Abstract
Blood pressure variability (BPV) and heart rate variability (HRV) have been associated with Alzheimer’s Disease and Related Dementias (ADRD) in rigorously controlled studies. However, the extent to which BPV and HRV may offer predictive information in real-world, routine clinical care is unclear. In a retrospective cohort study of 48,204 adults (age 54.9 ± 17.5 years, 60% female) receiving continuous care at a single center, we derived BPV and HRV from routinely collected clinical data. We use multivariable Cox models to evaluate the association of BPV and HRV, separately and in combination, with incident ADRD. Over a median 3 [2.4, 3.0] years, there were 443 cases of new-onset ADRD. We found that clinically derived measures of BPV, but not HRV, were consistently associated with incident ADRD. In combined analyses, only patients in both the highest quartile of BPV and lowest quartile of HRV had increased ADRD risk (HR 2.34, 95% CI 1.44–3.81). These results indicate that clinically derived BPV, rather than HRV, offers a consistent and readily available metric for ADRD risk assessment in a real-world patient care setting. Thus, implementation of BPV as a widely accessible tool could allow clinical providers to efficiently identify patients most likely to benefit from comprehensive ADRD screening.
Subject terms: Cardiology, Medical research
Introduction
Cardiovascular measures of autonomic dysfunction have been associated with Alzheimer’s Disease and Related Dementias (ADRD) in rigorously controlled studies and proposed as a method for identifying patients at risk for or experiencing subclinical cognitive dysfunction 1–4. Blood pressure variability (BPV) represents the most well studied of these factors, with higher BPV associated with incident ADRD in numerous prospective cohort studies 5–7. Importantly, obtaining BPV data requires repeated blood pressure measurements, with most studies spanning years. Further, blood pressure values used to determine BPV in these studies were captured using high-fidelity protocols that are not often used in clinical practice 8–11. Therefore, while promising, it remains unclear if BPV derived from clinically generated blood pressure data may offer similar or even any valuable information with respect to ADRD risk stratification in a real-world clinical care setting.
Heart rate variability (HRV) represents an alternative measure of autonomic tone that has been associated with cognitive dysfunction and which can be obtained over a much shorter period of time 12,13. Analysis of EKGs from the Multi-Ethnic Study of Atherosclerosis (MESA) Study found an association between higher HRV and better cognitive performance across multiple domains 14. Compared with BP assessment, EKG ascertainment is less susceptible to measurement error, potentially improving the ability of this modality to be utilized in clinical practice. Conversely, EKGs are not performed as frequently as blood pressure measurements, potentially limiting clinical utility.
There exists an urgent need to develop efficient and cost-effective approaches to identifying patients at high risk for developing ADRD, particularly earlier in the course of disease progression 15,16, given ongoing aging of the population 17,18 and amid concerns that existing approaches to disease screening are considered relatively inaccurate and burdensome 19–21. The rapid uptake of electronic health records and digital EKG capture has created an abundance of longitudinal blood pressure and heart rate measures that are typically collected and stored for individual patients as they age through their care within a healthcare system. As such, BPV and HRV represent potentially readily accessible clinical tools for identifying and prioritizing patients who could benefit from more comprehensive ADRD screening protocols, however, given the recognized limitations of each modality, which method maintains optimal predictive capacity in a real-world clinical setting remains unknown. To address this knowledge gap, we evaluated the association of BPV and HRV with subsequent diagnosis of ADRD, using clinical data longitudinally collected from a diverse cohort of patients cared for in a large urban multi-site health system.
Results
A total of 631,216 patients had at least 1 outpatient encounter at which a BP was recorded during the clinical assessment period, of whom 51,147 had an outpatient visit every calendar year from 2013 through 2016. Following exclusion of 2270 individuals under age 18, 335 with a history of ADRD, 334 who died during the final year of the clinical assessment period, and 4 with non-physiologic BP measurements, there was a total of 48,204 patients receiving consistent care during the clinical assessment period. Of these 7270 (15.1%) had at least 1 qualifying EKG (Supplemental Fig. 1). The median follow-up time for both the full and EKG cohorts was 3.0 [2.4, 3.0] years, during which time 443 new dementia cases occurred.
The average age of the BP cohort was 54.9 ± 17.5 years, of whom 29,011 (60.2%) were female. The most common comorbid condition was diabetes mellitus (11.9%) followed by coronary artery disease (11.3%), heart failure (8.6%), and kidney disease (7.4%). On average, patients had 15.4 ± 13.9 blood pressure measurements during the clinical assessment period, with a mean SBP of 124.0 ± 12.1 mmHg and DBP of 73.8 ± 7.2 mmHg, with 13,539 (28.1%) prescribed at least 1 antihypertensive medication. By comparison, the average age of the EKG Cohort was 68.1 ± 15.8 years of age, with 4015 (55.2%) females. Coronary artery disease was the most common comorbid condition (27.0%), followed by heart failure (23.5%), diabetes mellitus (19.4%), and atrial fibrillation/flutter (17.3%). Participants had on average 23.9 ± 18.4 BP measurements, with a mean SBP of 127.0 ± 11.8 mmHg and DBP of 73.0 ± 7.4 mmHg, with 3351 (46.1%) prescribed at least 1 antihypertensive medication. The median number of qualifying EKGs was 2.0 [1.0, 3.0], with an average heart rate of 73.9 ± 24.2 beats per minute (Table 1). By comparison, the BP and EKG cohorts were generally older, more frequently non-Hispanic White, and had more BP recordings in the EHR than patients not meeting inclusion criteria (Table 1). Frequency of BP measurements per patient can be found in Supplemental Table 1.
Table 1.
Baseline characteristics of blood pressure and EKG cohorts, as well as all ambulatory care patients prior to eligibility screening.
