Abstract
Introduction
This study aimed to investigate the association between scan frequency and intermittently scanned continuous glucose monitoring (isCGM) metrics and to clarify the factors affecting scan frequency in adults with type 1 diabetes mellitus (T1D).
Methods
We enrolled adults with T1D who used FreeStyle® Libre. Scan and self-monitoring of blood glucose (SMBG) frequency and CGM metrics from the past 90-day glucose data were collected. The receiver operating characteristic curve was plotted to obtain the optimal cutoff values of scan frequency for the target values of time in range (TIR), time above range (TAR), and time below range (TBR).
Results
The study was conducted on 211 adults with T1D (mean age, 50.9 ± 15.2 years; male, 40.8%; diabetes duration, 16.4 ± 11.9 years; duration of CGM use, 2.1 ± 1.0 years; and mean HbA1c, 7.6 ± 0.9%). The average scan frequency was 10.5 ± 3.3 scan/day. Scan frequency was positively correlated with TIR and negatively correlated with TAR, although it was not significantly correlated with TBR. Scan frequency was positively correlated with the hypoglycemia fear survey-behavior score, while it was negatively correlated with some glycemic variability metrics. Adult patients with T1D and good exercise habits had a higher scan frequency than those without exercise habits. The AUC for > 70% of the TIR was 0.653, with an optimal cutoff of 11 scan/day.
Conclusions
In real-world conditions, frequent scans were linked to improved CGM metrics, including increased TIR, reduced TAR, and some glycemic variability metrics. Exercise habits and hypoglycemia fear-related behavior might affect scan frequency. Our findings could help healthcare professionals use isCGM to support adults with T1D.
Clinical Trial Registry No. UMIN000039376.
Keywords: Blood glucose monitoring frequency, Flash glucose monitoring, Type 1 diabetes, Real-world data
Introduction
Type 1 diabetes mellitus (T1D) is a chronic autoimmune disease characterized by the destruction of pancreatic beta cells [1, 2]. For patients with T1D, continuous glucose monitoring (CGM) is a cost-effective adjunct to T1D management and has the potential to improve glycemic outcomes and quality of life [3–5]. Glycated hemoglobin (HbA1c) is a fundamental metric for monitoring blood glucose levels over several months and serves as an index of average glucose measurement. Despite being relatively inexpensive and simple to measure, it only provides an approximate estimate of glucose control and does not account for glycemic variability or hypoglycemic episodes. To overcome these limitations, CGM technology has been developed to aid clinicians and individuals with diabetes in managing their condition [6]. For most patients with T1D, a time in range (TIR; 70–180 mg/dL, or 3.9–10 mmol/L) target of more than 70% is recommended, with each incremental increase of 5% toward this target being clinically meaningful [7–9]. Randomized controlled trials (RCTs) using FreeStyle® Libre™ (Abbott Diabetes Care, Alameda, CA) have the benefit of factory calibration and reduced glucose variability in both continuous subcutaneous insulin infusion and multiple daily injection users [10].
In real-world settings, studies using anonymized reader data from intermittently scanned continuous glucose monitoring (isCGM) users indicate that a higher scanning frequency is associated with a lower HbA1c and a higher TIR in Saudi Arabia [11], Netherlands [12], Israel [13], Spain [14], Brazil [15], Poland [16], and Europe [17]. Because of the anonymous nature of the database used for these studies, detailed information concerning the characteristics of FreeStyle Libre users is lacking. Previous studies examining the relationship between the number of scans and TIR have relied on the internet-uploaded data to categorize scan frequency into 10 or 20 equal-sized groups, ultimately revealing a positive correlation between increased scanning and improved TIR. However, there exists no consensus on the optimal scanning frequency, and the impact of patient attributes, such as pathology, lifestyle, and type, remains largely unexplored.
In our current study, we utilized receiver operating characteristic (ROC) curves and area under curve (AUC) to determine the optimal scan frequency, a novel approach that has not been previously reported. Therefore, this study aimed to determine the optimal scan frequency for the target achievement defined as TIR more than 70% and to clarify the influential factors affecting scan frequency in adults with T1D.
Methods
Study design
The “FGM-Japan Study” was a cross-sectional investigation that aimed to assess the practical application of intermittently scanned CGM (isCGM) in a real-world setting. The primary endpoint was the target achievement defined as TIR more than 70%.
