Abstract
The M protein is one of the most important virulence factors of group A streptococci (Streptococcus pyogenes) and may play an important role in the first steps of streptococcal infection. Since acute pharyngitis is a frequently occurring infectious disease caused by these bacteria, we wished to know whether antibodies to the M protein or other surface components inhibit adherence and internalization of streptococci to pharyngeal cells. We investigated the role of whole human secretory immunoglobulin A (sIgA), M6 protein-specific sIgA, and M6 protein-specific serum IgG in the inhibition of streptococcal adherence and internalization to cultured human pharyngeal cells. S. pyogenes D471, which produces a type 6 M protein (M+), and its isogenic M-negative (M−) derivative JRS75 were tested. Purified whole sIgA, M protein-specific sIgA, and sIgA preabsorbed with M protein were able to decrease significantly the adherence of streptococci to pharyngeal cells. Purified IgG against the M6 protein did not diminish the attachment of streptococci to the pharyngeal cells but did reduce internalization. Thus, our data suggest that secretory IgA may play a key role in preventing streptococcal infection at mucosal surfaces by blocking adherence while affinity-purified anti-M protein-specific IgG blocks epitopes responsible for invasion.
Streptococcus pyogenes (group A streptococcus) is an important bacterial pathogen of humans. It is the most frequent bacterial cause of acute pharyngitis and can initiate the postinfection sequelae of rheumatic fever and acute glomerulonephritis. Adherence and colonization of the host cells are considered to be the initial event of any bacterial disease and involve specific interactions between molecules present on the surfaces of both host and bacterial cells (2). For group A streptococci, this initial step is not well understood. To date, several different proteins of adhesion have been proposed for group A streptococci. Published studies have implicated both the surface M protein (1) and lipoteichoic acid (30) in the adherence of these organisms to epithelial cells, but other data have shown that neither the M protein nor lipoteichoic acid is directly involved in the adherence process (27, 29). Indeed, it was demonstrated that a streptococcal strain which produces a type 6 M protein and its isogenic derivative lacking the structural gene for M protein (emm6.1) were equally able to bind to human buccal and tonsillar epithelial cells (7). In contrast, another study suggested that protein F, a fibronectin binding protein, may play an important role in adherence, as the mutant lacking this surface molecule had a much lower capacity to adhere to respiratory epithelial cells (14). Furthermore, a major surface protein on group A streptococci with multiple binding activity to various mammalian proteins was recently described by members of our laboratory (22). This surface protein is a glyceraldehyde-3-phosphate dehydrogenase and may play a role in the first step of infection (24). Thus, while the issue remains incompletely resolved, it is likely that group A streptococci possess several surface molecules which might enable them to colonize various types of tissues.
Until recently, streptococci usually were considered to be pathogens that limit their infection to the mucosa and skin. However, there are reports showing that group A streptococci are capable of invading cultured human respiratory epithelial cells, with some strains capable of invading at frequencies as high as those of other pathogens, such as salmonellae and listeriae (20). This subsequent intracellular invasion of attached bacteria might be important in pathogenesis and may lead to invasion of deeper tissue and blood. Since the numbers of reports of severe streptococcal diseases, including streptococcal toxic shock syndrome and necrotizing fasciitis, are increasing (6, 28), it is important to understand the pathogenic mechanism of the invasion process in order to devise strategies to prevent streptococcal diseases.