| Characteristic | All ambulatory care patients (n = 631,216) | BP cohort (n = 48,204) | EKG cohort (n = 7270) |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years, mean (SD) | 46.26 (20.65) | 54.87 (17.50) | 68.11 (15.77) |
| Age, ≥ 65 years, n (%) | 140,988 (22.3) | 15,397 (31.9) | 4517 (62.1) |
| Female, n (%) | 358,869 (56.9) | 29,011 (60.2) | 4015 (55.2) |
| Race/ethnicity, n (%) | |||
| Asian | 51,134 (8.1) | 4437 (9.2) | 483 (6.6) |
| Hispanic/Latinx | 75,291 (11.9) | 5322 (11.0) | 691 (9.5) |
| Non-Hispanic Black | 59,163 (9.4) | 6385 (13.2) | 1111 (15.3) |
| Non-Hispanic White | 357,461 (56.6) | 29,483 (61.2) | 4735 (65.1) |
| Othera | 28,359 (4.5) | 1423 (3.0) | 214 (2.9) |
| Smoking status, n (%) | |||
| Current | 39,683 (6.3) | 2266 (4.7) | 320 (4.4) |
| Former | 119,872 (19.0) | 14,011 (29.1) | 2832 (39.0) |
| Never | 410,407 (65.0) | 31,927 (66.2) | 4118 (56.6) |
| Clinical characteristics | |||
| Follow-up length, mean (SD), years | – | 2.45 (0.97) | 2.37 (1.03) |
| Follow-up length, median [IQR], years | – | 3.00 [2.36, 3.00] | 3.0 (2.0, 3.0) |
| Number of blood pressures recorded during study period, mean (SD) | 6.88 (11.17) | 15.39 (13.86) | 23.86 (18.38) |
| Number of blood pressures recorded during study period, median [IQR] | 3.00 [1.00, 8.00] | 12.00 [8.00, 18.00] | 19.0 [12.0, 30.0] |
| Diabetes mellitus, n (%) | – | 5721 (11.9) | 1409 (19.4) |
| Renal disease, n (%) | – | 3559 (7.4) | 1250 (17.2) |
| Atrial fibrillation or atrial flutter, n (%) | – | 3376 (7.0) | 1261 (17.3) |
| Coronary artery disease, n (%) | – | 5441 (11.3) | 1960 (27.0) |
| Metastatic malignancy, n (%) | – | 872 (1.8) | 318 (4.4) |
| Myocardial Infarction, n (%) | – | 1807 (3.7) | 814 (11.2) |
| Heart failure, n (%) | – | 4122 (8.6) | 1709 (23.5) |
| Stroke, n (%) | – | 2543 (5.3) | 851 (11.7) |
| Blood pressure characteristics | |||
| Antihypertensive use, n (%) | – | 13,539 (28.1) | 3351 (46.1) |
| Mean systolic blood pressure, mean (SD), mmHg | 122.69 (15.16) | 123.96 (12.09) | 126.99 (11.78) |
| Mean systolic blood pressure, median [IQR], mmHg | 121.67 [112.00, 132.00] | 123.43 [115.30, 131.80] | 127.0 [119.0, 134.0] |
| Mean diastolic blood pressure, mean (SD), mmHg | 74.03 (9.36) | 73.77 (7.18) | 72.97 (7.43) |
| Mean diastolic blood pressure, median [IQR], mmHg | 74.00 [68.00, 80.00] | 73.46 [68.81, 78.40] | 73.0 [68.0, 78.0] |
| Heart rate characteristics | |||
| Number of EKGs, mean (SD) | – | – | 2.74 (3.59) |
| Number of EKGs, median [IQR] | – | – | 2.0 [1.0, 3.0] |
| Heart rate, mean (SD) | – | – | 73.91 (24.18) |
| Heart rate, median [IQR] | – | – | 70.0 [62.0, 81.0] |
| Number of QRS complexes, mean (SD) | – | – | 10.76 (2.7) |
| Number of QRS complexes, median [IQR] | – | – | 10 [9, 12] |
aOther race includes American Indian/Alaska Native, Native Hawaiian or other Pacific Islander, and Other.
SD standard deviation, IQR interquartile range.
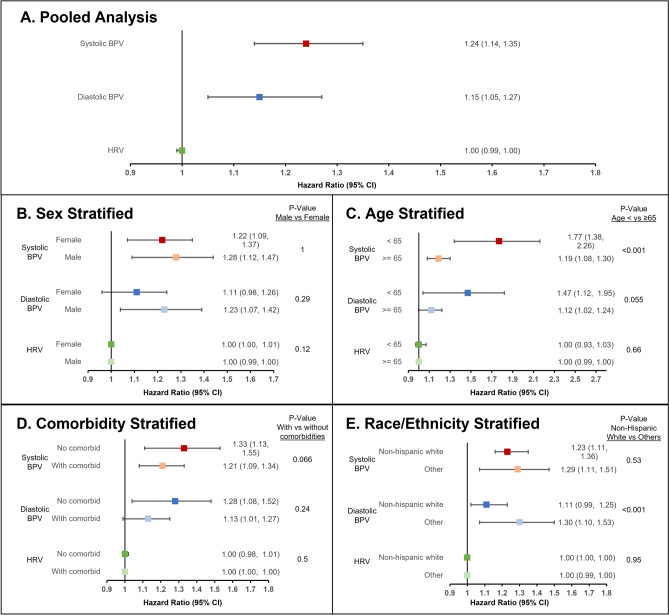
In multivariable Cox proportional hazards models, the risk of incident ADRD increased as BPV increased for both systolic (Hazard Ratio 1.24, 95% CI 1.14–1.35) and diastolic (1.15, 1.05–1.27) VIM in the BP cohort, while no association between ADRD and HRV was appreciated in the EKG cohort (1.00, 0.99–1.00) (Fig. 1A). Increasing age, more clinic visits, diabetes mellitus, prior myocardial infarction, and heart failure were all associated with increased risk of ADRD (Supplemental Table 2). Of note, mean systolic and diastolic BP were not associated with ADRD. Results were similar when BPV and HRV were included in the same model using the EKG cohort (Supplemental Table 3). Similar findings were noted following stratification by age and sex without significant risk difference between sexes or age strata (Fig. 1B,C). Similarly, BPV, particularly systolic VIM, remained significantly associated with incident ADRD following stratification by number of comorbid conditions, and race (Fig. 1D,E). There were generally not significant differences between groups, except for systolic BPV following age stratification (P-value for interaction < 0.001) and diastolic BPV following race stratification (P-value for interaction < 0.001). The c-statistics for the primary models can be found in Supplemental Table 4. Survival analysis demonstrated a significantly lower disease-free survival among patients with a systolic VIM above the median for the BP cohort (Supplemental Fig. 2). In secondary analyses, we repeated the BPV models using only the smaller EKG cohort with similar to slightly higher hazard ratios for both systolic (1.37, 1.16–1.61) and diastolic BPV (1.15, 1.05–1.27). Similar findings were again noted following stratification by age and sex, except for individuals < 65 years, for whom there were too few events for analysis (7 ADRD cases out of 2753, 0.25%) (Table 2).
Figure 1.
Association of blood pressure and heart rate variability with incident Alzheimer’s Disease and Related Dementias in the EKG cohort. Incident dementia risk associated with systolic BPV, diastolic BPV, and HRV (A) in the pooled cohort, and in stratified analyses by (B) sex, (C) age, (D) number of comorbidities (0 vs. ≥ 1), and (E) race/ethnicity (Non-Hispanic White vs. Others). Cox models adjusted for age, sex, race/ethnicity, number of visits, use of antihypertensive medications, smoking status, diabetes mellitus, chronic kidney disease, atrial fibrillation/atrial flutter, coronary artery disease, mean systolic and diastolic blood pressure, presence of any metastatic malignancy, myocardial infarction, heart failure, stroke, number of EKGs, and heart rate. Patients censored at last follow up visit or death prior to the end of the study period, whichever occurred later. Analyses exclude patients of unknown race due to model convergence. Abbreviations: BPV blood pressure variability, CI confidence interval, HRV heart rate variability.