Participants
Patients were recruited from a collaboration center between February 2020 and April 2021. The inclusion criteria were as follows: T1D, duration of isCGM use for ≥ 3 months, age ≥ 20 years, and regular attendance at the collaborating center. The exclusion criteria were non-insulin therapy, anti-dementia drug use, and inappropriate cases judged by the research director or coordinators.
Clinical characteristics
The self-reported number of severe hypoglycemia (SH) episodes in the preceding year, defined as “a hypoglycemic episode that you were unable to treat yourself,” was collected. The self-reported number of hypoglycemic episodes in the preceding month was recorded. Impaired awareness of hypoglycemia was determined using the Gold method. Data on clinical characteristics, HbA1c, liver enzymes (i.e., aspartate aminotransferase, alanine transaminase, and gamma-glutamyl transferase), and lipid profiles were collected from medical records. Diabetes distress and fear of hypoglycemia were assessed by the Problem Areas in Diabetes (PAID) survey and Hypoglycemia Fear Survey (HFS-B, for behavior, and HFS-W, for worry), respectively.
Lifestyle factors
Self-administered standardized questionnaires were used to extract data on lifestyle behaviors (current smoking, regular exercise, dietary habits, drinking habits, and sleeping habits) from the specific health check and guidance system. Exercise habits included three items: 1) regular exercise (≥ 2 times/week of exercise for ≥ 4 metabolic equivalents [METs]/h); 2) active physical activity (≥ 23 METs-h/week); and 3) walking pace (rapid or not rapid), an indicator of physical fitness. Excessive drinking was defined based on answers to the questions on drinking habits, i.e., both “occasionally or every day” and “ ≥ 180 mL of sake (equivalent to ≥ 20 g of alcohol).” The classification of lifestyle behaviors as “healthy” or “unhealthy” was based on adherence to a healthy diet and active exercise habits for the former, and on an unhealthy diet and behavior (i.e., late-night dinner consumption, current smoking, and excessive alcohol intake) for the latter [18, 19].
Continuous glucose monitoring derived metrics
CGM metrics, including the average daily risk range (ADRR), average glucose, glucose management indicator, high blood glucose index (HBGI), low blood glucose index (LBGI), mean amplitude of glycemic excursion (MAGE), mean of daily difference for inter-day variation, standard deviation (SD), time in range (TIR) 70–180 mg/dL, time below range (TBR) < 70 mg/dL or < 54 mg/dL, percent coefficient of variation (%CV), and time above range (TAR) > 180 mg/dL or > 250 mg/dL [20–23], were collected during the last 90 days using the FreeStyle Libre system (Abbott Diabetes Care Inc., Alameda, CA, USA). CGM metrics were calculated for adequate isCGM data (≥ 70%). Stable glucose levels were defined as a CV < 36%, and unstable glucose levels were defined as CV ≥ 36% according to the International Consensus on Use of CGM [22].
Statistical analysis
Continuous variables were compared using Student’s t-test or the Mann–Whitney U test. Correlations were assessed using Spearman’s rank correlation coefficient or Pearson’s correlation coefficient. Categorical variables were compared using the Fisher’s exact test. The Youden index was used to determine the optimal cutoff value for the target value. Diagnostic accuracy using the area under the receiver operating characteristic (ROC) curve was defined as follows: < 0.5 (test not useful), 0.5–0.6 (bad), 0.6–0.7 (sufficient), 0.7–0.8 (good), 0.8–0.9 (very good), and 0.9–1.0 (excellent) [24]. To identify the indirect effect of scan frequency on glycemic variability (GV), we employed structural equation modeling (SEM) through the use of TAR or TBR. Statistical analysis was conducted using the R software program, version 4.1.2 (the R Foundation for Statistical Computing, Vienna, Austria). Glycemic variability was calculated using the “Gluvarpro” and “iglu” R packages.
Results
The study was conducted on 211 adults with T1D (mean age, 50.9 ± 15.2 years; male, 40.8%; diabetes duration, 16.4 ± 11.9 years; duration of CGM use, 2.1 ± 1.0 years; mean TDD, 34.0 ± 15.4; and mean HbA1c, 7.6 ± 0.9%) (Table 1). The participants had 25.5% retinopathy, 22.0% neuropathy, and 15.2% nephropathy. The average scan frequency was 10.5 ± 3.3 scan/day. This study did not include pregnant women.
