Abstract
The Lyme disease spirochete, Borrelia burgdorferi, infects multiple tissues, such as the heart, joint, skin, and nervous system and has been shown to recognize heparan sulfate and dermatan sulfate proteoglycans. In this study, we examined the contribution of different classes of proteoglycans to the attachment of the infectious B. burgdorferi strain N40 to several immortalized cell lines and primary cultured cells, including endothelial cells and brain cells. Bacterial attachment was inhibited by exogenous proteoglycans or by treatment of host cells with inhibitors of proteoglycan synthesis or sulfation, indicating that proteoglycans play a critical role in bacterial binding to diverse cell types. Binding to primary bovine capillary endothelial cells or a human endothelial cell line was also inhibited by digestion with heparinase or heparitinase but not with chondroitinase ABC. In contrast, binding to glial cell-enriched brain cell cultures or to a neuronal cell line was inhibited by all three lyases. Binding of strain N40 to immobilized heparin could be completely inhibited by dermatan sulfate, and conversely, binding to dermatan sulfate could be completely blocked by heparin. As measured by 50% inhibitory dose, heparin was a better inhibitor of binding than dermatan sulfate, regardless of whether the substrate was heparin or dermatan sulfate. These results are consistent with the hypotheses that the species of proteoglycans recognized by B. burgdorferi vary with cell type and that bacterial recognition of different proteoglycans is mediated by the same bacterial molecule(s).
Lyme disease is a chronic, multisystemic infection caused by the tick-borne spirochete Borrelia burgdorferi (40). The spirochete initially establishes an infection at the site of the tick bite and then migrates through the skin, resulting in the characteristic expanding rash, erythema migrans. As the infection progresses, the bacterium can spread via the bloodstream to multiple sites, such as the joints, heart, skin, or nervous system. During this phase of the infection, Lyme disease patients may experience arthralgia, carditis, secondary erythema migrans lesions, or neurologic manifestations such as meningitis, cranial neuritis, or radiculoneuritis (40). Chronic infection may be established in at least some of these tissues, as reflected by the late manifestations of Lyme disease, which include arthritis and a variety of neurological syndromes, such as encephalopathy or polyradiculoneuropathy (15, 25, 30). The spirochete has been detected at a variety of these sites in Lyme disease patients and infected laboratory animals (2, 34, 36). Thus, although the pathophysiological mechanisms that result in the manifestations of Lyme disease are not known in detail, infection of the affected tissues is likely to be the critical trigger.
Most bacterial pathogens are able to attach to host cells in the target tissue, a step that is thought to contribute to the establishment of an infectious niche at that site (3, 4). During blood-borne dissemination from the site of tick inoculation, B. burgdorferi must cross the endothelial cell barrier. Reflecting this property, the spirochete has the ability to bind to and cross confluent endothelial cell monolayers in vitro (7, 8, 32, 42). Furthermore, the ability of B. burgdorferi to infect multiple tissues may result in part from its ability to bind to many different cell types, including neuroglia (15, 16), epithelial cells (44), fibroblasts (20, 28), lymphocytes (9), and platelets (5, 14).
B. burgdorferi binds to several classes of host cell molecules expressed on the cell surface or in the extracellular matrix. For example, the platelet-specific integrin αIIbβ3 and two widely expressed integrins, αvβ3 and α5β1, mediate bacterial attachment to human cells (5, 6), while galactocerebroside promotes attachment to Schwann cells (17). In addition, B. burgdorferi, like many other microbial pathogens (39), binds to proteoglycans (19, 23, 29). Proteoglycans consist of core proteins covalently linked to long, linear, negatively charged disaccharide repeats, termed glycosaminoglycans (27). They are widely expresed and involved in diverse biological phenomena, such as cell adhesion and migration, tumor metastasis, cell signaling, and hemostasis. Depending on the composition of the disaccharide repeat and the overall extent of sulfation, glycosaminoglycans can be classified into several different species, including heparin, heparan sulfate (formerly called heparitin sulfate), chondroitin-4-sulfate (chondroitin sulfate A), chondroitin-6-sulfate (chondroitin sulfate C), dermatan sulfate (chondroitin sulfate B), and keratan sulfate.
Because of their ubiquitous expression on the cell surface and in the extracellular matrix, proteoglycans could mediate spirochetal attachment to diverse tissues. B. burgdorferi attachment to monkey kidney (Vero) cells is mediated by heparan sulfate (29). The spirochete binds to heparan sulfate and dermatan sulfate on human epithelial (HeLa) cells (23) and to decorin, a dermatan sulfate/chondroitin sulfate proteoglycan associated with collagen fibrils (19). It is not clear whether binding to different proteoglycans is mediated by the same bacterial molecule(s) or by multiple molecules, each of which recognizes a distinct subset of proteoglycans. The present study was designed to investigate whether (i) the attachment of B. burgdorferi to diverse cell types is mediated by glycosaminoglycans, (ii) the host cell determinants of these interactions exhibit cell type specificity, and (iii) binding to different classes of glycosaminoglycans is likely to be mediated by the same bacterial molecule(s).
MATERIALS AND METHODS
Bacteria and mammalian cells.
