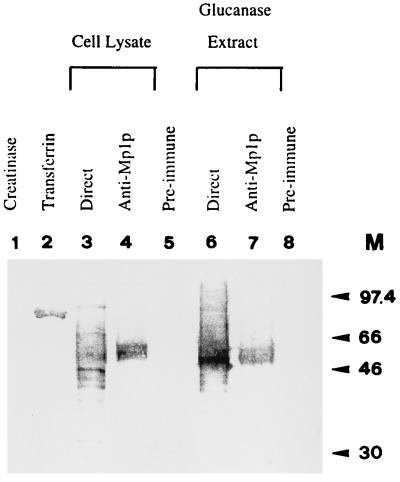
Since mannoproteins are the major cell wall components of S. cerevisiae and C. albicans (11, 16, 18, 36), we asked if Mp1p is also a mannoprotein. ConA was used for the test because it has high affinity for mannose-containing carbohydrates and has been used extensively for the study of mannoproteins of S. cerevisiae and C. albicans. In addition, ConA has also been used for purification of mannoproteins (44, 45). The above extracts and the immunoprecipitated proteins were tested for ConA staining. Although many ConA staining bands were visible with the total cell lysate and the glucanase extract fraction of the cell surface (Fig. 4, lanes 3 and 6), the 58-kDa protein was the primary ConA reactive band after immunoprecipitation with guinea pig anti-Mp1p (Fig. 4, lanes 4 and 7), indicating that Mp1p is a mannoprotein. The 90-kDa protein appeared to be absent in the immunoprecipitated lanes (Fig. 4, lanes 4 and 7). This was probably due to the fact that there was a significantly smaller amount of this protein in the precipitated fractions and that chromogenic substrates were used for detection instead of the more sensitive ECL fluorescence method, thereby failing to reveal the lower levels of reacting proteins at 90 kDa. Although there were many ConA reactive proteins in the total cell lysate and the glucanase extract, it appeared that the most abundant mannoprotein band from the total surface glucanase extract was comigrating with Mp1p (Fig. 4, lanes 4 and 7), suggesting that Mp1p could be one of the most abundant glucanase-extractable mannoproteins in P. marneffei cells.
FIG. 4.
Mp1p is a mannoprotein. All samples were loaded in the same order as in Fig. 3. ConA was used because it has high affinity for mannoproteins. M, molecular mass (in kilodaltons).
Similar results were obtained when the same protein samples were used in a total glycoprotein detection assay using the DIG glycan detection kit, which detects all glycoproteins based on the oxidation of adjacent hydroxyl groups in carbohydrates to aldehydes, suggesting that Mp1p is also a highly abundant cell surface glycoprotein in P. marneffei (data not shown).
Examination of the cell wall distribution of Mp1p in yeast, conidia, and hyphae of P. marneffei by electron microscopy.
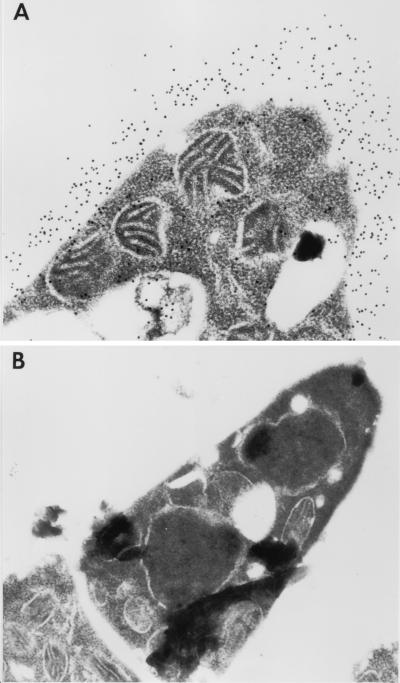
To examine the distribution of Mp1p in the yeast cell wall, fixed sections of P. marneffei yeast cells were immunogold stained with rabbit anti-Mp1p antibody. Electron micrographs demonstrated that Mp1p was specifically located in the walls of P. marneffei yeast cells (Fig. 5A). A negative control with preimmune rabbit serum showed no staining (Fig. 5B). Ultrastructural analysis further indicated that Mp1p was evenly spread throughout the entire thickness of the yeast cell wall of P. marneffei.
FIG. 5.