Table 1.
Clinical characteristics of FGM-Japan participants
| Variables | |
|---|---|
| Age, years | 50.9 (15.2) |
| Male sex, % | 40.8 |
| Diabetes duration, years | 16.4 (11.9) |
| HbA1c, % | 7.6 (0.9) |
| GA, % | 21.8 (4.5) |
| Insulin treatment | |
| Daily total, U | 34.0 (15.4) |
| Basal, U | 11.5 (7.2) |
| Basal, % | 33.6 (14.0) |
| CGM metrics | |
| TAR | 28.3 (16.6) |
| TIR | 62.0 (13.9) |
| TBR | 9.7 (9.2) |
| ADRR | 41.2 (10.8) |
| %CV | 34.1 (6.0) |
| MAGE | 142.9 (35.1) |
| HBGI | 6.4 (4.3) |
| LBGI | 2.4 (2.4) |
| SD | 58.2 (14.5) |
| MODD | 76.8 (27.6) |
| Psychological | |
| PAID, points | 29.9 (20.0) |
| HFS-B, points | 20.5 (5.6) |
| HFS-W, points | 14.9 (10.4) |
Mean (SD, standard deviation) or %
GA glycated albumin, CGM continuous glucose monitoring, TAR time above range, TIR time in range, TBR time below range, ADRR average daily risk range, CV coefficient of variation, MAGE mean amplitude of glycemic excursion, HBGI high blood glucose index, LBGI low blood glucose index, SD standard deviation, MODD mean daily difference for inter-day variation, PAID problem areas in diabetes, HFS-B hypoglycemia fear survey-behavior, and HFS-W hypoglycemia fear survey-worry
There was no difference in scan frequency between age, sex, BMI, HbA1c, diabetic complications, and treatment categories (Table 2). Scan frequency was positively correlated with TIR and negatively correlated with TAR, although it was not significantly correlated with TBR. The scan frequency showed a negative correlation with ADRR, %CV, MAGE, HBGI, and SD. Scan frequency showed a positive correlation with hypoglycemia fear survey (HFS)-behaviors, but did not correlate significantly with the HFS-worry score. There was no significant association between self-monitoring blood glucose (SMBG) frequency and any of the variables of interest, except for age (Table 3). There was no difference in scan frequency (10.3 ± 3.2 vs. 10.3 ± 3.3, p = 0.826) between isCGM-naïve user group (n = 11, 5.2%) or non-naïve user group (n = 200, 94.8%). There was no association between the duration of isCGM use and scan frequency (r = 0.027 [95% CI -0.109, 0.163], p = 0.696).
Table 2.
Scan frequency according to the interest variable category
| Variables | Yes | No | p value | ||
|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | ||
| ≥ 60 years | 29.0 | 10.4 (3.2) | 71.0 | 10.4 (3.3) | 0.980 |
| Male sex | 40.8 | 10.4 (3.6) | 59.2 | 10.5 (3.1) | 0.950 |
| ≥ 25 of BMI, kg/m2 | 22.9 | 9.9 (3.2) | 77.1 | 10.6 (3.3) | 0.205 |
| ≥ 7% of HbA1c | 76.3 | 10.2 (3.1) | 23.7 | 11.2 (3.7) | 0.059 |
| Diabetic complications | |||||
| Retinopathy | 25.5 | 9.9 (2.8) | 74.5 | 10.6 (3.4) | 0.217 |
| Nephropathy | 15.2 | 9.8 (3.0) | 84.8 | 10.6 (3.4) | 0.224 |
| Peripheral neuropathy | 22.0 | 10.5 (2.9) | 78.0 | 10.4 (3.4) | 0.894 |
| Coronary artery disease | 4.3 | 8.8 (3.3) | 95.7 | 10.5 (3.3) | 0.128 |
| Cerebrovascular disease | 4.7 | 10.5 (2.9) | 95.3 | 10.4 (3.3) | 0.937 |
| Peripheral arterial disease | 3.8 | 11.3 (3.3) | 96.2 | 9.6 (3.5) | 0.256 |
| IAH | 17.7 | 10.6 (3.1) | 82.3 | 10.4 (3.4) | 0.729 |
| SH | 11.1 | 10.1 (3.2) | 88.9 | 10.4 (3.3) | 0.664 |
| Diabetes treatment | |||||
| CSII | 23.2 | 10.4 (2.9) | 76.8 | 10.5 (3.4) | 0.864 |
% or mean (SD, standard deviation)
*1 p < 0.05 (vs. no)
BMI body mass index, IAH impaired awareness of hypoglycemia, SH severe hypoglycemia, and CSII continuous subcutaneous insulin infusion
Table 3.