Studies with humans have shown that serum type-specific antibodies against the M protein correlate with protection of group A streptococcal pharyngitis by the same serotype (19). Since this organism can colonize the pharyngeal mucosa without clinical signs, it was suggested that opsonic IgG is protective only after an established infection. At the mucosal site, affinity-purified, M protein-specific secretory immunoglobulin A (sIgA) proved to be protective in mice challenged intranasally with group A streptococci, whereas opsonic M protein-specific serum IgG was not (4). These results were the first to show that M protein-specific antibodies other than serum IgG are protective against group A infections and prompted later studies showing that mucosal immunization with M protein conserved regions were protective against streptococcal challenge (3, 5, 11). Since the majority of group A streptococcal infections occur at the pharyngeal mucosa, we investigated the role of sIgA and serum IgG in the inhibition of adherence and internalization of these organisms by cultured human pharyngeal cells. Our results show that purified sIgA against the M protein was able to significantly decrease adherence and internalization of pharyngeal cells. In addition, purified IgG against M protein diminished internalization but not adherence.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. pyogenes D471, which produces a type 6 M protein (M+), and its isogenic M-negative (M−) derivative JRS75 were grown overnight to stationary phase in Todd-Hewitt medium (Difco) supplemented with 0.2% yeast extract (THY). Numbers of CFU were determined by plating serial dilutions on Todd-Hewitt broth supplemented with 1.5% agar and counting individual colonies after incubation for 24 h at 37°C.
Purified proteins.
Recombinant M6 protein (rM6) was purified as described previously (12). The protein concentration of the purified M protein was determined by the bicinchoninic acid protein estimation method (Pierce Chemical Co., Rockford, Ill.).
Tissue cultures.
A pharyngeal carcinoma cell line (Detroit 562; ATCC CLL 138) was used for adherence and internalization assays of group A streptococci. Cells grew to confluence in 24-well plates containing HEPES-minimal essential medium supplemented with 10% fetal bovine serum (HiClone) without antibiotics in a CO2 incubator.
Preparation of immunoglobulins and absorptions.
Antisera to the whole M+ strain and to the M6 protein were prepared in New Zealand White rabbits as previously described (12). Salivary IgA and serum IgG antibodies were purified as reported elsewhere (4).
For absorptions, pooled human saliva was clarified by ultracentrifugation (50,000 × g, 2 h), passed over a column of jacalin immobilized on agarose (Pierce Chemical Co.), eluted with 0.1 M melibiose, dialyzed against phosphate-buffered saline (PBS), and concentrated by ultrafiltration. Antibodies directed to the M6 protein from this concentrated salivary IgA and hyperimmune rabbit serum (IgG) were purified by passing over a column of glutardialdehyde-activated glass beads (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) derivatized with recombinant M6 protein (ColiM6). The bound antibody was eluted with 0.1 M glycine, pH 2.1, and quickly adjusted to pH 7.4 with 1.0 M Tris. All samples were dialyzed against PBS supplemented with Ca2+ and Mg2+. Prior to the experiments, titers of the M protein were determined by enzyme-linked immunosorbent assay (ELISA). Aliquots (200 μl) of the different IgA preparations (whole sIgA, sIgA preabsorbed with M protein, and M-specific sIgA) and of the M-specific IgG were added with 100 μl of the bacterial suspension to the wells with the confluent pharyngeal cells and incubated as described previously.
ELISA.
Microtiter wells were coated with 100 μl of 1 and 5 μg of rM6 protein per ml for IgG and IgA, respectively, in 0.05 M carbonate buffer, pH 10, and incubated overnight at room temperature. The plates were then washed five times with PBS and blocked with 2% bovine serum albumin in PBS-Brij (0.05%) for 1 h at 37°C. Rabbit serum was diluted 1:100, purified human sIgA was diluted 1:2 in PBS-Brij, and 200 μl of each was added to the first row of wells, serially diluted twofold, and incubated 3 h at 37°C. All samples were run in duplicate. After washing, alkaline phosphatase-conjugated anti-rabbit IgG at a dilution of 1:2,000 (Sigma Immunochemicals) or monoclonal anti-human IgA secretory component at a dilution of 1:5,000 (IgA, mouse ascites fluid; Sigma Immunochemicals) was added and incubated for 3 h at 37°C. The plates were then washed with PBS, 1-mg/ml phosphatase substrate in 10% diethanolamine–3 mM MgCl2, pH 10.0, was added, and the plates were read at A405. ELISA titers are reported as the reciprocal of the highest dilution that gave a reading of 1.0 at 30 min. The ELISA titer of serum IgG against the whole M+ strain was 25,000, and that of serum IgG against the rM6 protein was 12,800. The ELISA titer of the affinity anti-M protein IgG used in the adherence assay with sIgA was 64,000. The ELISA titers of whole sIgA and purified IgA against the M protein were both 16 and were <2 for IgA absorbed with M protein.