B. burgdorferi N40, clone D10/E9, is an infectious B. burgdorferi (sensu stricto) isolate (5). These strains were cultured in MKP base medium (MKP-S) supplemented with human serum as described previously (5, 37). Briefly, 100 ml of 10× CMRL medium, 3 g of neopeptone, 6 g of HEPES, 0.7 g of sodium citrate, 3 g of glucose, 0.8 g sodium pyruvate, 0.8 g of N-acetylglucosamine, and 2 g of sodium bicarbonate were added to 900 ml of distilled H2O, and the pH was adjusted to 7.6. Then 200 ml of autoclaved 7% gelatin, 35 ml of 35% bovine serum albumin (BSA) filtered through a 0.45-μm-pore-size filter, and 70 ml of heat-inactivated human serum were added prior to filtration through a 0.22-μm-pore-size filter. Radiolabeled B. burgdorferi was prepared by growth in modified MKP medium supplemented with 100 μCi of [35S]methionine per ml, washed, and stored as aliquots at −80°C as previously described (5).
Vero cells were cultured in RPMI 1640 supplemented with 10% NuSerum (Collaborative Research). 293 human embryonic kidney cells were cultured in a 1:1 mix of Dulbecco modified Eagle medium (DMEM; low glucose; Gibco-BRL, Bethesda, Md.) and Ham’s F12 medium (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS). Primary bovine endothelial cells, provided by Judah Folkman, Catherine Butterfield, and Marsha Moses (13), were grown on gelatin-coated plastic in DMEM (low glucose; Gibco-BRL) supplemented with 10% newborn calf serum and 3 ng of basic fibroblastic growth factor (Gibco-BRL) per ml. EA-Hy926 is a human endothelial cell line that expresses a wide range of differentiated endothelial cell markers, such as von Willebrand factor antigen, Weibel-Palade bodies, and factor VIII-related antigen with morphological distributions similar to those of primary endothelial cells (11, 12). These cells, provided by Cora-Jean Edgell, were cultured in DMEM (high glucose; Gibco-BRL) supplemented with 1% hypoxanthine-aminopterin-thymidine (Gibco-BRL) and 10% FBS. Primary cultures of telencephalic cells, consisting primarily of astrocytes, with approximately 3% oligodendrocytes and 10% microglia, were prepared from 1- to 2-day-old Sprague-Dawley rats. This cell preparation responds to B. burgdorferi by the production of nitric oxide and interleukin-6 and was prepared as described previously (43). The CATH.a cell line, provided by Dona Chikaraishi, was derived from a tumor induced in mice by a transgene targeted to tyrosine hydroxylase-containing neurons and has phenotypic features of catacholaminergic neurons (41). These cells were grown in RPMI 1640 (Irvine Scientific) supplemented with 8% horse serum and 4% FBS, fed three times per week, and split when the monolayer reached 60% confluency. Penicillin (100 U/ml), streptomycin (100 μg/ml), and glutamine (2 mM) were added to all culture media. 293 cells were cultured in a 7% CO2 atmosphere, and capillary endothelial cells were cultured in a 10% CO2 atmosphere; all other cells were cultured in a 5% CO2 atmosphere.
Quantitation of bacterial attachment to mammalian cells.
One to two days prior to each assay, the mammalian cells that were to be tested were lifted and plated in Nunc 96-well break-apart microtiter plates coated with Yersinia pseudotuberculosis invasin protein, which promotes cell attachment by binding a subset β1-chain integrins (24). In pilot experiments with primary bovine endothelial cells, the levels of efficiency of bacterial binding to cells plated on invasin, gelatin, or invasin plus gelatin were indistinguishable. To assay bacterial attachment to these cells, frozen aliquots of radiolabeled bacteria were thawed, suspended at 1 × 108 to 2 × 108/ml in MKP-S, and incubated for 2 h at room temperature to allow for physiologic recovery of the bacteria. The spirochetes were then checked for intact morphology and vigorous motility and were diluted 1:3 into 10 mM HEPES–10 mM glucose–50 mM NaCl (pH 7.0) before addition to confluent cell monolayers that had been washed twice in phosphate-buffered saline (PBS). To promote host cell-bacterium contact, the microtiter plates were centrifuged at 190 × g for 5 min at 20°C and then rocked at 20°C for 1 h. Unbound bacteria were removed by washing the monolayers three times in PBS (150 mM NaCl, 16.9 mM K2HPO4, 4.8 mM KH2PO4 [pH 7.4]) supplemented with 0.2% BSA, and bound bacteria were quantitated by liquid scintillation.
To test the effect of exogenous proteoglycans or dextran sulfate on bacterial attachment, bacteria were incubated for 30 min at room temperature in MKP-S supplemented with chondroitin-4-sulfate, chondroitin-6-sulfate, heparin (porcine), dextran sulfate (500 kDa), dermatan sulfate (all purchased from Sigma Chemical Co., St. Louis, Mo.), or heparan sulfate (generous gift of Bis Lahiri) and diluted 1:3 into 10 mM HEPES–10 mM glucose–50 mM NaCl, pH 7.0 prior to infection of monolayers. The proteoglycan-binding chemokine platelet factor 4 (31) (Sigma) was added to bacteria at a final concentration of 5 μg/ml in the diluted MKP-S just prior to infection of the monolayer. Bound bacteria were quantitated as described above.