Correlation of scan and SMBG frequency per day with interest variables
| Scan frequency | SMBG frequency | |||
|---|---|---|---|---|
| r (95% CI) | p value | r (95% CI) | p value | |
| Age | 0.083 (− 0.053, 0.216) | 0.233 | 0.237 (0.105, 0.361) | < 0.001* |
| Diabetes duration | − 0.036 (− 0.171, 0.992) | 0.600 | 0.051 (− 0.085, 0.185) | 0.461 |
| HbA1c | − 0.150 (− 0.280, -0.016) | 0.029* | 0.031 (− 0.105, 0.165) | 0.655 |
| GA | − 0.169 (− 0.329, 0.001) | 0.051 | 0.061 (− 0.110, 0.228) | 0.486 |
| Insulin treatment | ||||
| Daily total | − 0.104 (− .236, 0.032) | 0.134 | − 0.056 (− 0.190, 0.080) | 0.423 |
| Basal | − 0.087 (− 0.251, 0.083) | 0.316 | − 0.083 (− 0.248, 0.087) | 0.338 |
| Basal, % | − 0.034 (− 0.170, 0.102) | 0.622 | − 0.070 (− 0.204, 0.067) | 0.314 |
| CGM metrics | ||||
| TAR | − 0.156 (− 0.285, − 0.021) | 0.024* | 0.055 (− 0.081, 0.103) | 0.635 |
| TIR | 0.196 (0.062, 0.322) | 0.004* | 0.003 (− 0.132, 0.138) | 0.970 |
| TBR | − 0.018 (− 0.152, 0.118) | 0.799 | 0.055 (− 0.081, 0.189) | 0.427 |
| ADRR | − 0.149 (− 0.279, − 0.015) | 0.030* | − 0.026 (− 0.160, 0.110) | 0.711 |
| %CV | − 0.160 (− 0.289, − 0.026) | 0.020* | − 0.034 (− 0.168, 0.102) | 0.625 |
| MAGE | − 0.214 (− 0.339, − 0.081) | 0.002* | − 0.111 (− 0.243, 0.024) | 0.108 |
| HBGI | − 0.151 (− 0.280, − 0.016) | 0.028* | 0.003 (− 0.133, 0.138) | 0.970 |
| LBGI | − 0.031 (− 0.165, 0.105) | 0.659 | 0.064 (− 0.072, 0.197) | 0.359 |
| SD | − 0.253 (− 0.376, − 0.122) | < 0.001* | − 0.072 (− 0.205, 0.064) | 0.299 |
| MODD | − 0.074 (− 0.209, 0.064) | 0.292 | − 0.045 (− 0.180, 0.093) | 0.526 |
| Psychological | ||||
| PAID | 0.062 (− 0.074, 0.196) | 0.371 | − 0.077 (− 0.210, 0.059) | 0.265 |
| HFS-B | 0.253 (0.122, 0.376) | < 0.001* | − 0.055 (− 0.189, 0.082) | 0.433 |
| HFS-W | − 0.009 (− 0.144, 0.126) | 0.895 | − 0.064 (− 0.197, 0.072) | 0.358 |
Mean (SD, standard deviation) or r (95% CI, confidence interval). * P < 0.05
SMBG self-monitoring of blood glucose, GA glycated albumin, CGM continuous glucose monitoring, TAR time above range, TIR time in range, TBR time below range, ADRR average daily risk range, CV coefficient of variation, MAGE mean amplitude of glycemic excursion; HBGI high blood glucose index, LBGI low blood glucose index, SD standard deviation, MODD mean daily difference for inter-day variation, PAID problem areas in diabetes, HFS-B hypoglycemia fear survey-behavior, and HFS-W hypoglycemia fear survey-worry