Immunoelectron microscopy of P. marneffei yeast cells stained with rabbit anti-Mp1p antibody. (A) P. marneffei yeast cells stained with rabbit anti-Mp1p antibody. Immunogold particles were specifically located throughout the entire thickness of the yeast cell wall. (B) Negative control stained with preimmune rabbit serum. Magnification, ×17,000.
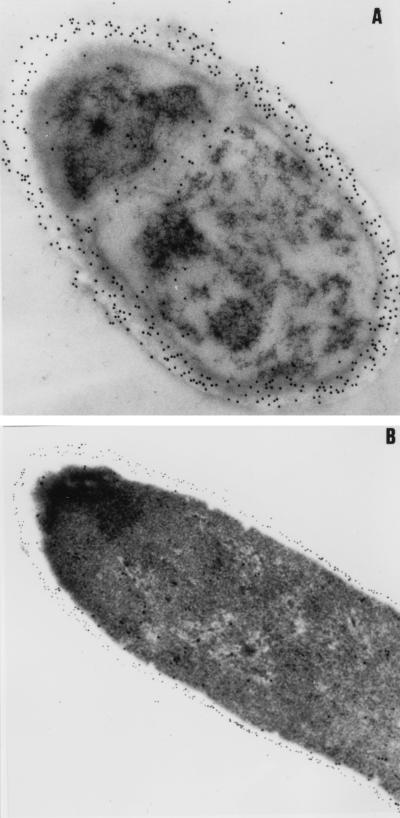
Both the conidia and hyphae of P. marneffei were also examined for Mp1p distribution. The electron microscopic results with the anti-Mp1p antibody showed that the protein was present throughout the thickness of the conidial cell walls (Fig. 6A). However, the distribution of Mp1p seemed to be different in the hyphae. It was localized on the outer layers of the hyphal cell walls (Fig. 6B). It is not known what contributes to such differences in cell wall localization for Mp1p.
FIG. 6.
Immunoelectron microscopy of P. marneffei conidia (A) and hyphae (B) stained with rabbit anti-Mp1p antibody.
Detection of Mp1p antibody in infected patients.
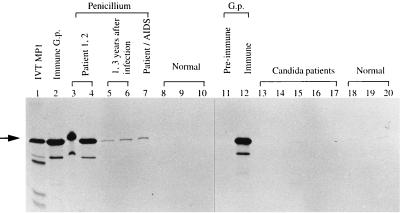
The presence of specific anti-Mp1p antibody in the sera of infected patients was investigated by immunoprecipitation with in vitro-translated Mp1p. Mp1p was specifically immunoprecipitated by sera from immunocompetent P. marneffei patients (Fig. 7, lanes 3 and 4) at a level similar to that of the guinea pig hyperimmune serum (Fig. 7, lanes 2 and 12). Thus, Mp1p is a highly immunogenic protein in penicilliosis patients who produce high levels of anti-Mp1p antibodies. Marked reduction of anti-Mp1p antibody levels was observed in people at 1 and 3 years after recovery from earlier P. marneffei infections (Fig. 7, lanes 5 and 6). An AIDS patient with penicilliosis had a lower but detectable level of anti-Mp1p antibody (Fig. 7, lane 7). No precipitated Mp1p was seen with sera from either healthy individuals or patients with documented C. albicans infection. This result suggests the presence of specific antibodies against Mp1p in the sera of penicilliosis patients.
FIG. 7.
Specific immunoprecipitation of Mp1p by penicilliosis patient sera. A major band of 50 kDa (arrow) was the in vitro-translated (IVT) full-length Mp1p. Mp1p can be immunoprecipitated by guinea pig (G.p.) immune serum (lanes 2 and 12) but not preimmune serum (lane 11). Mp1p protein was immunoprecipitated by sera from two immunocompetent patients with acute penicilliosis (lanes 3 and 4), from two people at 1 and 3 years after recovery from penicilliosis (lanes 5 and 6), and from an AIDS-penicilliosis patient (lane 7). Controls were sera from healthy blood donors (lanes 8 to 10 and 18 to 20) and from patients infected with C. albicans (lanes 13 to 17).
DISCUSSION
In this study, we describe a method of screening for genes encoding antigenic proteins in the pathogenic fungus P. marneffei by using guinea pig hyperimmune serum raised against the fungal cells. Previously we used a similar approach to screen an expression library of C. albicans and identified enolase genes in 47 of 50 independent isolates (unpublished results). Since it was known that enolase was the most immunogenic protein of C. albicans in infected patients (12, 23, 38), we reasoned that the same approach with P. marneffei should also produce results. Indeed, of the 10 clones isolated with guinea pig hyperimmune serum raised against P. marneffei cells, 7 were shown to contain a unique new gene, MP1.