The effect of enzymatic removal of different classes of proteoglycans on bacterial attachment was determined as previously described (29). Briefly, monolayers were incubated with 35 μl of 0.5 U of heparinase I, heparitinase (heparinase III), or chondroitinase ABC (all purchased from Sigma) per ml for 2 h at 37°C in RPMI 1640 supplemented with 1% BSA, 10−2 trypsin inhibitory units of aprotinin per ml, and 150 μg of phenylmethylsulfonyl fluoride per ml. The monolayers were then washed with PBS, incubated with radiolabeled bacteria, and processed as described above. To test the role of sulfation on bacterial attachment to proteoglycans, monolayers were cultured overnight in 10% dialyzed FBS (18) in Ham’s F12 medium supplemented with either 30 mM sodium chlorate, 30 mM sodium chloride, or 30 mM sodium chlorate plus 30 mM sodium sulfate as described previously (29). Sodium chlorate competitively inhibits proteoglycan sulfation, while the addition of sodium sulfate restores sulfation (1). To inhibit heparan sulfate and chondroitin sulfate glycosaminoglycan attachment to the protein core of proteoglycans, cells were cultured overnight in medium supplemented with 5 mM p-nitrophenyl-β-d-xyloside or, as a control, 5 mM p-nitrophenyl-α-d-galactoside (26). Monolayers were washed in PBS prior to infection with bacteria, and bacterial attachment to treated monolayers was quantitated as described above.
Inhibition of bacterial attachment to purified heparin or dermatan sulfate.
Nunc 96-well break-apart microtiter plates were coated overnight at 4°C with 5 mg of heparin or dermatan sulfate per ml in PBS. To assay bacterial attachment to these wells, frozen aliquots of radiolabeled bacteria were thawed and suspended at 1 × 108 to 2 × 108/ml in MKP-S as described above. Soluble proteoglycan was added to the bacteria at various concentrations, and the mixture was incubated for 30 min at room temperature. Spirochetes were diluted 1:3 into 10 mM HEPES–10 mM glucose–50 mM NaCl (pH 7.0) before addition to microtiter wells. The microtiter plates were centrifuged at 1,430 × g for 15 min at 20°C and then rocked at 20°C for 1 h. Unbound bacteria were removed by washing the wells three times in PBS supplemented with 0.2% BSA, and bound bacteria were quantitated by liquid scintillation.
RESULTS
Bacterial attachment to Vero and 293 cells is mediated by different species of glycosaminoglycans.
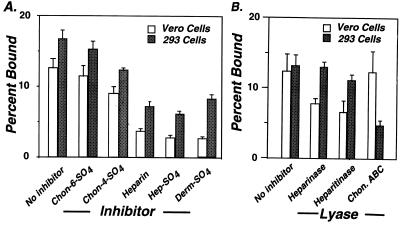
To investigate proteoglycan-mediated attachment of B. burgdorferi to host cells, Vero cells and 293 cells were infected with the infectious B. burgdorferi strain N40 (clone D10/E9). Patterns of inhibition of N40 binding to both cell lines by different proteoglycans were remarkably similar: heparin, heparan sulfate, and dermatan sulfate showed better inhibitory activity than chondroitin-4-sulfate or chondroitin-6-sulfate (Fig. 1A). As previously shown (29), Vero cell binding was inhibited by digestion of the monolayer with heparinase, which cleaves heparin-related glycosaminoglycans, or with heparitinase, which cleaves heparan sulfate proteoglycans (Fig. 1B). Chondroitinase ABC, which cleaves chondroitin-4-sulfate, dermatan sulfate, and chondroitin-6-sulfate, had no effect on binding to this cell line. In contrast, parallel digestions with heparinase or heparitinase had no significant effect on N40 binding to 293 cells, while chondroitinase ABC digestion inhibited attachment by more than 60% (Fig. 1B). These results indicate that glycosaminoglycans play an important role in recognition of both cell lines by B. burgdorferi, but that the specific class of proteoglycans that plays the major role in bacterial attachment varies with host cell.
FIG. 1.
Attachment of B. burgdorferi to Vero cells is sensitive to heparinase or heparitinase digestion, while attachment to 293 cells is sensitive to chondroitinase ABC digestion. Bacterial attachment of the infectious B. burgdorferi strain N40, clone D10/E9, to confluent monolayers was determined as described in Materials and Methods. (A) Attachment of N40 to Vero cells or 293 cells was determined (from left to right) in the absence of inhibitor or in the presence of 500 μg of chondroitin-6-sulfate, chondroitin-4-sulfate, heparin, heparan sulfate, or dermatan sulfate per ml. (B) Bacterial attachment was quantitated (from left to right) after no treatment or after a 2-h treatment of the monolayers with 0.5 U of heparinase, heparitinase, or chondroitinase ABC per ml.
Binding of B. burgdorferi to heparan sulfate on endothelial cells.