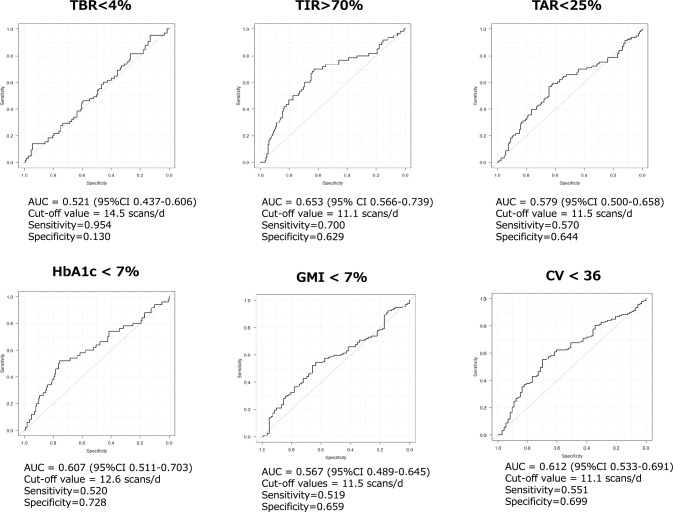
Adults with T1D and good exercise habits had a higher scan frequency than sedentary adults (Table 4). There was no difference in the scan frequency between patients with healthy vs. unhealthy eating habits. The AUC for > 70% TIR was 0.653 with an optimal cutoff of 11 scan/day, although the AUC for < 25% TAR or < 4% TBR was not sufficient for diagnostic accuracy (Fig. 1). For each additional scan per day, the mean GMI decreased 0.03% and TIR increased 0.83%. The SEM model revealed that scan frequency had an indirect influence on GV in adults with T1D via TAR (with a standardized β-coefficient of -0.784, p < 0.001), but not TBR.
Table 4.
Scan frequency according to the eating, exercise, and lifestyle categories
| Variables | Healthy | Unhealthy | P value | ||
|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | ||
| Eating habits | |||||
| Skipping breakfast | 89.0 | 10.5 (3.3) | 11.0 | 10.1 (3.6) | 0.615 |
| Fast eating | 68.1 | 10.6 (3.2) | 31.9 | 10.2 (3.4) | 0.409 |
| Late-night dinner eating | 73.3 | 10.6 (3.3) | 26.7 | 10.1 (3.2) | 0.336 |
| Snack and sweetened beverage | 29.3 | 10.8 (3.0) | 70.7 | 10.3 (3.4) | 0.373 |
| Fruits | 30.0 | 10.9 (3.7) | 70.0 | 10.2 (3.1) | 0.154 |
| Milk | 67.1 | 10.3 (3.3) | 32.9 | 10.7 (3.2) | 0.389 |
| Fish | 11.5 | 10.5 (3.2) | 88.5 | 10.4 (3.3) | 0.884 |
| Vegetable | 3.3 | 12.3 (3.0) | 96.7 | 10.4 (3.3) | 0.132 |
| Exercise habit | |||||
| Exercise | 34.8 | 11.1 (2.7) | 65.2 | 10.1 (3.5) | 0.043* |
| Physical activity | 38.1 | 10.8 (3.0) | 61.9 | 9.9 (3.6) | 0.044* |
| Fast walking | 45.2 | 10.4 (3.4) | 54.8 | 10.4 (3.2) | 0.982 |
| Lifestyle factors | |||||
| Over work | 82.4 | 10.5 (3.2) | 17.6 | 10.1 (3.7) | 0.559 |
| Current smoking | 91.0 | 10.5 (3.3) | 9.0 | 9.3 (3.5) | 0.104 |
| Drinking everyday | 79.0 | 10.6 (3.3) | 21.0 | 9.7 (3.2) | 0.109 |
| Excessive drinking | 87.6 | 10.6 (3.2) | 12.4 | 9.3 (3.5) | 0.638 |
| Non-restorative sleep | 73.2 | 10.5 (3.3) | 26.8 | 10.4 (3.3) | 0.846 |
% or mean (SD, standard deviation)
*p < 0.05
Fig. 1.