DNA sequence analysis of MP1 revealed that MP1 encodes a unique protein (Mp1p) of 462 amino acid residues. Although BLAST analysis of MP1 failed to identify any homolog at either the DNA or protein level, examination of the Mp1p sequence identified several features that are common to cell wall proteins in S. cerevisiae (20, 27, 29, 31, 40, 44) and C. albicans (1, 33). These include an N-terminal signal peptide, a serine- and threonine-rich region in the C-terminal half that acts as a site for O glycosylation, and a C-terminal GPI membrane attachment signal. The GPI signal peptide is utilized by many proteins to anchor themselves to eukaryotic cell membranes (2, 42). Once anchored to the cell membrane, these cell surface proteins may fulfill many important physiological functions, including cell-cell recognition, cell adhesion, and service as receptors and nutrient and ion transporters. In S. cerevisiae, interestingly, the GPI signal has been shown to be necessary for cell wall localization, and the removal of this sequence leads to secretion of the protein into the medium (35, 43, 48).
As predicted from the protein sequence, we show that Mp1p is a highly abundant cell wall mannoprotein. It can be effectively extracted from cell surfaces by treating P. marneffei yeast cells with glucanase that digests cell wall glucan. Mp1p has high affinity for ConA, indicative of a mannoprotein, and it is also glycosylated. Finally, immunoelectron microscopy demonstrates the cell wall localization of Mp1p protein in the yeast, conidia, and hyphal cells of P. marneffei.
Mannoproteins are one of the major structural components of the fungal cell wall. Extensive work with yeast has suggested roles for mannoproteins in a variety of diverse biological functions: determining cell shape, supporting cell growth and morphological change, serving a protective role, allowing sex agglutination, and limiting the porosity of the cell wall (9, 11, 16, 18, 33, 36). With S. cerevisiae, experiments have indicated that AGα1 and AGA1 are involved in sexual mating (20–22, 31), that FLO1 is related to flocculation (40), and that GAS1 participates in cell growth and aggregation (29). Recently, it has also been shown that mutants carrying deletions of a number of newly identified putative mannoprotein genes, including CWP1, CWP2, TIP1, and ICWP (27, 44), produce increased sensitivity to Congo red that disturbs the cell wall. In C. albicans, genes for several cell wall proteins have also been identified, including a pH-regulated PHR1 gene required for morphogenesis (33) and a hypha-specific HYR1 gene that appears to be a nonessential component of the hyphal cell wall (1). Mp1p is the first such protein from P. marneffei and appears to be one of the most abundant cell wall mannoproteins. At present, it is not possible to carry out gene replacement work with P. marneffei, due to the lack of a genetic system; therefore, the biological function of Mp1p remains to be addressed.
Ultrastructural analysis by immunogold staining with anti-Mp1p antibody reveals that Mp1p is specifically located in the cell wall and spans the entire thickness of the wall in yeast cells. Although the predicted Mp1p sequence contains a GPI domain that is commonly used for cytoplasmic membrane attachment (2, 42), both the ultrastructural results and the fact that Mp1p can be extracted with glucanase indicate that Mp1p is embedded in the yeast cell wall layer with glucan and therefore is unlikely to be directly associated with the cytoplasmic membrane. This result supports the hypothesis that in fungus the GPI-anchored proteins may be intermediates in the transfer of this type of cell wall protein to the cell wall glucan layer. Interestingly, this staining pattern of yeast cells is similar to a result obtained with C. albicans when a monoclonal antibody recognizing an oligomannoside epitope was used (6), suggesting that this distribution may be typical for many mannoproteins. Electron microscopic analysis of yeast cells also revealed staining granules in cytosolic vesicles (not shown). It is likely that these vesicles contain processing Mp1p that is exported to the outer surface of the cytoplasmic membrane.