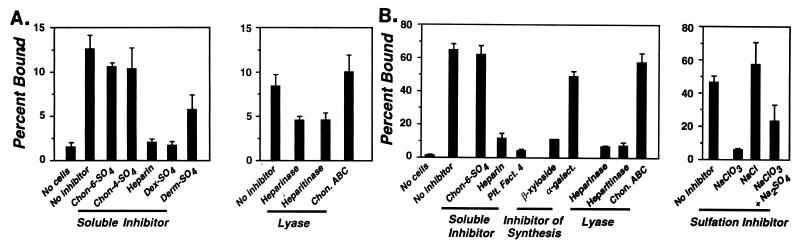
To determine which, if any, class of glycosaminoglycan might play a role in bacterial interactions with the endothelium, we investigated bacterial binding to both primary bovine capillary endothelial cells and to a human endothelial cell line. Binding of N40 to primary endothelial cells was almost completely inhibited by heparin or dextran sulfate, partially inhibited by dermatan sulfate, but only minimally inhibited by chondroitin-4-sulfate or chondroitin-6-sulfate (Fig. 2A, Soluble Inhibitor). Pretreatment of the cells with heparinase or heparitinase diminished N40 binding by 50%, whereas digestion with chondroitinase ABC had no effect (Fig. 2A, Lyase).
FIG. 2.
Heparin/heparan sulfate proteoglycans contribute to binding of B. burgdorferi to endothelial cells. Bacterial attachment by the infectious B. burgdorferi strain N40 to endothelial cell monolayers was determined as described in Materials and Methods. “No cells” denotes attachment to wells without cells; “No inhibitor” denotes binding to endothelial cells in the absence of inhibitor. Inhibitors tested included chondroitin-6-sulfate (Chon-6-SO4), chondroitin-4-sulfate (Chon-4-SO4), heparin, dextran sulfate (Dex-SO4), dermatan sulfate (Derm-SO4), and platelet factor 4 (Plt. Fact. 4). “Lyase” indicates the degree of binding after a 2-h incubation with 0.5 U of heparinase, heparitinase, or chondroitinase ABC (Chon. ABC) per ml. (A) Bacterial attachment to primary capillary endothelial cells. Inhibitors were tested at a concentration of 20 μg/ml. (B) Bacterial attachment to EA-Hy926, an endothelial cell line. Chondroitin-6-sulfate and heparin were tested at a concentration of 10 μg/ml, and platelet factor 4 was tested at 5 μg/ml. “Inhibitor of Synthesis” denotes binding to cells treated overnight with p-nitrophenyl-β-d-xyloside, an inhibitor of glycosaminoglycan attachment to the protein core (26), or treatment with the control sugar, p-nitrophenyl-α-d-galactoside, each at 5 mM. “Sulfation Inhibitor” indicates treatment with chlorate, an inhibitor of host cell sulfation of proteoglycans (1). Monolayers were cultured overnight in medium supplemented with either 30 mM sodium chlorate, 30 mM sodium chloride, or 30 mM sodium chlorate plus 30 mM sodium sulfate.
EA-Hy926 is a human endothelial cell line that expresses a wide range of differentiated endothelial cell markers (11, 12). N40 attachment to this cell line was inhibited by platelet factor 4, a chemokine that binds to glycosaminoglycans, and by heparin but not by chondroitin-6-sulfate (Fig. 2B, Soluble Inhibitor). Bacterial binding was blocked by pretreatment of EA-Hy926 cells with β-d-xyloside, which inhibits linkage of the heparin/heparan sulfate and dermatan/chondroitin sulfate chains to the protein core of proteoglycans (26). Pretreatment with a control sugar, α-d-galactoside, had no effect (Fig. 2B, Inhibitor of Synthesis). Sulfation is apparently required for attachment of B. burgdorferi to EA-Hy926 cells, because pretreatment of EA-Hy926 cells with chlorate, an inhibitor of proteoglycan sulfation (1), reduced N40 attachment by almost 90% (Fig. 2B, Inhibitor of Sulfation). This effect was specific for chlorate: chloride treatment had no effect, and the addition of sulfate along with chlorate partially reversed the inhibition of binding. Pretreatment of EA-Hy926 cells with heparinase or heparitinase reduced binding by 90%, while chondroitinase ABC treatment had little effect (Fig. 2B, Lyase). Taken together with the analysis of primary endothelial cells, these experiments provide evidence that proteoglycans are critical in the attachment of B. burgdorferi to endothelial cells and that heparin/heparan sulfate in particular play an important role in this recognition process. In contrast, there was no evidence that chondroitin or dermatan sulfate proteoglycans were required for efficient bacterial binding to endothelial cells.