A series of ROC analysis demonstrating optimal cutoff values of scan frequency for achieving HbA1c and CGM-derived metrics target. ROC, receiver operating characteristic; CGM, continuous glucose monitoring; TBR, time below range; TIR, time in range; TAR, time above range; GMI, glucose management indicator; CV, coefficient of variation, AUC, area under curve; and CI, confidential interval
Discussion
This is the first study to examine the association between scan frequency and CGM metrics and to identify the optimal cutoff for achieving the glycemic target (> 70% of TIR) in Japanese adults with T1D. Furthermore, scan frequency was significantly associated with the HFS-B score, and exercise habits was associated with an increased the scan frequency.
The average scan frequency (10.5 scan/day) was relatively lower than that described in previous studies (12 in Israel [13], 13 in Spain [14], 14 in Brazil [15], and 16.3 in Europe [17]). In 85 Japanese children and adolescents with T1D, frequent scanning decreased hyperglycemia with increased TIR, but did not reduce TBR [25]. In 163 Denmark people with T1D, increased scanning frequency was associated with a higher TIR with no change in TBR [26]. These results were consistent with our own. In a study by Dunn et al., a scanning frequency of > 20 scans/day was associated with an estimated HbA1c level close to 7.0% [17]. The study by Leelarathna et al. [27] recommended scanning as much as possible, aiming > 15 per day. The ISCHIA study advised frequent scanning (≥ 10 times per day) [28]. Our ROC analysis showed that the optimal cutoff for achieving the glycemic target (> 70% of TIR) was 11 scans per day. SEM demonstrated the indirect correlation between scan frequency and GV. Therefore, in future investigations, a comprehensive examination with a large sample size will be necessary to validate both the direct and indirect effects of scan frequency on GV.
In this study, T1D adults with exercise habits had more frequent scanning. They might scan frequently because of worrying hypoglycemia during the exercise. But the accuracy of the isCGM diminished during the exercise and using isCGM to monitor glucose levels during exercise is not recommended [29]. Moreover, the accuracy decreased when carbohydrate was consumed before exercise. Careful attention should be paid for the use of isCGM during the exercise. Adults with T1D can read a glucose trend arrow and a graph of glucose readings over the preceding 8 h [30]. However, we did not check the degree of consideration trend arrow for T1D management. Recently, the use of isCGM with optional alarms for high and low blood glucose levels decreased HbA1c levels in 156 British adults with T1D [27]. Now, Japanese adults with T1D did not use the isCGM system with optional alarms.
In this study, scan frequency was associated with HFS-B scores but not HFS-W scores. In 61 American youth with T1D, consistent CGM use was associated with treatment adherence and improved glycemic control without an increase in psychosocial distress [31]. Also, isCGM use with the structured education and regular support by healthcare professionals improved quality of life in British 52 children with T1D [32]. The use of isCGM produced a reduction in the frequency and severity of diabetic ketoacidosis (DKA) events in 47 Saudi Arabian T1D people with recurrent DKA [33]. Further examination including a trend arrow, isCGM with optional alarms [34], and longer follow-up are required to confirm these issues.
Limitation of the study
The strengths of this study include real-world data and detailed information on the T1D treatment, lifestyle, and psychological factors. There were several limitations, including the cross-sectional design of this study precluding conclusions concerning causality, and the target population comprised only adult patients with T1D. Generalizability was limited by the target population, i.e., Japanese adults with T1D. COVID-19 lockdown may have affected scan frequency, but the participants were only followed for three months, making it difficult to analyze the impact. Systematic review indicated that glycemic values in people with T1D significantly improved during COVID-19 lockdown, which may be associated with positive changes in self-care and digital diabetes management [35]. Further examinations including COVID-19 lockdown periods are required to confirm these issues. In this study, we did not measure the frequency of insulin administration including correction bolus. In future, it will be necessary to investigate the relationship between the frequency of insulin administration, especially correction by insulin regimen, and various indicators.
Conclusions
In real-world conditions, frequent scans are linked to improved CGM metrics, including increased TIR, reduced TAR, and glycemic variability indicators. Exercise habits and hypoglycemia fear-related behavior might affect scan frequency. This information will help diabetes health professionals support adults with T1D and isCGM.
Acknowledgements
The authors are grateful to RN Yukiko Tsuchida (Tokyo Women’s Medical University).
Funding
The FGM-Japan study was completed with funding from the Japan Agency for Medical Research and Development (AMED), Japan (Grant number: 18ek0210104h0001, 19ek0210104h0002, 20ek0210104h0003).