It is interesting that the most antigenic protein in P. marneffei is a cell wall mannoprotein. Mannoproteins have been implicated both in activating nonspecific immunity (30, 34, 46) and in eliciting cell-mediated immunity (26, 41). From our results, they are further shown to be closely associated with humoral immunity. Although cell-mediated immunity plays a major protective role against opportunistic fungal infections, antibodies have also been suggested to be important against certain extracellular opportunistic fungi (4, 24, 25). It has been shown that antibodies against mannan of C. albicans protect against intravenously injected C. albicans cells (14). Similarly, monoclonal antibodies against the capsular polysaccharide glycomannan of Cryptococcus neoformans prolonged survival when mice were inoculated with the fungal pathogen (3, 28). Although P. marneffei is an intracellular pathogen for most of its life cycle in humans, the initial stage of infection is likely mediated through the inhalation of its conidia. Elevation of the antibody response via active immunization might stimulate the complement pathway and facilitate phagocytosis of the conidia, thereby preventing infection. Since Mp1p is commonly present in the cell walls of conidia, it is worth investigating whether anti-Mp1p antibodies have a protective role at the initial stage of infection, which is likely mediated by the conidia.
The cloning of MP1 should have direct implications for the clinical diagnosis of P. marneffei infections. P. marneffei causes progressive systemic diseases in normal and immunocompromised patients. Clinical diagnosis is often difficult because most patients present with fever without any localizing symptom. Since it is rare to isolate this pathogen from blood cultures of these patients, the diseases are routinely treated as tuberculosis (10, 49). Definitive diagnosis requires invasive procedures to obtain biopsy specimens from bone marrow, lymph node, and spleen (39, 49), and they are often delayed (49). An immunofluorescence serological test using fixed P. marneffei cells (49), though useful in many cases, is relatively nonspecific. Therefore, an enzyme-linked immunosorbent assay using purified Mp1p should greatly enhance the sensitivity and specificity of the serological test for P. marneffei infections.
ACKNOWLEDGMENTS
We are grateful for the excellent technical assistance of W. L. Yao in performing immunogold electron microscopic work. We thank P. Y. Chau and M. H. Ng for very helpful discussions and Joan Marsh for reading the manuscript.
This work was supported by a CRCG grant from the University of Hong Kong, Hong Kong Industry Support Fund (AF/55/96) and a Hong Kong RGC grant (HKU489/96M).
REFERENCES
- 1.Bailey D A, Feldmann P J F, Bovey M, Gow N A R, Brown A J P. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–5360. doi: 10.1128/jb.178.18.5353-5360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caras I W, Weddell G N. Signal peptide for protein secretion directing glycophospholipid membrane anchor attachment. Science. 1989;243:1196–1198. doi: 10.1126/science.2466338. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassone A, Conti S, De Bernardis F, Polonelli L. Antibodies, killer toxins and antifungal immunoprotection: a lesson from nature? Immunol Today. 1997;18:164–169. doi: 10.1016/s0167-5699(97)84662-2. [DOI] [PubMed] [Google Scholar]
- 5.Cogliati M, Roverselli A, Boelaert J R, Taramelli D, Lombardi L, Viviani M A. Development of an in vitro macrophage system to assess Penicillium marneffei growth and susceptibility to nitric oxide. Infect Immun. 1997;65:279–284. doi: 10.1128/iai.65.1.279-284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bernardis F, Molinari A, Boccanera M, Stringaro A, Robert R, Senet J-M, Arancia G, Cassone A. Modulation of cell surface-associated mannoprotein antigen expression in experimental candidal vaginitis. Infect Immun. 1994;62:509–519. doi: 10.1128/iai.62.2.509-519.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng Z L, Ribas J L, Gibson D W, Connor D H. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis. 1988;10:640–652. doi: 10.1093/clinids/10.3.640. [DOI] [PubMed] [Google Scholar]