Adherence and internalization assays.
Streptococcal adherence and internalization experiments with Detroit pharyngeal cells were determined by a method originally described by Isberg and Falkow (16) and modified by Rubens et al. (25). Confluent monolayers were infected with various numbers of group A streptococci. Bacterial overnight cultures were washed in PBS (15 mM Na2HPO4–145 mM NaCl, pH 7.2) supplemented with Ca2+ and Mg2+ and finally diluted to the appropriate inoculum density. The bacterial suspension was added to the wells and incubated for 3 h at 37°C in a 5% CO2-enriched atmosphere with 100% relative humidity. The infected monolayers were then washed three times with PBS supplemented with Ca2+ and Mg2+ to remove unattached bacteria. The monolayers were then removed from the plate surface with 0.25% trypsin–1 mM EDTA, streptococci were released from the cells by lysis with sterile distilled water, and the number of CFU was scored. For the internalization experiments, 500 μl of minimal essential medium containing gentamicin (100 μg/ml) and penicillin (5 μg/ml) was added to the wells and incubated for another 2 to 3 h prior to cell lysis. Neither antibiotic penetrates eukaryotic cells (20). Monolayers were then treated as described above, and the number of CFU was determined by plating serial dilutions of the lysate on Todd-Hewitt agar supplemented with 5% sheep blood. The petri dishes were incubated for 24 h at 37°C, and the colonies were counted.
Inhibition of internalization by cytochalasin D and colchicine.
Cytochalasin D inhibits actin polymerization of the eukaryotic cell cytoskeleton, and colchicine inhibits microtubule formation (both were obtained from Sigma). Various concentrations of these inhibitors were added to the pharyngeal cells 30 min prior to the infection, as described by Rubens et al. (25). The inhibitors were present for the whole invasion assay period until the medium with the bacteria was replaced by fresh cell culture medium containing antibiotics as in the adherence assay. The streptococcal M+ strain (D471) was prepared as for the adherence and internalization assays (described above).
Transmission electron microscopy.
Group A streptococci were grown for 15 h in Todd-Hewitt broth, and the monolayers in six-well plates were inoculated with 8 × 107 CFU per well. Three hours after incubation, the wells were washed with PBS and fixed in 2.5% glutaraldehyde in 0.1 cacodylate buffer, pH 7.4. The fixed cells were then postfixed in 1% osmium tetroxide in 0.25% uranyl acetate. Electron microscopy was performed at the Rockefeller University Central Biotechnology Facility.
Statistical analysis.
Differences between groups were tested by Student’s t test. The results are expressed as means ± standard deviations (SD).
RESULTS
Localization of M+ streptococci in infected pharyngeal cells.
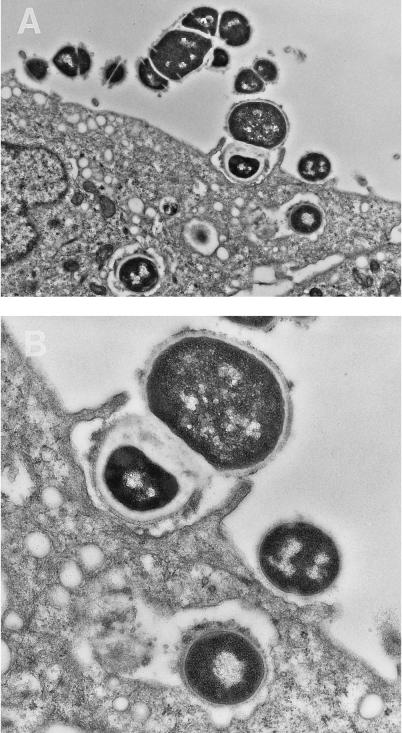
Electron microscopy was performed to verify the adherence and internalization process of the M+ strain. After initial contact of the streptococci with the eukaryotic cell surface, membrane extensions appear to surround the organisms to initiate bacterial uptake (Fig. 1). In all cases, the observed contact between the pharyngeal cell membrane and the bacteria appears to be through streptococcal surface molecules. Internalized streptococci are seen within vacuoles in both micrographs.