Table 2.
Association of combined systolic blood pressure and heart rate variability with incident Alzheimer’s Disease and Related Dementias in the EKG cohort, overall and stratified by sex and age.
| Outcome | Overall (n = 7270) | Sex stratified | Age stratified | ||||
|---|---|---|---|---|---|---|---|
| Female (n = 4015) | Male (n = 3255) | p-valueb | Age ≥ 65 (n = 4517) | Age < 65 (n = 2753)c | |||
| Crude HR (95% CI) | Adjusted HR (95% CI)a | Adjusted HR (95% CI)a | Adjusted HR (95% CI)a | Adjusted HR (95% CI)a | Adjusted HR (95% CI)a | ||
| Systolic VIM | 1.56 (1.36, 1.79) | 1.37 (1.16, 1.61) | 1.35 (1.08, 1.70) | 1.41 (1.11, 1.79) | 0.84 | 1.38 (1.16, 1.63) | – |
| Diastolic VIM | 1.26 (1.09, 1.47) | 1.18 (0.99, 1.40) | 1.09 (0.85, 1.38) | 1.34 (1.05, 1.71) | 0.14 | 1.18 (0.99, 1.41) | – |
CI confidence interval, HR hazard ratio, VIM variation independent of the mean.
aCox models adjusted for age, sex, race/ethnicity, number of visits, use of antihypertensive medications, smoking status, diabetes mellitus, chronic kidney disease, atrial fibrillation/atrial flutter, coronary artery disease, mean systolic and diastolic blood pressure, presence of any metastatic malignancy, myocardial infarction, heart failure, stroke, number of EKGs, and heart rate. Patients censored at last follow up visit or death prior to the end of the study period, whichever occurred later. Analyses exclude patients of unknown race due to model convergence.
bP-values for sex interaction, i.e. difference in adjusted HRs between males and females.
cToo few events (n = 7) for modeling.
Significant values are in bold.
Following categorization based on quartile of systolic BPV and HRV, a total of 4165 (57.3%) patients were categorized as low BPV/high HRV (reference group with presumed lowest risk hemodynamic profile), 1287 (17.7%) as low BPV/low HRV, 1285 (17.7%) as high BPV/high HRV, and 533 (7.3%) as high BPV/low HRV (presumed highest risk hemodynamic profile). In multivariable adjusted Cox models, an association between BPV/HRV category during the clinical assessment period and incident ADRD during the outcome surveillance period was only appreciated among those with high BPV and low HRV (2.34, 1.44–3.81) (Table 3). Similar findings were appreciated when quartiles of BPV and HRV were examined independently (Supplemental Table 5A,B). In sex stratified analyses, the high BPV/low HRV category was associated with future ADRD among males (3.21, 1.60–6.46), but not females (1.61, 0.80–3.23), though the risk difference between sexes did not reach statistical significance (P-value for sex interaction 0.18).
Table 3.
Association of combined systolic blood pressure and heart rate variability with incident Alzheimer’s Disease and Related Dementias in the EKG cohort using all available EKGs, overall and stratified by sex.
| Outcome | Overall (n = 7270) | Sex stratified | p-valueb | ||
|---|---|---|---|---|---|
| Female (n = 4015) | Male (n = 3255) | ||||
| Crude HR (95% CI) | Adjusted HR (95% CI)a | Adjusted HR (95% CI)a | Adjusted HR (95% CI)a | ||
| Systolic VIM + HRV | |||||
| Low VIM, high HRV (n = 4165) | Ref | Ref | Ref | Ref | – |
| Low VIM, low HRV (n = 1287) | 0.95 (0.58, 1.56) | 0.98 (0.59, 1.63) | 0.65 (0.29, 1.50) | 1.34 (0.69, 2.62) | 0.188 |
| High VIM, high HRV (n = 1285) | 1.94 (1.31, 2.86) | 1.32 (0.88, 1.97) | 1.17 (0.68, 2.03) | 1.58 (0.86, 2.91) | 0.482 |
| High VIM, low HRV (n = 533) | 3.03 (1.91, 4.82) | 2.34 (1.44, 3.81) | 1.61 (0.80, 3.23) | 3.21 (1.60, 6.46) | 0.176 |
| Diastolic VIM + HRV | |||||
| Low VIM, high HRV (n = 4155) | Ref | Ref | Ref | Ref | – |
| Low VIM, low HRV (n = 1297) | 1.53 (1.03, 2.28) | 1.54 (1.01, 2.33) | 1.02 (0.53, 1.96) | 2.09 (1.19, 3.68) | 0.103 |
| High VIM, high HRV (n = 1295) | 1.39 (0.92, 2.09) | 1.22 (0.80, 1.86) | 1.00 (0.56, 1.76) | 1.59 (0.84, 3.00) | 0.288 |
| High VIM, low HRV (n = 523) | 0.93 (0.45, 1.93) | 0.99 (0.47, 2.09) | 0.91 (0.35, 2.38) | 0.94 (0.28, 3.12) | 0.979 |
BPV blood pressure variability, CI confidence interval, HR hazard ratio, HRV heart rate variability, VIM variation independent of the mean.
aCox models adjusted for age, sex, race/ethnicity, number of visits, use of antihypertensive medications, smoking status, diabetes mellitus, chronic kidney disease, atrial fibrillation/atrial flutter, coronary artery disease, mean systolic and diastolic blood pressure, presence of any metastatic malignancy, number of EKGs, myocardial infarction, heart failure, stroke, and heart rate. Patients censored at last follow up visit or death prior to the end of the study period, whichever occurred later. Analyses exclude patients of unknown race due to model convergence.
bP-values for sex interaction, i.e. difference in adjusted HRs between males and females.
Significant values are in bold.
In further secondary analyses, we repeated all testing with the population narrowed only to individuals age ≥ 65 years at the time of first qualifying visit with qualifying EKGs (n = 4517). As was appreciated in the cohort with all adults, an association between BPV/HRV category during the clinical assessment period and incident ADRD during the outcome surveillance period was only appreciated among those with high systolic BPV and low HRV (2.27, 1.38–3.74) (Supplemental Table 6). As above, there were insufficient events among those age < 65 years with an EKG for analysis.