Declarations
Conflict of interest
YH received lecture fees from Eli Lilly, Sanofi, Abbott, Terumo and Sumitomo Pharma, and research expenses and grants from Sumitomo Pharma. JM received lecture fees from Terumo. MT received lecture fees from Abbott, Terumo and Sumitomo Pharma, Novo Nordisk, and Boehringer Ingelheim and subsides or donations from Cocokara fine Healthcare Inc., LifeScan Japan, Roche DC Japan, and SUPER LIGHT WATER CO., LTD. AT receives lecture fees from Eli Lilly, Sanofi, Abbott, and Terumo.TM received lecture fees from Eli Lilly. Other members declare no competing interests.
Ethical Approval
This study conformed to the standards of the Declaration of Helsinki. The present study was approved by the Ethics Committee of the National Hospital Organization Kyoto Medical Center (No.19–072, approval date: January 20, 2020).
Informed consent
Informed consent or substitute for it was obtained from all patients for being included in the study. Approval date of Registry and the Registration No. of the study/trial: Trial registration number: University hospital Medical Information Network (UMIN) Center: UMIN000039376).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vanderniet JA, Jenkins AJ, Donaghue KC. Epidemiology of type 1 diabetes. Curr Cardiol Rep. 2022;24(10):1455–1465. doi: 10.1007/s11886-022-01762-w. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia. 2006;49(5):828–836. doi: 10.1007/s00125-006-0213-8. [DOI] [PubMed] [Google Scholar]
- 3.Bidonde J, Fagerlund BC, Frønsdal KB, Lund UH, Robberstad B. FreeStyle Libre Flash Glucose Self-Monitoring System: A Single-Technology Assessment [Internet]. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2017 Aug 21. Report from the Norwegian Institute of Public Health No. 2017–07. [PubMed]
- 4.Leelarathna L, Wilmot EG. Flash forward: a review of flash glucose monitoring. Diabet Med. 2018;35(4):472–482. doi: 10.1111/dme.13584. [DOI] [PubMed] [Google Scholar]
- 5.Lin R, Brown F, James S, Jones J, Ekinci E. Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabet Med. 2021;38(5):e14528. doi: 10.1111/dme.14528. [DOI] [PubMed] [Google Scholar]
- 6.Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther. 2019;10(3):853–863. doi: 10.1007/s13300-019-0619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson AL, Criego AB, Martens TW, Bergenstal RM. HbA1c: the glucose management indicator, time in range, and standardization of continuous glucose monitoring reports in clinical practice. Endocrinol Metab Clin North Am. 2020;49(1):95–107. doi: 10.1016/j.ecl.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Dovc K, Battelino T. Time in range centered diabetes care. Clin Pediatr Endocrinol. 2021;30(1):1–10. doi: 10.1297/cpe.30.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slattery D, Choudhary P. Clinical use of continuous glucose monitoring in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(S2):S55–S61. doi: 10.1089/dia.2017.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomized controlled trial. Lancet. 2016;388(10057):2254–2263. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 11.Al-Harbi MY, Albunyan A, Alnahari A, et al. Frequency of flash glucose monitoring and glucose metrics: real-world observational data from Saudi Arabia. Diabetol Metab Syndr. 2022;14(1):66. doi: 10.1186/s13098-022-00831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lameijer A, Lommerde N, Dunn TC, et al. Flash Glucose Monitoring in the Netherlands: Increased monitoring frequency is associated with improvement of glycemic parameters. Diabetes Res Clin Pract. 2021;177:108897. doi: 10.1016/j.diabres.2021.108897. [DOI] [PubMed] [Google Scholar]