- 8.De Nobel H, Lipke P N. Is there a role for GPIs in yeast cell-wall assembly? Trends Cell Biol. 1994;4:42–45. doi: 10.1016/0962-8924(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 9.Douglas L J. Adhesion of Candida albicans to host surface. FEMS Symp. 1991;50:43–48. [Google Scholar]
- 10.Duong A D. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 11.Fleet G H. Cell walls. In: Rose A H, Harrison J S, editors. The yeasts. 2nd ed. Vol. 4. London, United Kingdom: Academic Press; 1991. pp. 199–277. [Google Scholar]
- 12.Franklyn K M, Warmington J R. Cloning and nucleotide sequence analysis of the Candida albicans enolase gene. FEMS Microbiol Lett. 1993;111:101–108. doi: 10.1111/j.1574-6968.1993.tb06368.x. [DOI] [PubMed] [Google Scholar]
- 13.Gavel Y, von Heijne G. Sequence differences between glucosylated and non-glycosylated Asn-X-Ser/Thr acceptor sites: implications for protein engineering. Protein Eng. 1990;3:433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han T, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 16.Hearn V M. Structure and function of the fungal cell wall. In: Jacobs P H, Nall L, editors. Fungal disease. New York, N.Y: Marcel Dekker; 1997. pp. 27–60. [Google Scholar]
- 17.Imwidthaya P. Update of penicilliosis marneffei in Thailand. Mycopathologia. 1994;127:135–137. doi: 10.1007/BF01102912. [DOI] [PubMed] [Google Scholar]
- 18.Klis F M. Review: cell wall assembly in yeast. Yeast. 1994;10:851–869. doi: 10.1002/yea.320100702. [DOI] [PubMed] [Google Scholar]
- 19.Kudeken N, Kawakami K, Kusano N, Saito A. Cell-mediated immunity in host resistance against infection caused by Penicillium marneffei. J Med Vet Mycol. 1996;34:371–378. doi: 10.1080/02681219680000671. [DOI] [PubMed] [Google Scholar]
- 20.Lipke P N, Wojciechowicz D, Kurjan J. AGα1 is the structural gene for the Saccharomyces cerevisiae α-agglutinin, a cell surface glycoprotein involved in cell-cell interactions during mating. Mol Cell Biol. 1989;9:3155–3165. doi: 10.1128/mcb.9.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C-F, Kurjan J, Lipke P N. A pathway for cell wall anchorage of Saccharomyces cerevisiae α-agglutinin. Mol Cell Biol. 1994;14:4825–4833. doi: 10.1128/mcb.14.7.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu C F, Montijn R C, Brown J L, Klis F, Kurjan J, Lipke P N. Glycosyl phosphatidylinositol-dependent crosslinking of α-agglytinin and β-1,6-glucan in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1995;128:333–340. doi: 10.1083/jcb.128.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason A B, Buckley H R, Gorman J A. Molecular cloning and characterization of the Candida albicans enolase gene. J Bacteriol. 1993;175:2632–2639. doi: 10.1128/jb.175.9.2632-2639.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews R, Hodgetts S, Burnie J. Preliminary assessment of a human recombinant antibody fragment to hsp90 in murine candidiasis. J Infect Dis. 1995;171:1668–1671. doi: 10.1093/infdis/171.6.1668. [DOI] [PubMed] [Google Scholar]
- 25.Matthews R C, Burine J P, Howart D, Rowland T, Walton F. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidosis. Immunology. 1991;74:20–24. [PMC free article] [PubMed] [Google Scholar]
- 26.Mencacci A, Torosantucci A, Spaccapelo R, Romani L, Bistoni F, Cassone A. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect Immun. 1994;62:5353–5360. doi: 10.1128/iai.62.12.5353-5360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moukadira I, Armero J, Abad A, Sentandreu R, Zueco J. Identification of a mannoprotein present in the inner layer of the cell wall of Saccharomyces cerevisiae. J Bacteriol. 1997;179:2154–2162. doi: 10.1128/jb.179.7.2154-2162.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee S, Lee S C, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuoffer C, Jenö P, Conzelmann A, Riezman H. Determinants for glycophospholipid anchoring of the Saccharomyces cerevisiae GAS1 protein to the plasma membrane. Mol Cell Biol. 1991;11:27–37. doi: 10.1128/mcb.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palma C, Serbousek D, Torosantucci A, Cassone A, Djeu J Y. Identification of a mannoprotein fraction from Candida albicans that enhances human polymorphonuclear leukocyte (PMNL) functions and stimulates lactoferrin in PMNL inhibition of candidal growth. J Infect Dis. 1992;166:1103–1112. doi: 10.1093/infdis/166.5.1103. [DOI] [PubMed] [Google Scholar]