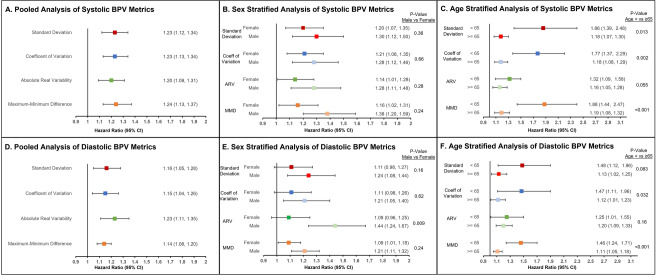
In sensitivity analyses, we examined the association of ADRD using alternative EHR derived BPV measures. All four evaluated metrics of systolic BPV demonstrated a similar significantly positive association with incident ADRD (SD 1.23, 1.12–1.34; CoV 1.23, 1.13–1.34; AVR 1.20, 1.09–1.31; MMD 1.24, 1.13–1.37) (Fig. 2A). These associations persisted following stratification by sex (Fig. 2B) and age (Fig. 2C). While no sex differences were appreciated (P-values for interaction all > 0.05), systolic BPV demonstrated a larger association with incident ADRD among those < 65 years of age when assessed by SD (P-value for age strata interaction 0.013), CoV (P-value for age strata interaction 0.002), and MMD (P-value for age strata interaction < 0.001), though not ARV (P-value for age strata interaction 0.06). Similar, though slightly lower, risk associations were appreciated with ADRD and all metrics of diastolic BPV (SD 1.16, 1.05–1.28, CoV 1.15, 1.04–1.26; AVR 1.23, 1.11–1.35; MMD 1.14, 1.08–1.20) (Fig. 2D), including following sex (Fig. 2E) and age (Fig. 2F) stratification.
Figure 2.
Association of multiple blood pressure variability metrics with incident Alzheimer’s Disease and Related Dementias. Incident dementia risk associated with systolic measures of BPV (A) in the pooled cohort, (B) stratified by sex, and (C) stratified by age, as well as diastolic measures of BPV with the same stratification (D–F). Cox models adjusted for age, sex, race/ethnicity, number of visits, use of antihypertensive medications, smoking status, diabetes mellitus, chronic kidney disease, atrial fibrillation/atrial flutter, coronary artery disease, mean systolic and diastolic blood pressure, presence of any metastatic malignancy, myocardial infarction, heart failure, stroke, number of EKGs, and heart rate. Patients censored at last follow up visit or death prior to the end of the study period, whichever occurred later. Analyses exclude patients of unknown race due to model convergence. Abbreviations: ARV absolute real variability, CI confidence interval, MMD maximum–minimum difference, SD standard deviation.
In sensitivity analyzes using HRV derived from all qualifying EKGs, we found similar results, without a significant association between HRV and incident ADRD alone (Supplemental Table 7) or in combination with systolic BPV (Supplemental Table 8).
Discussion
In this analysis of nearly 50,000 patients receiving continuous care, we found that increased BPV derived from real-world, clinically generated data was associated with incident ADRD over the ensuing 3 years. Notwithstanding the variability of clinical data routinely collected in patient care settings, BPV was effective in identifying patients are increased risk of developing ADRD in the intermediate term. Further, the association between clinically derived BPV and incident ADRD was robust to BPV metric and held across sex and age strata. By comparison, HRV in the clinical setting was not informative on its own and did not substantively add information on top of BPV. Taken together, these results indicate that clinically derived BPV, without need for considering HRV, may represent a potentially clinically useful marker of ADRD risk for a broad patient population.
Numerous mechanistic underpinnings have been proposed as driving the association between BPV, HRV, and ADRD 22,23. One set of hypotheses focuses on the potential for neurovascular damage imposed by both BPV and HRV which may result in neurocognitive dysfunction. Specifically, reduced HRV and increased BPV may result in reduced cerebral perfusion, microvascular damage, and white matter lesions 6,24–27. Alternatively, others postulate an alternative directionality linking dementia and BPV and HRV. Specifically, ADRD often results in autonomic dysfunction, particularly reduced parasympathetic and increased sympathetic tone, with patients suffering from orthostasis, dry mouth, and constipation 28. This school of thought hypothesizes that this abnormal and variable autonomic tone results in high BPV and low HRV, rather than BPV and HRV resulting in neurovascular damage and subsequent cognitive dysfunction 7,29–32. In this theory, high BPV and low HRV may serve rather as autonomic nervous system markers for subclinical cognitive impairment which becomes clinically recognized as the disease state progresses.
The rapidly expanding incidence and prevalence of ADRD demands novel, tailored approaches to population level screening. Age is well recognized as the single largest risk factor for the development of ADRD 33, however, as the number of individuals over the age of 65 grows, expected to reach 80 million by 2040, the healthcare system is under-resourced to enable global ADRD screening 34. Thus, targeted screening approaches and tools re needed to facilitate early diagnosis and, in turn, benefit patients, caregivers, and society alike. For patients, early diagnosis may allow for recognition of potentially reversible causes of cognitive impairment or treatment with disease modifying therapies. Additionally, early diagnosis allows patients to participate in clinical trials for novel treatment approaches and provides ample time for patient education and goal-setting discussions. For caregivers, early diagnosis affords opportunities for caregiver education, care planning, and access to community-based resources. At a societal level, early identification and treatment may help to limit downstream costs of care and identify patients eligible for future clinical studies 35,36. Overcoming the resource-demand mismatch in screening for ADRD necessitates a readily available marker of increased risk, allowing providers to direct screening efforts to those most likely to benefit, akin to AAA screening only among those at highest risk (males, over age 65, with a smoking history) 37. Based on our results, BPV represents an accessible clinical measure that could well fill this gap.
At the outset, we examined both BPV and HRV in association with ADRD risk in the clinical setting based on their ability to represent pathophysiologically related vascular as well as autonomic dysfunction and the abundant prior evidence linking these measures to ADRD outcomes. Importantly, previous reports of BPV and HRV associations with ADRD were primarily derived from clinical trial and cohort studies in which these metrics were assessed and recorded under ideal research conditions 1–3. It is well recognized that standardized methods used in clinical trials are rarely adhered to in clinical practice, potentially limiting the signal to noise ratio of an association with ADRD in clinically generated data 9–11,38–41. Further, no study of which we are aware has attempted to maximize on BPV and HRV as dual measures of autonomic function in ADRD risk assessment.
Our results demonstrate the use of clinically generated data does indeed provide sufficient signal to noise to assess ADRD risk. In particular, we found that individuals in both the highest quartile of BPV and lowest quartile of HRV were at twice the risk of developing future ADRD compared with those in the lowest and highest BPV and HRV quartiles, respectively. Importantly, when examined separately, BPV alone provided a similar ADRD risk estimate as combined BPV/HRV measures. In fact, use of HRV from single or pooled EKGs did not demonstrate predictive capacity for intermediate term incident ADRD risk. There are several potential explanations for our findings, including the possibility that higher BPV reflects vascular dysregulation that contributes mechanistically to brain atrophy and dysfunction 42–46 whereas HRV is a downstream manifestation of dysautonomia that develops after ADRD has already advanced 28,42,47–49. Another factor possibly contributing to lack of an HRV association may have been reduced sample size, with an 85% absolute reduction in the number of patients with eligible BP measurements compared to those with eligible EKGs. From a pragmatic perspective, any requirement for EKG data would also limit scalability for developing useful screening tools. Thus, our results indicating that a simplified and more accessible method of ADRD risk screening based on BPV alone is promising for broad implementation in the clinical environment. Notably, the association between BPV was consistent across race and sex strata, without significant between-group differences observed. Importantly, results were less robust among individuals < 65 years of age, largely related to the relatively small number of events in this cohort. While future larger studies may clarify the association between clinically derived BPV and ADRD among younger patients, our results emphasis the strength of this association predominately among those 65 and older. These findings further underscore the potential of using clinically generated BPV data to risk stratify patients across large patient populations. Given increasing evidence emphasizing the role of mid-life compared to later-life BP levels, along with the possibility of a U-shaped relationship between mean BP and ADRD risk, our findings also contribute to the growing literature on the importance as well as complexity of variations in BP measures in relation to longer-term outcomes. In this context, our study results highlight the need to further understand the dynamic combinations of risk traits predisposing to ADRD over the life course—as part of efforts to advance precision medicine approaches to disease screening and management. Our results overall also suggest that additional, less computationally complex measures may be promising for further development of screening approaches.