FIG. 1.
Electron microscopy demonstrating the attachment and internalization of streptococci by human cultured pharyngeal cells. Bacteria were observed to associate with microvilli upon initial contact with the pharyngeal cells. Membrane extension is observed for the internalization process. Close observation indicates that surface proteins are involved with this initial interaction. Intracellular streptococci are found enclosed in cytoplasmic vacuoles with bacterial surface proteins still in contact with the membrane surface. The bacterial inoculum was prepared as described for the standard internalization assay. Magnification, ×12,700 (A) and 24,300 (B).
Effect of cytochalasin D and colchicine on streptococcal internalization.
Since electron microscopy results suggest the occurrence of active bacterial uptake, we tested the effect of cytochalasin D and colchicine on the internalization of group A streptococci in the pharyngeal cells. In the presence of cytochalasin D at doses of 0.5 and 0.25 μg/ml, internalization was significantly inhibited (Table 1). Even at a dose of 0.125 μg/ml, uptake was lower than that in the control wells. In contrast, internalization was not affected by colchicine, even at the highest tested dose of 40 μg/ml, suggesting that polymerized actin and not microtubule formation plays an important role in the uptake of streptococci by the pharyngeal cells.
TABLE 1.
Internalization of M+ streptococci in the presence of cytochalasin D and colchicinea
| Inhibitor | Concn (μg/ml) | Internalizationb (CFU) | % Inhibition of internalization | P (internalized CFU) |
|---|---|---|---|---|
| Cytochalasin D | 0.0 | 4,000 ± 1,025 | ||
| 0.125 | 2,300 ± 1,000 | 42 | NS | |
| 0.25 | 283 ± 134 | 93 | <0.01 | |
| 0.5 | 416 ± 324 | 90 | <0.01 | |
| Colchicine | 0.0 | 3,800 ± 2,000 | ||
| 10 | 2,000 ± 654 | 47 | NS | |
| 20 | 3,000 ± 1,400 | 21 | NS | |
| 40 | 2,600 ± 1,200 | 32 | NS |
The inhibitor was added 30 min prior to the infection of 24-well plates with confluently cultured pharyngeal cells. The bacterial inoculum was prepared as described for the adherence and internalization assays, and each well was infected with approximately 5 × 105 CFU of the streptococcal strain M+ (S. pyogenes D471). NS, not significant.
Data represent the means ± SD of three independent experiments with two infected wells per inhibitor concentration per experiment.
Effect of immunoglobulins on adherence to pharyngeal cells.