B. burgdorferi binding to heparinase- and chondroitinase-sensitive proteoglycans expressed by cultured brain cells.
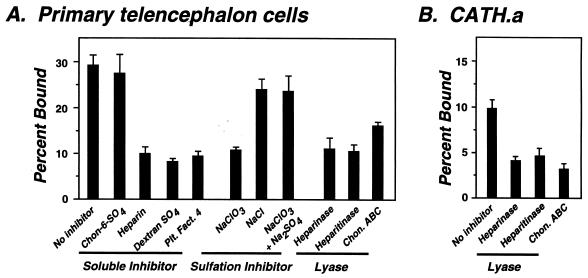
To investigate the interaction of B. burgdorferi with a second potential target tissue, a glial cell-enriched population of primary mixed telencephalic brain cells was cultured from neonatal rats (43). Heparin, dextran sulfate, and platelet factor 4 reduced N40 binding, as did inhibition of sulfation by pretreatment with chlorate (Fig. 3A). Digestion of cultured telencephalic cells with heparinase or heparitinase diminished bacterial attachment by about 60% (Fig. 3A, Lyase), indicating that heparan sulfate proteoglycans expressed by telencephalic cells contribute to bacterial binding, as was the case for binding to endothelial cells. In contrast to the results with endothelial cells however, chondroitinase ABC digestion of telencephalic cells inhibited N40 attachment by about 45%. These results suggest that for this population of neural cells, chondroitinase-sensitive as well as heparinase-sensitive proteoglycans contribute to bacterial recognition.
FIG. 3.
Attachment of B. burgdorferi to neural cells is mediated by both heparinase- and chondroitinase ABC-sensitive proteoglycans. Attachment of N40 to primary rat telencephalic cells (43) (A) or the mouse midbrain neuronal cell line CATH.a (41) (B) was assessed in assays using exogenous proteoglycans, platelet factor 4, chlorate inhibition of proteoglycan sulfation, or lyase digestion of glycosaminoglycans as described in the legend to Fig. 1.
To determine which species of proteoglycans contribute to B. burgdorferi attachment to neuronal cells, we assayed the effect of lyase digestion on bacterial binding to the catacholaminergic neuron-derived CATH.a cells (41). Heparinase, heparitinase, and chondroitinase ABC digestion of CATH.a cells each resulted in a significant inhibition of N40 attachment (Fig. 3B), indicating that a chondroitinase-sensitive component of CATH.a cells promotes attachment of B. burgdorferi. Lyase digestion of the pheochromocytoma cell line, PC12, gave similar results (data not shown). Thus, in this sampling of two neuronal cell lines and central nervous system-derived primary neurons and glia, chondroitinase ABC-sensitive proteoglycans contributed to B. burgdorferi attachment.
Dermatan sulfate inhibits bacterial binding to immobilized heparin, and heparin inhibits binding to immobilized dermatan sulfate.
The analysis of lyase-treated Vero and 293 cells (Fig. 1B) is consistent with the suggestion that heparin/heparan sulfate mediates B. burgdorferi attachment to Vero cells, while a chondroitinase ABC-sensitive glycosaminoglycan, e.g., dermatan sulfate, mediates attachment to 293 cells. The observed cell-specific difference in the species of glycosaminoglycans recognized by B. burgdorferi could be due to the expression of a single glycosaminoglycan-binding molecule that recognizes both heparan sulfate and dermatan sulfate. Alternatively, the spirochete could utilize two independent mechanisms, one recognizing heparin/heparan sulfate and the other recognizing dermatan sulfate. In the latter case, one might expect that Vero cell binding would be inhibited most efficiently by heparin, whereas dermatan sulfate would be the more effective inhibitor of attachment to 293 cells. Heparin, dermatan sulfate, and chondroitin-6-sulfate were titrated as inhibitors of strain N40 binding to Vero or 293 cells. Both heparin and dermatan sulfate blocked binding to both cell lines, and regardless of cell line, heparin was the best inhibitor of N40 attachment, with a 50% inhibitory concentration (IC50) 4- to 45-fold lower than that for dermatan sulfate (Table 1).
TABLE 1.
Comparison of heparin and dermatan sulfate as inhibitors of bacterial attachment to immobilized proteoglycans or mammalian cells
| Inhibitor | IC50 (μg/ml)a
|
|||
|---|---|---|---|---|
| Vero cells | 293 cells | Heparin | Dermatan sulfate | |
| Heparin | 8.0 | 1.3 | 0.2 | 0.5 |
| Dermatan sulfate | 32 | 60 | 0.6 | 3.2 |
| Chondroitin-6-sulfate | >500 | >500 | >500 | 18 |
Bacterial attachment of radiolabeled B. burgdorferi N40 to mammalian cells or immobilized proteoglycans was determined in the presence of various concentrations of heparin, dermatan sulfate, or chondroitin-6-sulfate (see Materials and Methods). At the concentration of inhibitor indicated, bacterial attachment was 50% of the level of binding in the absence of inhibitor (Fig. 4).