There are several limitations of our study that merit consideration. First, this was a retrospective analysis of patients receiving continuous care at a single center. Thus, the extent to which our results may represent causal relationships or are generalizable to other populations remains unclear and warrants further investigation. Importantly, given that we focused our study on patients receiving continuous care, the implications of our findings may be limited across the continuum of patients with less regular access to or utilization of healthcare services. While causation cannot be extrapolated from the results, this was also not the purpose of the study. Specifically, given the clear need for a tailored approach to ADRD screening, we sought to evaluate 2 clinical markers, together and independently, for their association with future dementia diagnoses. The causative nature and directionality of the relationship between BPV and HRV with ADRD remains to be fully explored, with intriguing and pathologically plausible explanations in both directions. We look forward to future work that further delineates the mechanisms underlying this association, while recognizing the immediate potential use of the finding for risk stratification. Concurrently, while both BPV and HRV are recognized as markers of autonomic tone, the degree to which long-term BPV and short-term HRV assess the same underlying biologic mechanisms remains unclear. Additionally, we cannot account for external factors such as patient positioning, recent exertion, or incorrect BP cuff size which may contribute to BPV and HRV. These factors exist as part of routine clinical practice and the aim of this study was specifically evaluate if the association between BPV, HRV, and ADRD demonstrated in controlled clinical trials is maintained even when ‘noise’ from these external variables is present. Finally, we relied on administrative data for the identification of both comorbid conditions and ADRD outcomes. Fortunately, these codes have been validated previously 50,51; further, the ADRD is more frequently under coded, which would balance our findings to the null 52.
Our findings indicate that clinically generated and readily available BPV data, without the need for additional HRV data, represents a novel metric by which to assess individual patient-level risk of future ADRD. This widely accessible and feasible use of EHR data offers a potential mechanism by which providers may efficiently identify patients most likely to benefit from comprehensive ADRD screening, the need for which will continue to burgeon with continued aging of the population.
Methods
Cohort development
Using data from the electronic health record (EHR), we identified patients receiving consistent ambulatory care in our health system, a large academic medical center in Southern California, during a ‘clinical assessment period’ from 2013 through 2016. Consistent care was defined as having at least 1 ambulatory care visit each calendar year during the clinical assessment period in which BP was documented. Systolic blood pressures < 60 mmHg or > 250 mmHg and diastolic blood pressures < 20 mmHg and > 200 mmHg were considered spurious and excluded. For this cohort, we extracted self-reported age, sex, race/ethnicity, and smoking status at the time of first qualifying visit. We further used ICD-9 and ICD-10 codes to identify baseline comorbid conditions including diabetes mellitus, chronic kidney disease, coronary artery disease, cancer metastases, myocardial infarction, heart failure, stroke, and atrial fibrillation or flutter (Supplemental Table 9). Dyslipidemia was not assessed due to previously recognized limitations in the accuracy of administrative coding, even in combination with laboratory data, when using EHR data to identify presence of this condition 53. We also determined if patients were prescribed an antihypertensive medication at any time during the clinical assessment period. We excluded individuals less than 18 years of age or with a history of ADRD (based on ICD codes or prescription for a dementia medication) prior to or during the clinical assessment period.
From this cohort, we identified a cohort of patients with an EKG from which HRV could be determined, excluding EKGs with evidence of atrial fibrillation or flutter, premature atrial or ventricular contractions, atrial or ventricular pacing, or missing leads (Supplemental Fig. 1). HRV was determined from EKGs rather than visit to visit heart rates as the former, but not the latter, has been shown to be positively associated with dementia 3,6.
Blood pressure variability
Among qualifying individuals, we extracted all systolic (SBP) and diastolic (DBP) BPs, measured in mmHg, from every outpatient visit during the clinical assessment period; if multiple BP readings were recorded for a single visit, the SBPs and DBPs for that visit were averaged. Based on previously published literature examining multiple methods for the evaluation of BPV (i.e. standard deviation, coefficient of variation, and mean real variability), we elected to use variability independent of the mean (VIM) as our primary measure of visit-to-visit BPV, as alternate measures have been shown to be highly correlated with the mean BP, thus limiting their ability to differentiate from effects of mean BP 54,55.
Systolic and diastolic VIM were calculated separately. As previously described 56, VIM is calculated first as the standard deviation of BP readings divided by the mean BP raised to the power of x, where x is obtained from fitting a nonlinear regression model among the entire sample where standard deviation = a*meanx. This quantity is then multiplied by the sample mean BP raised to the power of x. As such,
where
Since VIM is derived from the distribution of BP within the sample itself, the values of VIM in a given sample cannot be compared to the values from a population with a different distribution of BP values. In general, the value of the VIM is a considered a relative, rather than an absolute measure of BP variability given that it is calculated in reference to values derived from mean BP; a higher value of VIM represents greater variability of visit-to-visit BP readings 55.
For use in sensitivity analyses, we calculated other commonly utilized BPV metrics for each patient including standard deviation (SD), coefficient of variation (CoV), average real variability (ARV), and maximum to minimum difference (MMD) 57. Calculation methods for additional BPV metrics is located in Supplemental Table 10. Assessments of the distributions and intercorrelations between measures of BPV can be found in Supplemental Tables 11 and 12.
Heart rate variability
Among individuals with a qualifying EKG, QRS complexes were identified using peak finding algorithms from scikit-learn, from which we calculated Q-Q intervals in milliseconds, from all qualifying EKGs 58. We then calculated the root mean square of successive differences (RMSSD) for each qualifying EKG 59. Our primary analysis examined HRV using the most recent EKG from the clinical assessment period only. In sensitivity analyses, RMSSD was calculated as the mean RMSSD across all qualifying EKGs for each patient.