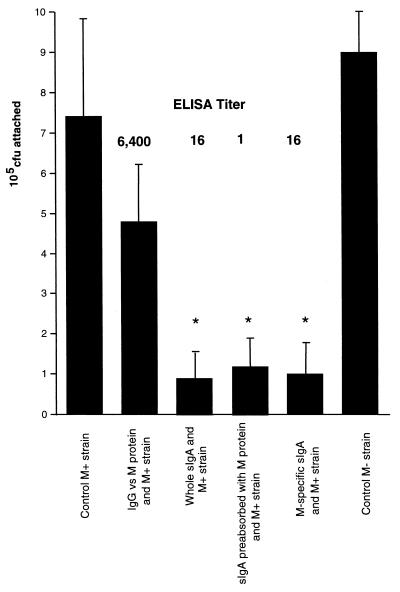
To investigate the role of sIgA in the adherence of group A streptococci to pharyngeal cells, wells of confluent cells were infected with 106 CFU of streptococci in the presence of various immunoglobulin preparations. Both the M+ and control M− strains could bind equally well to the pharyngeal cells (Fig. 2). In the presence of purified rabbit IgG against the M protein, at a final titer of 6,400, adherence of the M+ strain was not significantly altered compared to that of the controls. In contrast, all three preparations of the purified IgAs (whole purified human sIgA, sIgA preabsorbed with M protein, and M protein-specific sIgA, at titers of 16, 1, and 16, respectively) significantly decreased the attachment of the M+ group A streptococci to the pharyngeal cells (P < 0.05 compared to the controls). This decreased attachment of M+ streptococci in the presence of sIgA occurred despite the significantly lower immunoreactivity of the sIgA to purified M protein (<400-fold) compared with that of purified rabbit serum IgG. The absorption experiment suggests that sIgAs to other surface proteins, and perhaps even to M protein, are responsible for blocking the attachment of the streptococci to pharyngeal cells.
FIG. 2.
Effect of purified rabbit IgG and different preparations of human sIgAs on the adherence of group A streptococci to pharyngeal cells. The M+ strain (106 CFU) was incubated with purified rabbit IgG, purified sIgA, purified sIgA preabsorbed with M protein, or purified M protein-specific sIgA. All were at the final ELISA titer/well shown. Untreated M+ and M− strains were used as controls. Infected monolayers were incubated for 3 h, dispersed by addition of 0.25% trypsin–1 mM EDTA, and lysed with H2O. Error bars, SD; ∗, P < 0.05 compared to controls.
Effect of hyperimmune IgG against M protein on adherence and internalization.
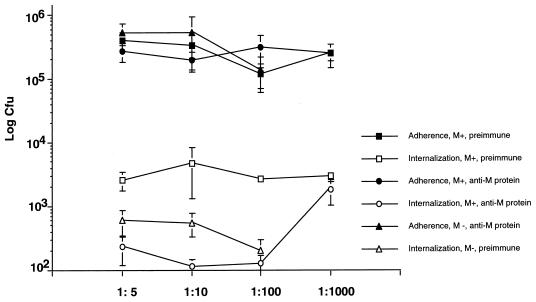
To determine if antibodies specific to M protein also play a role in blocking internalization, hyperimmune rabbit serum raised against the M protein was tested in the adherence and internalization assay. Both the M+ and M− strains attached equally well to the pharyngeal cells in the presence of preimmune rabbit serum. In the presence of hyperimmune serum against the rM6 protein, the M+ strain adhered similarly to controls (Fig. 3). In contrast, internalization of the M+ strain was significantly decreased (P < 0.001) by the hyperimmune serum against the rM6 protein at dilutions from 1:5 to 1:100 and was comparable to that of the M− strain, which is unable to invade eukaryotic cells.
FIG. 3.
Adherence and internalization of M+ and M− strains in the presence of preimmune rabbit serum and hyperimmune rabbit serum raised against the M6 protein. Thirty minutes prior to the inoculation of the confluent monolayers, bacteria (5 × 105 CFU) were incubated at various dilutions of the preimmune rabbit serum or the hyperimmune rabbit serum in PBS supplemented with Mg2+ and Ca2+. The adherence was performed as described in the legend to Fig. 2. For the internalization assay, monolayers were infected for 3 h, treated with antibiotics for 2 to 3 h, dispersed by addition of 0.25% trypsin–1 mM EDTA, and lysed with H2O. Data represent the means ± SD (error bars) of two to three independent experiments with two infected wells per experiment.