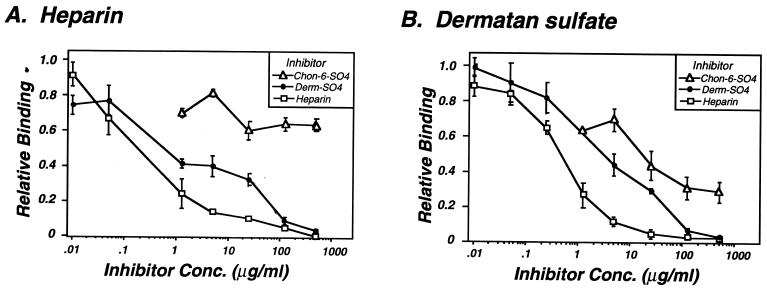
Because intact mammalian cells present a complex mixture of potential receptors for B. burgdorferi, we also determined whether bacterial binding to immobilized heparin could be inhibited by dermatan sulfate and, conversely, whether binding to immobilized dermatan sulfate could be inhibited by heparin. Each proteoglycan inhibited binding of B. burgdorferi N40 to the other (Fig. 4). Heparin was the more potent inhibitor regardless of which glycosaminoglycan was immobilized, with an IC50 three- to sixfold lower than that for dermatan sulfate (Table 1). Chondroitin-6-sulfate showed some inhibitory activity but was the poorest inhibitor of the three tested. That heparin was the most potent inhibitor of B. burgdorferi N40 attachment to purified heparin, dermatan sulfate, Vero cells, or 293 cells is consistent with the hypothesis that a single glycosaminoglycan-binding pathway recognizes multiple species of glycosaminoglycans.
FIG. 4.
Inhibition of bacterial attachment to dermatan sulfate by heparin and to heparin by dermatan sulfate. Bacterial attachment of B. burgdorferi N40 to immobilized heparin or dermatan sulfate was determined in the presence of various concentrations of exogenous proteoglycan. Binding is expressed relative to the level of binding in the absence of inhibitor. (A) B. burgdorferi N40 attachment to immobilized heparin sulfate; (B) N40 attachment to immobilized dermatan sulfate.
DISCUSSION
Given that endothelial damage is one of the hallmarks of Lyme disease (2, 10) and that neurologic manifestations are a prominent feature of this illness (30), it is likely that the interactions of B. burgdorferi with endothelial cells and cells in the nervous system play an important role in vivo. We analyzed bacterial attachment to primary endothelial cells, primary central nervous system-derived glial cells, and cell lines of endothelial or neural origin and found that binding of B. burgdorferi to all of these cells was mediated by proteoglycans. Binding to all cells tested was inhibited by the addition of heparin, heparan sulfate, or dermatan sulfate and by platelet factor 4, a chemokine that binds glycosaminoglycans. In addition, cell attachment was diminished by pretreatment of these cells with an inhibitor of proteoglycan synthesis (β-d-xyloside) or sulfation (sodium chlorate) or by digestion of cell surface glycosaminoglycans with lyases. Given the widespread expression of proteoglycans, it is likely that proteoglycan binding by the spirochete contributes to the recognition of other cell types as well.
Although glycosaminoglycans mediate attachment to many cell types, removal of specific classes of glycosaminoglycans with lyases indicated that the particular populations of glycosaminoglycans that contribute to spirochetal attachment vary with cell type. Heparin/heparan sulfate appeared to play the most critical role for spirochetal binding to primary endothelial cells, the EA-Hy926 endothelial cell line, and Vero cells. A chondroitinase ABC-sensitive glycosaminoglycan, presumably dermatan sulfate, mediated attachment to 293 cells. Binding to primary telencephalon cells and two neuronal cell lines appeared to be mediated by both heparin/heparan sulfate and dermatan sulfate. It was previously shown that binding of B. burgdorferi 297 to HeLa cells was inhibited by digestion with either heparinase or chondroitinase ABC (23). Of the glycosaminoglycans that are substrates for chondroitinase ABC (chondroitin-4-sulfate, chondroitin-6-sulfate, and dermatan sulfate), only dermatan sulfate is a potent inhibitor of bacterial attachment to mammalian cells. Thus, it is likely that the chondroitinase ABC-sensitive glycosaminoglycan that is critical for B. burgdorferi recognition is dermatan sulfate. Consistent with this hypothesis, chondroitinase AC, which does not cleave dermatan sulfate, had no effect on B. burgdorferi attachment to HeLa cells (23).
At the present time, we do not know whether the relative importance of a given species of glycosaminoglycan in bacterial binding to a particular cell type reflects its relative affinity for the bacterium, its abundance on the cell surface, or both. Heparan sulfate and chondroitin sulfate are expressed to a variable extent on virtually all cells, while dermatan sulfate is less common (27). Although dermatan sulfate did not appear to participate in bacterial binding to endothelial cells in this study, cultured bovine aortic endothelial cells have been shown to express this glycosaminoglycan (35). Dermatan sulfate appeared to promote bacterial attachment to cultured rat brain cells, even though it is apparently poorly expressed in rat brain (21, 33). It is difficult, however, to directly compare these previous studies to our results, because proteoglycan expression can vary considerably with culture conditions. For this reason, multiple representatives of both endothelial and neural cells were evaluated in this study.
The recognition of multiple classes of proteoglycans by B. burgdorferi could reflect the expression of several proteoglycan receptors, or the expression of a single receptor that recognizes different species of glycosaminoglycans. We found no evidence for independent mechanisms for binding dermatan sulfate and heparin by strain N40: heparin was a better inhibitor than dermatan sulfate, regardless of whether the substrate was heparin, dermatan sulfate, Vero cells, or 293 cells. Previous results demonstrating that 100 μg of heparan sulfate or dermatan sulfate per ml partially (46 or 59%, respectively) inhibits heparin binding by B. burgdorferi also suggest a promiscuous glycosaminoglycan-binding pathway that binds heparin with the highest affinity (23). It is not uncommon for glycosaminoglycan-binding receptors to recognize multiple species of glycosaminoglycans, because the polyanionic nature of these molecules is a critical determinant in these interactions (27).