Outcomes
We defined an ‘outcomes surveillance period’ of 2017 through 2019 during which we assessed for the development of incident ADRD based on ICD-9 and ICD-10 codes or the prescription of dementia medication (Supplemental Table 9). Of the n = 443 patients who developed ADRD during the surveillance period, a relevant dementia ICD code was present for 127 (28.7%), new dementia medication for 354 (79.9%), and both a dementia ICD and medication were present for 38 (8.6%). We identified all-cause death using vital status documented in the EHR. All study protocols were approved by the Cedars-Sinai Institutional Review Board with requirement for individual informed consent waived. All research was performed in accordance with the relevant guidelines and regulations.
Statistical analyses
We performed multivariable Cox proportional hazards regression to compute hazard ratios (HRs) examining the association between BPV (separately for SBP and DBP), HRV, and a combination of BPV and HRV during the clinical assessment period and the development of incident dementia during the outcome surveillance period. For the combined BPV and HRV measure, we categorized patients into one of four groups, similar to work done by others 47,60–62: low BPV, high HRV (reference group); high BPV, high HRV; low BPV, low HRV; and high BPV, low HRV. High BPV values were those with VIM in the 75th percentile or above for the sample, and low HRV values were those with RMSSD in the 25th percentile or below for the sample. Analyzes were run using systolic BPV and performed on the largest qualifying sample (full qualifying cohort for models of BPV alone and EKG cohort for models including HRV).
Patients were censored at time of last recorded outpatient visit during which BP was measured or at the end of the outcome surveillance period (December 31, 2019), whichever came first. All analyses adjusted for age, sex, race/ethnicity, and smoking status along with presence of diabetes mellitus, chronic kidney disease, coronary artery disease, myocardial infarction, heart failure, stroke, or atrial fibrillation or flutter; all analyses also adjusted for use of antihypertensive medications, the number of visits at which a BP was recorded, number of qualifying EKGs, heart rate, and mean SBP and DBP. In secondary analyses, we repeated the primary outcome analyses in subgroups stratified by age (less than or ≥ 65 years of age at the time of first qualifying visit) and sex.
In sensitivity analyses, we repeated the primary BPV outcome analyses using various measures of BPV (SD, CoV, ARV, and MMD). We similarly repeated the primary HRV analyzes using the average RMSSD across all EKGs for each patient during the clinical assessment period. We conducted all statistical analyses using R (v3.6.1) and considered statistical significance as a two-tailed P value < 0.05.
Standard protocol approvals, registrations, and patient consents
Study procedures were reviewed and approved by the Cedars-Sinai institutional review board (Study 00000603), with a waiver of informed consent.
Supplementary Information
Author contributions
J.E.E. contributed to the conception, design, acquisition of data, analysis of data, interpretation of data, drafting of the manuscript and revision of manuscript. M.P.D. contributed to the design, analysis of data, and interpretation of data. T.Y.H. and J.M. contributed to the analysis of data and interpretation of data. P.G.B. contributed to the acquisition of data and analysis of data. M.W. contributed to the analysis of data and interpretation of data. P.-S.C. contributed to the design, interpretation of data, drafting of the manuscript, and manuscript revision. N.A.B. contributed to the analysis of data, interpretation of data, drafting of the manuscript, and manuscript revision. D.O. contributed to the acquisition of data, interpretation of data, drafting of the manuscript, and manuscript revision. J.T. contributed to the acquisition of data and analysis of data. S.C. contributed to the design, analysis of data, interpretation of data, drafting of the manuscript, and manuscript revision. Z.T. contributed to the conception, design, acquisition of data, analysis of data, interpretation of data, drafting of the manuscript and revision of manuscript. All authors reviewed the manuscript.
Funding
This work was supported in part by Cedars-Sinai Medical Center, the Erika J Glazer Family Foundation, and NIH grants R01-HL131532 and K23-HL153888. No funders had a role in the design/conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability
Due to the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in protocols on the protection of human subjects may be sent to Cedars-Sinai Medical Center at biodatacore@cshs.org. JE and SC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-52406-8.
References
- 1.Heus RAAd, Tzourio C, Lee EJL, Opozda M, Vincent AD, Anstey KJ, Hofman A, Kario K, Lattanzi S, Launer LJ, Ma Y, Mahajan R, Mooijaart SP, Nagai M, Peters R, Turnbull D, Yano Y, Claassen JAHR, Tully PJ. Association between blood pressure variability with dementia and cognitive impairment: A systematic review and meta-analysis. Hypertension. 2021;78:1478–1489. doi: 10.1161/HYPERTENSIONAHA.121.17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo JE, Shin DW, Han K, Kim D, Lee S-P, Jeong S-M, Lee J, Kim S. Blood pressure variability and the risk of dementia. Hypertension. 2020;75:982–990. doi: 10.1161/HYPERTENSIONAHA.119.14033. [DOI] [PubMed] [Google Scholar]
- 3.Rouch, L., Vidal, J.-S., Hanon, O. and Investigators SA. Visit-to-visit heart rate variability is associated with cognitive decline in older adults: The S.AGES cohort. Alzheimer's & Dementia 16:e044024 (2020).