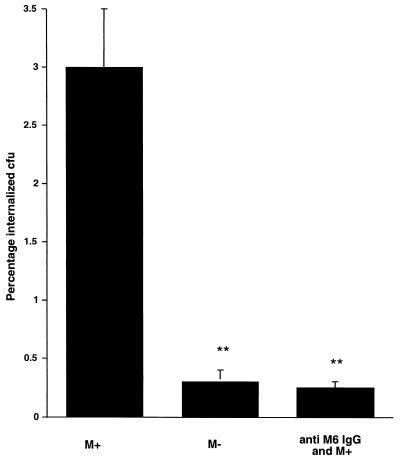
To eliminate the possibility of serum components interfering with internalization, an additional experiment was performed in which inhibition of internalization was tested by affinity-purified serum IgG to M protein (Fig. 4). As seen in earlier experiments, about 3 to 4% of attached M+ streptococci became internalized, compared to the M− strain, in which nearly 10 times fewer organisms (0.4%) were internalized (P < 0.01). The purified anti-M6 IgG at a concentration of 0.15 mg/ml inhibited internalization to levels comparable to that of the M− strain. However, as shown in Fig. 2, this M protein-specific antibody was unable to block adherence.
FIG. 4.
Effect of purified M protein-specific IgG on the internalization process of M+ streptococci. Purified IgG at a protein concentration of 0.15 mg/ml was added to approximately 5 × 105 CFU and incubated at room temperature for 30 min and then added to the pharyngeal cells. The M+ and M− strains alone were used as controls. The internalization assay was performed as described in the legend to Fig. 3. Data represent the means ± SD (error bars) of two independent experiments with two infected wells per experiment. ∗∗, P < 0.01.
DISCUSSION
Group A streptococci are important bacterial pathogens due to their frequency as a cause of pharyngotonsillar infection and the nonpurulent sequelae of rheumatic fever and acute glomerulonephritis. Although this bacterium is generally considered a noninvasive pathogen causing localized infection of the nasopharyngeal mucosal surfaces and skin, there are increasing reports of invasive infections resulting in necrotizing fasciitis and death (6, 28). Invasion of cultured human cells has been performed with both group A and group B streptococci (20, 25). However, whether invasion of these cell lines is a measure of the ability of these organisms to cause invasive disease has not yet been shown. In a recent report, group A streptococci of the M1 serotype containing the pyrogenic exotoxin A (speA) gene were found to efficiently invade cultured lung epithelial cells (20). In the present study we have demonstrated that speA-negative group A streptococci of the M6 serotype adhered to a cultured human pharyngeal cell line and had the capacity to invade these eukaryotic cells. The internalized bacteria were found to be contained within vacuoles in the eukaryotic cells, suggesting an involvement of cytoskeletal rearrangement of the host cells. In support of this, we found that internalization could be inhibited with cytochalasin D but not colchicine. These results agree with the findings of Rubens et al. (25), who found that invasion of group B streptococci into alveolar epithelial cells could be inhibited in a similar way. However, our findings do not support those of LaPenta et al. (20) showing that group A streptococcal invasion of cultured human lung cells could be inhibited by both cytochalasin D and colchicine. The fact that we do not see significant inhibition with colchicine in our experiments (using a human pharyngeal cell) could reflect the differences in the cell lines used in these experiments. This is supported by a recent study (24) in which differences were seen in the signal transduction pattern induced by surface dehydrogenase (SDH) on group A streptococci and the cells used for induction.
Results of earlier studies with mice showed that purified anti-M6 IgA administered intranasally passively protected mice from challenge with type 6 M streptococci. In contrast, anti-M6 serum IgG was not protective when delivered at this site (4). To help explain these results, in the present study we investigated the effect of different preparations of IgA on adherence of group A streptococci in an in vitro model of cultured pharyngeal cells. Affinity-purified anti-M protein sIgA could significantly decrease bacterial attachment to the eukaryotic cells. In contrast, hyperimmune IgG against the whole M+ strain (data not shown) and the affinity-purified M protein-specific IgG did not affect attachment, although the immunoreactivity of the IgG preparation against the M protein was significantly higher than that of the IgA preparation. These results confirm the idea that sIgA alone can protect against bacterial adherence and, therefore, the initiation of infection (4), whereas opsonic IgG is likely protective only after infection (19). Interestingly, bacterial adherence could also be decreased by the IgA preparation without the population of IgA specific for M protein, suggesting that IgAs against other surface molecules are also important for blocking adhesion of group A streptococci. This is supported by the fact that in our experiment, the M− strain could adhere as well as the M+ strain to the pharyngeal cells. Several previous studies investigated the role of the M protein as an adhesion molecule, with controversial results (7, 15, 21, 31). It is probable that group A streptococci possess different adhesion molecules, depending on both environmental conditions, such as levels of CO2 or O2, and the tissue they contact, such as the mucosal surface or skin (21).