Although our results are consistent with a single proteoglycan-binding mechanism, we cannot rule out the possibility that binding of one proteoglycan to the surface of the spirochete can inhibit attachment to other molecules nonspecifically, e.g., by steric hindrance, or by conferring a strong negative charge to the bacterial surface. Guo et al. showed that binding of B. burgdorferi B31 to decorin, a dermatan sulfate/chondroitin sulfate proteoglycan, was not inhibited by 10 μg of heparin per ml, whereas the same concentration of exogenous decorin blocked binding (19). This finding could indicate that the decorin binding and heparin binding are mediated by different bacterial molecules. Alternatively, the specificity of proteoglycan binding varies somewhat among strains of Lyme disease spirochete (35a), and strain B31 may express a proteoglycan-binding receptor that binds to decorin with much higher affinity than it does to heparin. Resolution of the question of one versus multiple proteoglycan-binding pathways awaits further characterization of the bacterial molecules that mediate the varied interactions that have been described to date.
While it is clear that proteoglycan recognition promotes bacterial attachment to a wide variety of cells, these results do not preclude the involvement of other host molecules, and the degree of residual binding upon inhibition of the proteoglycan pathway may reflect the activity of additional binding pathways. For example, integrins contribute to bacterial attachment to platelets (5) and other cell types (6), while galactocerebroside promotes binding to Schwann cells (17). Glycosaminoglycans often act in concert with other cell surface receptors to promote ligand binding (22, 38, 45), and attachment to proteoglycans by the Lyme disease spirochete could facilitate binding to other classes of molecules. The precise sequence of events that occur during bacterial attachment to host cells, as well as the way in which this interaction may promote colonization of specific tissues, will be the subject of future investigations.
ACKNOWLEDGMENTS
We thank Judah Folkman, Catherine Butterfield, and Marsha Moses for providing primary capillary endothelial cells, Bis Lahiri for consultation and for heparan sulfate proteoglycan, and Allen Steere for supporting part of this work. Dona Chikaraishi provided CATH.a cells, and Cora-Jean Edgell provided EA-Hy926 cells. We received helpful advice and discussion from Eduardo Ortega-Barria, Robert Kokenyesi, Louis Rosenfeld, and Meircio Pereira.
This work was supported by NIH grant R01-AI 37601-01 awarded to J.M.L., by NIH grant R01-MH 44694 awarded to J.B.T., and by the Center for Gastroenterology Research on Absorptive and Secretory Processes, PHS grant 1 P30DK39428 awarded by NIDDK. H.W. and J.C. were supported by an NIH training grant AR-07570. P.E.M. received a summer medical student fellowship from the American College of Rheumatology. J.M.L. was a Pew Scholar in the Biomedical Sciences, and J.C. was a Genentech Fellow of the Life Sciences Research Foundation and later received support in part from a grant from the Lincoln National Foundation of Fort Wayne, Ind., and from the English, Bonter, Mitchell Foundation of Fort Wayne, Ind.
REFERENCES
- 1.Baeuerle P A, Huttner W B. Chlorate: a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986;141:870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- 2.Barthold S W, de Souze M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 3.Beachey E H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Bloch C A, Orndorff P E. Impaired colonization by and full invasiveness of Escherichia coli K1 bearing a site-directed mutation in the type 1 pilin gene. Infect Immun. 1990;58:275–278. doi: 10.1128/iai.58.1.275-278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn J, Leong J M, Erban J. Integrin αIIbβ3 mediates binding of the Lyme disease agent, Borrelia burgdorferi, to human platelets. Proc Natl Acad Sci USA. 1993;90:7058–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coburn, J., L. Magoun, S. C. Bodary, and J. M. Leong. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 7.Coleman J L, Sellati T J, Testa J E, Kew R R, Furie M B, Benach J L. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comstock L E, Thomas D D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989;57:1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorward, D. W., E. R. Fischer, and D. M. Brooks. 1997. Invasion and cytopathic killing of human lymphocytes by spirochetes causing Lyme disease. Clin. Infect. Dis. 25(Suppl. 1):S2–S8. [DOI] [PubMed]
- 10.Duray P H. The surgical pathology of human Lyme disease. An enlarging picture. Am J Surg Pathol. 1987;11:47–60. doi: 10.1097/00000478-198700111-00005. [DOI] [PubMed] [Google Scholar]
- 11.Edgell C J, Haizlip J E, Bagnell C R, Packenham J P, Harrison P, Wilbourn B, Madden V J. Endothelium specific Weibel-Palade bodies in a continuous human cell line, EA.hy926. In Vitro Cell Dev Biol. 1990;26:1167–1172. doi: 10.1007/BF02623694. [DOI] [PubMed] [Google Scholar]