- 13.Eldor R, Roitman E, Merzon E, Toledano Y, Alves C, Tsur A. Flash glucose monitoring in israel: understanding real-world associations between self-monitoring frequency and metrics of glycemic control. Endocr Pract. 2022;28(5):472–478. doi: 10.1016/j.eprac.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Peralta F, Dunn T, Landuyt K, Xu Y, Merino-Torres JF. Flash glucose monitoring reduces glycemic variability and hypoglycemia: real-world data from Spain. BMJ Open Diabetes Res Care. 2020;8(1):e001052. doi: 10.1136/bmjdrc-2019-001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calliari LEP, Krakauer M, Vianna AGD, et al. Real-world flash glucose monitoring in Brazil: can sensors make a difference in diabetes management in developing countries? Diabetol Metab Syndr. 2020;7(12):3. doi: 10.1186/s13098-019-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohendorff J, Gumprecht J, Mysliwiec M, Zozulinska-Ziolkiewicz D, Malecki MT. intermittently scanned continuous glucose monitoring data of polish patients from real-life conditions: more scanning and better glycemic control compared to worldwide data. Diabetes Technol Ther. 2021;23(8):577–585. doi: 10.1089/dia.2021.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. 2018;137:37–46. doi: 10.1016/j.diabres.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Mazza E, Ferro Y, Pujia R, Mare R, Maurotti S, Montalcini T, Pujia A. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25(9):1076–1083. doi: 10.1007/s12603-021-1675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zareiyan A. (2017) Healthy or Unhealthy Lifestyle: A Thematic Analysis of Iranian Male Adolescents' Perspectives. Iran J Nurs Midwifery Res 22(1):1–7. [DOI] [PMC free article] [PubMed]
- 20.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7(2):253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 22.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19(4):203–211. [PMC free article] [PubMed] [Google Scholar]
- 25.Urakami T, Yoshida K, Kuwabara R, Mine Y, Aoki M, Suzuki J, Morioka I. Significance of “time below range” as a glycemic marker derived from continuous glucose monitoring in japanese children and adolescents with type 1 diabetes. Horm Res Paediatr. 2020;93(4):251–257. doi: 10.1159/000510454. [DOI] [PubMed] [Google Scholar]
- 26.Hansen KW, Bibby BM. The frequency of intermittently scanned glucose and diurnal variation of glycemic metrics. J Diabetes Sci Technol. 2022;16(6):1461–1465. doi: 10.1177/19322968211019382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leelarathna L, Evans ML, Neupane S, et al. Intermittently scanned continuous glucose monitoring for type 1 diabetes. N Engl J Med. 2022;387(16):1477–1487. doi: 10.1056/NEJMoa2205650. [DOI] [PubMed] [Google Scholar]
- 28.Murata T, Sakane N, Kuroda A, et al. Effect of intermittent-scanning CGM to glycaemic control including hypoglycaemia and quality of life of patients with type 1 diabetes (ISCHIA study) Diabetologia. 2021;64(Suppl 1):S123. [Google Scholar]
- 29.Clavel P, Tiollier E, Leduc C, Fabre M, Lacome M, Buchheit M. Concurrent validity of a continuous glucose-monitoring system at rest and during and following a high-intensity interval training session. Int J Sports Physiol Perform. 2022;17(4):627–633. doi: 10.1123/ijspp.2021-0222. [DOI] [PubMed] [Google Scholar]
- 30.Kudva YC, Ahmann AJ, Bergenstal RM, et al. Approach to using trend arrows in the freestyle libre flash glucose monitoring systems in adults. J Endocr Soc. 2018;2(12):1320–1337. doi: 10.1210/js.2018-00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giani E, Snelgrove R, Volkening LK, Laffel LM. Continuous glucose monitoring (CGM) adherence in youth with type 1 diabetes: associations with biomedical and psychosocial variables. J Diabetes Sci Technol. 2017;11(3):476–483. doi: 10.1177/1932296816676280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pintus D, Ng SM. Freestyle libre flash glucose monitoring improves patient quality of life measures in children with Type 1 diabetes mellitus (T1DM) with appropriate provision of education and support by healthcare professionals. Diabetes Metab Syndr. 2019;13(5):2923–2926. [DOI] [PubMed]
- 33.Al Hayek AA, Al Dawish MA. Frequency of diabetic ketoacidosis in patients with type 1 diabetes using freestyle libre: a retrospective chart review. Adv Ther. 2021;38(6):3314–3324. doi: 10.1007/s12325-021-01765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller E, Midyett LK. Just Because You Can, Doesn't Mean You Should … Now. A Practical Approach to Counseling Persons with Diabetes on Use of Optional CGM Alarms. Diabetes Technol Ther. 2021;23(S3):S66-S71. [DOI] [PubMed]
- 35.Eberle C, Stichling S. Impact of COVID-19 lockdown on glycemic control in patients with type 1 and type 2 diabetes mellitus: a systematic review. Diabetol Metab Syndr. 2021;13(1):95. doi: 10.1186/s13098-021-00705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]