For our experiments, we used a human pharyngeal cell line, since acute streptococcal pharyngitis is the most common streptococcal infection. Bacterial adherence and colonization are thought to be the first step in infection (2, 9). A large number of microorganisms are able to invade eukaryotic cells (8), and the molecular and genetic bases of this entry in gram-negative enteric bacteria (10) and the gram-positive bacterium Listeria monocytogenes (13) were recently investigated and described. The importance of intracellular invasion in the pathogenesis of group A streptococci, a typical extracellular pathogen, is less clear and is debated. LaPenta et al. (20) described an M1 serotype strain that was speA positive and could enter human lung carcinoma cells at about 1 CFU per target cell, a frequency corresponding to the number of internalized Salmonella typhimurium (18) and L. monocytogenes (13) bacteria. In our experiments, we used an M6 serotype speA-negative strain (isolated from a pharyngitis patient) which invaded the eukaryotic cells at a frequency of about 1 CFU per 10 cells. Similar numbers of internalized bacteria were found by LaPenta et al. (20) and others (17) using speA-negative organisms. A recently published study showed that the hyaluronic acid capsule as a major virulence factor of group A streptococci did not play a role in invasion. The highly encapsulated group A streptococcus resisted internalization into a human keratinocyte cell line (26), leading the investigators to conclude that bacterial internalization was not crucial for systemic infection.
Comparison of the isogenic M+ and M− strains of S. pyogenes indicates that the M protein mediates internalization into pharyngeal cells. Hyperimmune anti-M protein IgG could block invasion, decreasing it to the same level as that observed with the M− strain. This inhibition was dependent on the concentration of hyperimmune IgG and could be confirmed by affinity-purified anti-M6 IgG, suggesting that the pharyngeal cell surface interacts specifically with the M protein to initiate the internalization process. Streptococcal SDH, another important surface molecule on group A streptococci with multiple binding activity, was recently described (22, 23). SDH, a surface glyceraldehyde-3-phosphate dehydrogenase, has recently been shown to enable communication through signal transduction between host and bacterium during the first steps of infection (24). It is conceivable that both the M protein and SDH work in concert with eukaryotic surface molecules to initiate streptococcal infection.
Overall, our in vitro model using a cultured human pharyngeal cell line proved to be a useful tool to investigate both invasion and the ability of immunoglobulins to block adherence and internalization of group A streptococci. Our data confirm previous results obtained in animals (4) and may be useful for testing vaccine candidates for group A streptococci and other pathogens. It is unknown at this time if invasion of cultured pharyngeal cells resembles an event in the human host. If it does occur, it may be a phase in the infection cycle of the streptococcus that allows the organism to be maintained in the tissues for extended periods. Whether this is what occurs during the so-called streptococcal carrier state, in which the organisms may be isolated in small numbers from the pharynx of asymptomatic individuals, or is the initial stage of a streptococcal infection will require further investigation.
ACKNOWLEDGMENTS
We thank Vijaykumar Pancholi for helpful discussion and Mary Windels and Clara Eastby for technical assistance.
This study was supported in part by the USPHS (grant AI11822) and SIGA Pharmaceuticals (V.A.F.). U.F. was in part supported by a grant from the Jubiläumsstiftung Ciba-Geigy, Basel, Switzerland.