- 31.Roy A, Lu C F, Marykwas D L, Lipke P N, Kurjan J. The AGA1 product is involved in cell surface attachment of the Saccharomyces cerevisiae cell adhesion glycoprotein a-agglutinin. Mol Cell Biol. 1991;11:4196–4206. doi: 10.1128/mcb.11.8.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Saporito-Irwin S M, Birse C E, Sypherd P S, Fonzi W A. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15:601–613. doi: 10.1128/mcb.15.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scaringi L, Marconi P, Boccanera M, Tissi L, Bostoni F, Cassone A. Cell wall components of Candida albicans as immunomodulators: induction of natural killer and macrophage-mediated peritoneal cell cytotoxicity in mice by mannoprotein and glucan fractions. J Gen Microbiol. 1988;134:1265–1274. doi: 10.1099/00221287-134-5-1265. [DOI] [PubMed] [Google Scholar]
- 35.Schreuder M P, Brekelmans S, Van den Ende H, Klis F M. Targeting of a heterologous protein to the cell wall of Saccharomyces cerevisiae. Yeast. 1993;9:399–409. doi: 10.1002/yea.320090410. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd M G, Gopal P K. Nature and control of cell wall biosynthesis. In: Bossche H V, Odds F C, Kerridge D, editors. Dimorphic fungi in biology and medicine. New York, N.Y: Plenum Press; 1992. pp. 153–167. [Google Scholar]
- 37.Shibata N, Mizugami K, Takano K, Suzuki S. Isolation of mannan-protein complexes from viable cells of Saccharomyces cerevisiae X2180-1A wild type and Saccharomyces cerevisiae X2180-1A-5 mutant strains by the action of Zymolyase-60,000. J Bacteriol. 1983;156:552–558. doi: 10.1128/jb.156.2.552-558.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundstrom P, Aliaga G R. Molecular cloning of cDNA and analysis of protein secondary structure of Candida albicans enolase, an abundant, immunodominant glycolytic enzyme. J Bacteriol. 1992;174:6789–6799. doi: 10.1128/jb.174.21.6789-6799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Supparatpinyo K, Khamwan C, Baosoung V, Nelson K, Sirisanthana T. Dissemination of Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 40.Teunissen A W R H, Holub E, Van Der Hucht J, Van Den Berg J A, Steensma H Y. Sequence of the open reading frame of the FLO1 gene from Saccharomyces cerevisiae. Yeast. 1993;9:423–427. doi: 10.1002/yea.320090413. [DOI] [PubMed] [Google Scholar]
- 41.Torosantucci A, Bromuro C, Gomez M J, Ausiello C M, Urbani F, Cassone A. Identification of a 65-kDa mannoprotein as a main target of human cell-mediated immune response to Candida albicans. J Infect Dis. 1993;168:427–435. doi: 10.1093/infdis/168.2.427. [DOI] [PubMed] [Google Scholar]
- 42.Udenfriend S, Kodukula K. How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem. 1995;64:563–591. doi: 10.1146/annurev.bi.64.070195.003023. [DOI] [PubMed] [Google Scholar]
- 43.Van Berkel M A A, Caro L H P, Montijin R C, Klis F M. Glucosylation of chimeric proteins in the cell wall of Saccharomyces cerevisiae. FEBS Lett. 1994;349:135–138. doi: 10.1016/0014-5793(94)00631-8. [DOI] [PubMed] [Google Scholar]
- 44.Van Der Vaart J M, Caro L H P, Chapman J W, Klis F M, Verrips C T. Identification of three mannoproteins in the cell wall of Saccharomyces cerevisiae. J Bacteriol. 1995;177:3104–3110. doi: 10.1128/jb.177.11.3104-3110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Rinsum J, Klis F M, Van Den Ende H. Cell wall glucanmannoproteins of Saccharomyces cerevisiae mnn9. Yeast. 1991;7:717–726. doi: 10.1002/yea.320070707. [DOI] [PubMed] [Google Scholar]
- 46.Vecchiarelli A, Puliti M, Torosantucci A, Cassone A, Bostoni F. In vitro production of tumor necrosis factor by murine splenic macrophages stimulated with mannoprotein constituents of Candida albicans cell wall. Cell Immunol. 1991;134:65–76. doi: 10.1016/0008-8749(91)90331-5. [DOI] [PubMed] [Google Scholar]
- 47.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wojciechowicz D, Lu C-F, Kurjan J, Lipke P N. Cell surface anchorage and ligand-binding domains of the Saccharomyces cerevisiae cell adhesion protein α-agglutinin, a member of the immunoglobulin superfamily. Mol Cell Biol. 1993;13:2554–2563. doi: 10.1128/mcb.13.4.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuen K Y, Wong S S, Tsang D N, Chau P Y. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344:444–445. doi: 10.1016/s0140-6736(94)91771-x. [DOI] [PubMed] [Google Scholar]