- 4.Liu KY, Elliott T, Knowles M, Howard R. Heart rate variability in relation to cognition and behavior in neurodegenerative diseases: A systematic review and meta-analysis. Age. Res Rev. 2022;73:101539. doi: 10.1016/j.arr.2021.101539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y, Lim J-S, Oh MS, Yu K-H, Lee JS, Park J-H, Kim Y-J, Rha J-H, Hwang Y-H, Heo SH, Ahn SH, Lee J-H, Kwon SU. Blood pressure variability is related to faster cognitive decline in ischemic stroke patients: PICASSO subanalysis. Sci. Rep. 2021;11:5049. doi: 10.1038/s41598-021-83945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaich CL, Malaver D, Chen H, Shaltout HA, Hazzouri AZA, Herrington DM, Hughes TM. Association of heart rate variability with cognitive performance: The multi‐ethnic study of atherosclerosis. J. Am. Heart Assoc. 2020;9:e013827. doi: 10.1161/JAHA.119.013827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allan LM, Ballard CG, Allen J, Murray A, Davidson AW, McKeith IG, Kenny RA. Autonomic dysfunction in dementia. J. Neurol. Neurosurg. Psychiatry. 2007;78:671–677. doi: 10.1136/jnnp.2006.102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drawz PE, Agarwal A, Dwyer JP, Horwitz E, Lash J, Lenoir K, McWilliams A, Oparil S, Rahbari-Oskoui F, Rahman M, Parkulo MA, Pemu P, Raj DS, Rocco M, Soman S, Thomas G, Tuot DS, Whelton PK, Pajewski NM. Concordance between blood pressure in the systolic blood pressure intervention trial and in routine clinical practice. JAMA Intern. Med. 2020;180:1655–1663. doi: 10.1001/jamainternmed.2020.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang O, Juraschek SP, Appel LJ, Cooper LA, Charleston J, Boonyasai RT, Carson KA, Yeh HC, Miller ER., 3rd Comparison of automated clinical and research blood pressure measurements: Implications for clinical practice and trial design. J. Clin. Hypertens. 2018;20:1676–1682. doi: 10.1111/jch.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients' resting blood pressure in clinical settings: A systematic review. J. Hypertens. 2017;35:421–441. doi: 10.1097/HJH.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: Where and how many measures? Ann. Intern. Med. 2011;154:781–8. doi: 10.7326/0003-4819-154-12-201106210-00005. [DOI] [PubMed] [Google Scholar]
- 12.Imbimbo C, Spallazzi M, Ferrari-Pellegrini F, Villa A, Zilioli A, Mutti C, Parrino L, Lazzeroni D. Heart rate variability and cognitive performance in adults with cardiovascular risk. Cereb. Circ. Cognit. Behav. 2022;3:100136. doi: 10.1016/j.cccb.2022.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silva VP, Ramalho Oliveira BR, Tavares Mello RG, Moraes H, Deslandes AC, Laks J. Heart rate variability indexes in dementia: A systematic review with a quantitative analysis. Curr. Alzheimer Res. 2018;15:80–88. doi: 10.2174/1567205014666170531082352. [DOI] [PubMed] [Google Scholar]
- 14.Schaich CL, Malaver D, Chen H, Shaltout HA, Hazzouri AZA, Herrington DM, Hughes TM. Association of heart rate variability with cognitive performance: The Multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2020;9:e013827. doi: 10.1161/JAHA.119.013827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logsdon RG, McCurry SM, Teri L. Evidence-based interventions to improve quality of life for individuals with dementia. Alzheimers Care Today. 2007;8:309–318. [PMC free article] [PubMed] [Google Scholar]
- 16.Barnett JH, Lewis L, Blackwell AD, Taylor M. Early intervention in Alzheimer’s disease: A health economic study of the effects of diagnostic timing. BMC Neurol. 2014;14:101. doi: 10.1186/1471-2377-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Academies of Sciences, Engineering Medicine, Division of, Behavioral Social, Sciences Education, Board on Behavioral, Cognitive Sensory, Sciences Committee on Developing a, Behavioral Social Science Research Agenda on Alzheimer’s. The National Academies Collection: Reports funded by National Institutes of Health. In: T. Winters, ed. Alzheimer’s Disease and Related Dementias: Experience and Caregiving, Epidemiology, and Models of Care: Proceedings of a Workshop—in Brief Washington (DC): National Academies Press (US); 2020. [PubMed]
- 18.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster NL, Bondi MW, Das R, Foss M, Hershey LA, Koh S, Logan R, Poole C, Shega JW, Sood A, Thothala N, Wicklund M, Yu M, Bennett A, Wang D. Quality improvement in neurology. Mild Cogn. Impair. Qual. Meas. Set. 2019;93:705–713. doi: 10.1212/WNL.0000000000008259. [DOI] [PubMed] [Google Scholar]
- 20.van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J. Neurol. Neurosurg. Psychiatry. 2005;76:v2–v7. doi: 10.1136/jnnp.2005.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Force UPST. Screening for cognitive impairment in older adults: US preventive services task force recommendation statement. JAMA. 2020;323:757–763. doi: 10.1001/jama.2020.0435. [DOI] [PubMed] [Google Scholar]
- 22.Forte G, Favieri F, Casagrande M. Heart rate variability and cognitive function: A systematic review. Front. Neurosci. 2019;13:710. doi: 10.3389/fnins.2019.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Heus RAA, Olde Rikkert MGM, Tully PJ, Lawlor BA, Claassen J. Blood pressure variability and progression of clinical Alzheimer disease. Hypertension. 2019;74:1172–1180. doi: 10.1161/HYPERTENSIONAHA.119.13664. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J. Appl. Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez-Angelats E, de La Sierra A, Sierra C, Parati G, Mancia G, Coca A. Blood pressure variability and silent cerebral damage in essential hypertension. Am. J. Hypertens. 2004;17:696–700. doi: 10.1016/j.amjhyper.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 26.van Buchem MA, Biessels GJ, Brunner la Rocca HP, de Craen AJM, van der Flier WM, Ikram MA, Kappelle LJ, Koudstaal PJ, Mooijaart SP, Niessen W, van Oostenbrugge R, de Roos A, van Rossum AC, Daemen MJAP. The heart–brain connection: A multidisciplinary approach targeting a missing link in the pathophysiology of vascular cognitive impairment. J. Alzheimer's Dis. 2014;42:S443–S451. doi: 10.3233/JAD-141542. [DOI] [PubMed] [Google Scholar]
- 27.Beishon LC, Hosford P, Gurung D, Brassard P, Minhas JS, Robinson TG, Haunton V, Panerai RB. The role of the autonomic nervous system in cerebral blood flow regulation in dementia: A review. Autonom. Neurosci. 2022;240:102985. doi: 10.1016/j.autneu.2022.102985. [DOI] [PubMed] [Google Scholar]
- 28.Allan LM. Diagnosis and management of autonomic dysfunction in dementia syndromes. Curr. Treat. Options Neurol. 2019;21:38–38. doi: 10.1007/s11940-019-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aharon-Peretz J, Harel T, Revach M, Ben-Haim SA. Increased sympathetic and decreased parasympathetic cardiac innervation in patients with Alzheimer's disease. Arch. Neurol. 1992;49:919–922. doi: 10.1001/archneur.1992.00530330041013. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein G, Davis-Plourde K, Beiser AS, Seshadri S. Autonomic imbalance and risk of dementia and stroke: The Framingham study. Stroke. 2021;52:2068–2076. doi: 10.1161/STROKEAHA.120.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins O, Dillon S, Finucane C, Lawlor B, Kenny RA. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol. Aging. 2012;33:2324–2333. doi: 10.1016/j.neurobiolaging.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y-C, Huang Y-C, Huang W-L. Heart rate variability in patients with dementia or neurocognitive disorders: a systematic review and meta-analysis. Aust. NZ J Psychiatry. 2022;56:16–27. doi: 10.1177/0004867420976853. [DOI] [PubMed] [Google Scholar]
- 33.Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern. Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bureau USC. 2017 National Population Projections Tables: Main Series (2021).
- 35.Bernstein Sideman A, Chalmer R, Ayers E, Gershon R, Verghese J, Wolf M, Ansari A, Arvanitis M, Bui N, Chen P, Chodos A, Corriveau R, Curtis L, Ehrlich AR, Tomaszewski Farias SE, Goode C, Hill-Sakurai L, Nowinski CJ, Premkumar M, Rankin KP, Ritchie CS, Tsoy E, Weiss E, Possin KL. Lessons from Detecting Cognitive Impairment Including Dementia (DetectCID) in Primary Care. J. Alzheimers Dis. 2022;86:655–665. doi: 10.3233/JAD-215106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Geriatrics Review Syllabus: A Core Curriculum in Geriatric Medicine. 11th ed (American Geriatrics Society, 2022).