REFERENCES
- 1.Alkan M, Ofek I, Beachey E H. Adherence of pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977;18:555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beachey E H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 3.Bessen D, Fischetti V A. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988;56:2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessen D, Fischetti V A. Passive acquired mucosal immunity to group A streptococci by secretory immunoglobulin A. J Exp Med. 1988;167:1945–1950. doi: 10.1084/jem.167.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessen D, Fischetti V A. Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J Immunol. 1990;145:1251–1256. [PubMed] [Google Scholar]
- 6.Bisno A L, Stevens D L. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 7.Caparon M G, Stephens D S, Olsen A, Scott J R. Role of M protein in adherence of group A streptococci. Infect Immun. 1991;59:1811–1817. doi: 10.1128/iai.59.5.1811-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991;65:1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- 9.Finlay B B. Cell adhesion and invasion mechanisms in microbial pathogenesis. Curr Opin Cell Biol. 1990;2:815–820. doi: 10.1016/0955-0674(90)90078-s. [DOI] [PubMed] [Google Scholar]
- 10.Finlay B B, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989;53:210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischetti V A, Hodges W M, Hruby D E. Protection against streptococcal pharyngeal colonization with a vaccinia:M protein recombinant. Science. 1989;244:1487–1490. doi: 10.1126/science.2660266. [DOI] [PubMed] [Google Scholar]
- 12.Fischetti V A, Jones K F, Manjula B N, Scott J R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification and comparison with streptococcal-derived M protein. J Exp Med. 1984;159:1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard J-L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 14.Hanski E, Horwitz P A, Caparon M G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isberg R R, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 17.Jadoun, J., E. Burnstein, E. Hanski, and S. Sela. 1996. Proteins M and F are required for efficient invasion of group A streptococci into cultured epithelial cells, abstr. L19. XIII Lancefield International Symposium on Streptocci and Streptococcal Diseases
- 18.Kusters J G, Mulders-Kremers G A W M, Van Doornik E M, Van Der Zeijst B A M. Effects of multiplicity of infection, bacterial protein synthesis, and growth phase on adhesion to and invasion of human cell lines by Salmonella typhimurium. Infect Immun. 1993;61:5013–5020. doi: 10.1128/iai.61.12.5013-5020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancefield R C. Persistence of type specific antibodies in man following infection with group A streptococci. J Exp Med. 1959;110:271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPenta D, Rubens C, Chi E, Cleary P P. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada N, Pentland A P, Falk P, Caparon M G. M protein and protein F act as important determinants of cell-specific tropism of Streptococcus pyogenes in skin tissue. J Clin Invest. 1994;94:965–977. doi: 10.1172/JCI117463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pancholi V, Fischetti V A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pancholi V, Fischetti V A. Glyceraldehyde-3-phosphate dehydrogenase on the surface of group A streptococci is also an ADP-ribosylating enzyme. Proc Natl Acad Sci USA. 1993;90:8154–8158. doi: 10.1073/pnas.90.17.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pancholi V, Fischetti V A. Regulation of the phosphorylation of human pharyngeal cell proteins by group A streptococcal surface dehydrogenase (SDH): signal transduction between streptococci and pharyngeal cells. J Exp Med. 1997;186:1633–1643. doi: 10.1084/jem.186.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubens C E, Smith S, Hulse M, Chi E Y, van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992;60:5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrager H M, Rheinwald J G, Wessels M R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Invest. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson W A, Beachey E H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983;39:275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens D L. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 29.Talay S R, Valentin-Weigand P, Jerlstrom P G, Timmis K N, Chhatwal G S. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;60:3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tylewska S K, Fischetti V A, Gibbons R J. Binding selectivity of Streptococcus pyogenes and M-protein to epithelial cells differs from that of lipoteichoic acid. Curr Microbiol. 1988;16:209–216. [Google Scholar]
- 31.Wang J-R, Stinson M W. M protein mediates streptococcal adhesion to HEp-2 cells. Infect Immun. 1994;62:442–448. doi: 10.1128/iai.62.2.442-448.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]