- 12.Edgell C J, McDonald C C, Graham J B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folkman J, Haudenschild C C, Zetter B R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci USA. 1979;76:5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbe J L, Guy E, Zapatero J M, Peerschke E I, Benach J L. Vascular clearance of Borrelia burgdorferi in rats. Microb Pathog. 1993;14:187–201. doi: 10.1006/mpat.1993.1019. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Monco J C, Benach J L. Lyme neuroborreliosis. Ann Neurol. 1995;37:691–702. doi: 10.1002/ana.410370602. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Monco J C, Fernandez-Villar B, Benach J L. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989;160:497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Monco J C, Fernandez-Villar B, Rogers R C, Szczepanski A, Wheeler C M, Benach J L. Borrelia burgdorferi and other related spirochetes bind to galactocerebroside. Neurology. 1992;42:1341–1348. doi: 10.1212/wnl.42.7.1341. [DOI] [PubMed] [Google Scholar]
- 18.Guimond S, Maccarana M, Olwin B, Lindahl U, Rapraeger A. Activating and inhibitory heparin sequences for FGF-2 (basic FGF) J Biol Chem. 1993;268:23906–23914. [PubMed] [Google Scholar]
- 19.Guo B P, Norris S J, Rosenberg L C, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hechemy K E, Samsonoff W A, Harris H L, McKee M. Adherence and entry of Borrelia burgdorferi in Vero cells. J Med Microbiol. 1992;36:229–238. doi: 10.1099/00222615-36-4-229. [DOI] [PubMed] [Google Scholar]
- 21.Herndon M E, Lander A D. A diverse set of developmentally regulated proteoglycans is expressed in the rat central nervous system. Neuron. 1990;4:949–961. doi: 10.1016/0896-6273(90)90148-9. [DOI] [PubMed] [Google Scholar]
- 22.Herold B, WuDunn D, Soltys N, Spear P. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isaacs R. Borrelia burgdorferi bind to epithelial cell proteoglycan. J Clin Invest. 1994;93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isberg R R, Leong J M. Cultured mammalian cells attach to the invasin protein of Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 1988;85:6682–6686. doi: 10.1073/pnas.85.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalish R A. Lyme disease. Rheum Dis Clin North Am. 1993;19:399–426. [PubMed] [Google Scholar]
- 26.Kato Y, Kimata K, Ito K, Karasawa K, Suzuki S. Effect of β-d-xyloside and cycloheximide on the synthesis of two types of proteochondroitin sulfate in chick embryo cartilage. J Biol Chem. 1978;253:2784–2789. [PubMed] [Google Scholar]
- 27.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 28.Klempner M S, Noring R, Rogers R A. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1993;167:1074–1081. doi: 10.1093/infdis/167.5.1074. [DOI] [PubMed] [Google Scholar]
- 29.Leong J, Morrissey P, Ortega-Barria E, Pereira M, Coburn J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:874–883. doi: 10.1128/iai.63.3.874-883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logigian E L, Kaplan R F, Steere A C. Chronic neurologic manifestations of Lyme disease. N Engl J Med. 1990;323:1438–1444. doi: 10.1056/NEJM199011223232102. [DOI] [PubMed] [Google Scholar]
- 31.Loscalzo J, Melnick B, Handin R. The interaction of platelet factor four and glycosaminoglycans. Arch Biochem Biophys. 1985;240:446–455. doi: 10.1016/0003-9861(85)90049-9. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Sturrock A, Weis J J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun. 1991;59:671–678. doi: 10.1128/iai.59.2.671-678.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolis R U, Margolis R K. Nervous tissue proteoglycans. Dev Neurosci. 1989;11:276–288. doi: 10.1159/000111906. . (Review.) [DOI] [PubMed] [Google Scholar]
- 34.Nocton J, Dressler F, Rutledge B, Rys P, Persing D, Steere A. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–283. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 35.Oohira A, Wight T N, Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983;258:2014–2021. [PubMed] [Google Scholar]
- 35a.Parveen, N., and J. Leong. Unpublished observations.
- 36.Philipp M, Johnson B. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 1994;2:431–436. doi: 10.1016/0966-842x(94)90800-1. [DOI] [PubMed] [Google Scholar]
- 37.Preac-Mursic V, Wilske B, Schierz G. European Borrelia burgdorferi isolated from humans and ticks: culture conditions and antibiotic susceptibility. Zentralbl Bakteriol Hyg Reihe A. 1986;263:112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- 38.Rapraeger A, Krufka A, Olwin B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 39.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 41.Suri C, Fung B P, Tischler A S, Chikaraishi D M. Catecholaminergic cell lines from the brain and adrenal glands of tyrosine hydroxylase-SV40 T antigen transgenic mice. J Neurosci. 1993;13:1280–1291. doi: 10.1523/JNEUROSCI.13-03-01280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szczepanski A, Furie M B, Benach J L, Lane B P, Fleit H B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990;85:1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tatro J B, Romero L I, Beasley D, Steere A C, Reichlin S. Borrelia burgdorferi and Escherichia coli lipopolysaccharides induce nitric oxide and interleukin-6 production in cultured rat brain cells. J Infect Dis. 1994;169:1014–1022. doi: 10.1093/infdis/169.5.1014. [DOI] [PubMed] [Google Scholar]
- 44.Thomas D D, Comstock L E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989;57:1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yayon A, Klagsbrun M, Esko J, Leder P, Ornitz D. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]