- 37.Force UPST. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211–2218. doi: 10.1001/jama.2019.18928. [DOI] [PubMed] [Google Scholar]
- 38.McVicker JT. Blood pressure measurement—Does anyone do it right? An assessment of the reliability of equipment in use and the measurement techniques of clinicians. J. Fam. Plann. Reprod. Health Care. 2001;27:163–164. doi: 10.1783/147118901101195407. [DOI] [PubMed] [Google Scholar]
- 39.Vongpatanasin W. Accurate blood pressure in the office. Circulation. 2018;138:1771–1773. doi: 10.1161/CIRCULATIONAHA.118.036209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burkard T, Mayr M, Winterhalder C, Leonardi L, Eckstein J, Vischer AS. Reliability of single office blood pressure measurements. Heart. 2018;104:1173–1179. doi: 10.1136/heartjnl-2017-312523. [DOI] [PubMed] [Google Scholar]
- 41.Hwang KO, Aigbe A, Ju HH, Jackson VC, Sedlock EW. Barriers to accurate blood pressure measurement in the medical office. J. Prim. Care Community Health. 2018;9:2150132718816929. doi: 10.1177/2150132718816929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Tully PJ, Hofman A, Tzourio C. Blood pressure variability and dementia: A state-of-the-art review. Am. J. Hypertens. 2020;33:1059–1066. doi: 10.1093/ajh/hpaa119. [DOI] [PubMed] [Google Scholar]
- 43.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow–Robin spaces on magnetic resonance images: Normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48:745–754. doi: 10.1007/s00234-006-0112-1. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Qin W, Yang L, Fan H, Li Y, Yin J, Hu W. The relationship between ambulatory blood pressure variability and enlarged perivascular spaces: A cross-sectional study. BMJ Open. 2017;7:e015719. doi: 10.1136/bmjopen-2016-015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeil CJ, Myint PK, Sandu AL, Potter JF, Staff R, Whalley LJ, Murray AD. Increased diastolic blood pressure is associated with MRI biomarkers of dementia-related brain pathology in normative ageing. Age Ageing. 2018;47:95–100. doi: 10.1093/ageing/afx102. [DOI] [PubMed] [Google Scholar]
- 46.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021;17:639–654. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeki Al Hazzouri A, Elfassy T, Carnethon MR, Lloyd-Jones DM, Yaffe K. Heart rate variability and cognitive function in middle-age adults: The coronary artery risk development in young adults. Am. J. Hypertens. 2017;31:27–34. doi: 10.1093/ajh/hpx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Britton A, Singh-Manoux A, Hnatkova K, Malik M, Marmot MG, Shipley M. The association between heart rate variability and cognitive impairment in middle-aged men and women. The Whitehall II cohort study. Neuroepidemiology. 2008;31:115–121. doi: 10.1159/000148257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frewen J, Finucane C, Savva GM, Boyle G, Coen RF, Kenny RA. Cognitive function is associated with impaired heart rate variability in ageing adults: The Irish longitudinal study on ageing wave one results. Clin. Auton. Res. 2013;23:313–323. doi: 10.1007/s10286-013-0214-x. [DOI] [PubMed] [Google Scholar]
- 50.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ Elixhauser Comorbidity Index. Med. Care. 2017;55:698–705. doi: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 51.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 52.Cappetta K, Lago L, Potter J, Phillipson L. Under-coding of dementia and other conditions indicates scope for improved patient management: A longitudinal retrospective study of dementia patients in Australia. Health Inf. Manag. 2022;51:32–44. doi: 10.1177/1833358319897928. [DOI] [PubMed] [Google Scholar]
- 53.Oake J, Aref-Eshghi E, Godwin M, Collins K, Aubrey-Bassler K, Duke P, Mahdavian M, Asghari S. Using electronic medical record to identify patients with dyslipidemia in primary care settings: International classification of disease code matters from one region to a national database. Biomed. Inform. Insights. 2017;9:1178222616685880. doi: 10.1177/1178222616685880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nwabuo CC, Yano Y, Moreira HT, Appiah D, Vasconcellos HD, Aghaji QN, Viera A, Rana JS, Shah RV, Murthy VL, Allen NB, Schreiner PJ, Lloyd-Jones DM, Lima JAC. Association between visit-to-visit blood pressure variability in early adulthood and myocardial structure and function in later life. JAMA Cardiol. 2020;5:795–801. doi: 10.1001/jamacardio.2020.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yano Y. Visit-to-visit blood pressure variability—What is the current challenge? Am. J. Hypertens. 2017;30:112–114. doi: 10.1093/ajh/hpw124. [DOI] [PubMed] [Google Scholar]
- 56.Ebinger JE, Driver M, Ouyang D, Botting P, Ji H, Rashid MA, Blyler CA, Bello NA, Rader F, Niiranen TJ, Albert CM, Cheng S. Variability independent of mean blood pressure as a real-world measure of cardiovascular risk. EClinicalMedicine. 2022;48:101442. doi: 10.1016/j.eclinm.2022.101442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yano Y. Visit-to-visit blood pressure variability—What is the current challenge? Am. J. Hypertens. 2016;30:112–114. doi: 10.1093/ajh/hpw124. [DOI] [PubMed] [Google Scholar]
- 58.Holmstrom, L., Christensen, M., Yuan, N., Hughes, J.W., Theurer, J., Jujjavarapu, M., Fatehi, P., Kwan, A., Sandhu, R.K., Ebinger, J., Cheng, S., Zou, J., Chugh, S.S., & Ouyang, D. Deep learning based electrocardiographic screening for chronic kidney disease. medRxiv. 2022:2022.03.01.22271473. [DOI] [PMC free article] [PubMed]
- 59.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front. Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu M, Chen X, Zhang S, Lin J, Wang L, Liao X, Zhuang X. Assessment of visit-to-visit blood pressure variability in adults with optimal blood pressure: A new player in the evaluation of residual cardiovascular risk? J. Am. Heart. Assoc. 2022;11:e022716. doi: 10.1161/JAHA.121.022716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S-R, Choi Y-J, Choi E-K, Han K-D, Lee E, Cha M-J, Oh S, Lip GYH. Blood pressure variability and incidence of new-onset atrial fibrillation. Hypertension. 2020;75:309–315. doi: 10.1161/HYPERTENSIONAHA.119.13708. [DOI] [PubMed] [Google Scholar]
- 62.Yang X, Hidru TH, Han X, Zhang X, Liu Y, Wang B, Li H, Wu S, Xia YL. Link between elevated long-term resting heart rate variability and pulse pressure variability for all-cause mortality. J. Am. Heart Assoc. 2020;9:e014122. doi: 10.1161/JAHA.119.014122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in protocols on the protection of human subjects may be sent to Cedars-Sinai Medical Center at biodatacore@cshs.org. JE and SC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.