Introduction
Maintaining the functional integrity of respiratory muscles is crucial for critically ill patients to be weaned from mechanical ventilation and decannulated if tracheotomized. Dysfunction of the diaphragm is frequent in patients under mechanical ventilation and has been associated with a longer duration of weaning.[1], [2] Studies on expiratory muscles are scarce. Two recent studies demonstrated the feasibility and reproducibility of ultrasound in measuring the thickness of abdominal muscles,[3], [4] with one study showing abdominal muscle atrophy in 22% of mechanically ventilated critically ill patients during the first week of mechanical ventilation.[4]
We are unaware, however, of the modifications of trophicity in patients with prolonged ventilation and their impact on weaning. We report the case of a patient with coronavirus disease 2019 (COVID-19) related acute respiratory distress syndrome (ARDS), which led to prolonged mechanical ventilation and dependence on tracheotomy and massive but reversible atrophy of abdominal muscles.
Case report
A 25-year-old man was admitted with hypoxemic acute respiratory failure following a COVID-19 infection. After the failure of high-flow nasal oxygen to correct hypoxemia, the patient was intubated several hours after the admission. The initial days were characterized by severe ARDS, requiring neuromuscular blockade, prone positioning, and inhaled nitric oxide. The thickness of abdominal muscles at end-inspiration and diaphragm at end-expiration measured by ultrasound on days 1 and 7 of mechanical ventilation are reported in Table 1. On day 7, the patient developed life-threatening hypoxemia with a partial pressure of oxygen (PaO2) /fraction of inspired oxygen (FiO2) ratio at 60 mmHg on 100% FIO2, leading to implement extracorporeal membrane oxygenation (ECMO). High-dose corticosteroid treatment was added on day 12 due to poor compliance and difficulties in optimizing oxygenation despite ECMO. COVID-associated pulmonary aspergillosis and plurimicrobial nosocomial pneumonia were documented and treated. The condition of the patient improved progressively, enabling the discontinuation of sedation: revealing severe acquired weakness with tetraparesis.
Table 1.
Patient data of muscle thickness and MRC score.
| Measurement | Day 1 | Day 7 | Day 48 | Day 89 |
|---|---|---|---|---|
| Intubation | Weaning from MV | Decannulation | ||
| Rectus abdominis (mm) | 9.5 | 8.5 | 4.7 | 10.3 |
| External oblique (mm) | 4.4 | 4.8 | 2.1 | 3.4 |
| Internal oblique (mm) | 5.3 | 5.1 | 4 | 4 |
| Transversus abdominis (mm) | 2.5 | 2.1 | 1 | 2.6 |
| CTAM (mm) | 21.7 | 20.5 | 11.8 | 20.3 |
| Diaphragm (mm) | 2.3 | 2.2 | 1.3 | 2 |
| MRC score | NA | NA | 24 | 56 |
CTAM: Cumulated thickness of abdominal muscles; MRC: Medical Research Council; MV: Mechanical ventilation; NA: Not available due to sedation.
A tracheotomy was performed on day 40, the first spontaneous breathing separation was performed on day 46, and weaning from mechanical ventilation was completed after 48 h. Decannulation, however, was then impossible: cough effort was absent and immediate sputum retention have occurred when the cuff was deflated. Measuring thickness revealed a profound atrophy of all four abdominal muscles (Table 1), notably of the rectus abdominis (Figure 1). Diaphragm thickness was also reduced. Concomitant severe limb weakness persisted with a Medical Research Council (MRC) score of 24.
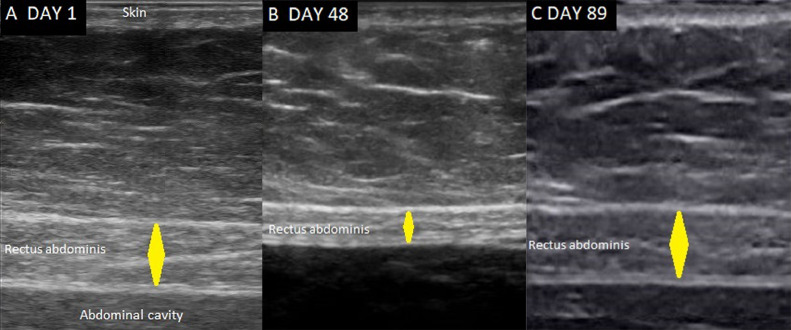
Figure 1.
Ultrasound images of the rectus abdominis muscle. The muscle thickness (yellow diamond) was measured at the end-inspiration and perpendicular to the inner side of the muscle fasciae. A: on the first day of mechanical ventilation; B: on day 48, when the patient was weaned from mechanical ventilation but decannulation was unsuccessful; C: on day 89, when decannulation was successful. The probe is located approximately 2 cm above the umbilicus and 2–3 cm lateral from the midline.
The patient was discharged to a rehabilitation unit, where progressive improvement in limb muscular strength was observed. On day 89, the MRC score was 56, his cough was efficient, and the decannulation was successful. Additionally, the thickness of the abdominal muscles (Figure 1) and diaphragm increased—the cumulative thickness of the four abdominal muscles was similar to values on day 1 of admission (Table 1).
Discussion
The efficiency of cough and airway clearance is crucial for successful extubation,[5], [6] especially after prolonged mechanical ventilation.[7] In tracheotomized patients, the effectiveness of cough and swallowing function is usually required as a prerequisite for decannulation in patients weaned from mechanical ventilation.[8], [9] In this case, cough strength and the ability to manage airway secretions seemed closely related to the trophicity of respiratory muscles—diaphragm and abdominal muscles—since efficient coughing that allowed successful decannulation was only observed when trophicity had recovered. Abdominal muscles are crucial in generating expiratory pressures and flows required for an effective cough. The link between abdominal muscle atrophy and cough weakness is likely since the cumulated thickness of abdominal muscles has been correlated with their ability to generate strength, as measured by gastric pressure.[10] Diaphragm atrophy may have contributed to the weakness of the cough since maximal inspiratory pressure and capacity also correlate with cough strength by the magnitude of inspired volume.[11], [12] However, persistent diaphragm dysfunction is frequent at the time of extubation[13] and is not associated with extubation failure.[13], [14]
Abdominal muscle atrophy, defined by a decrease in muscle thickness by at least 15%, was documented using ultrasound in the first days of mechanical ventilation[4] and was moderate. In this case, there was no significant reduction in abdominal muscle thickness on day 7. However, atrophy was profound on day 48, with a reduction in cumulative thickness of 50%. This degree of atrophy was much more severe than atrophy observed in chronic tetraplegic patients after cervical spinal cord injury.[10] Nevertheless, the atrophy presented in this report was reversible to complete concomitant with the recovery of the trophicity of the diaphragm and improvement in limb muscular strength. This observation is likely exceptional due to the severity of the disease. Moreover, we did not evaluate other factors involved in cough strength and airway clearance, such as intercostal muscles and glottis function.
Measuring abdominal muscle thickness using ultrasound may be routinely performed at the bedside, the rectus abdominis being the easier to visualize (Figure 1). When confronted with a weak cough and difficult extubation or decannulation, ultrasound allows the detection of abdominal muscle atrophy, which may explain expiratory muscle weakness and impaired cough strength.
Conclusions
Patients under prolonged mechanical ventilation may exhibit profound atrophy of abdominal muscles. This may contribute to severe impairment of cough strength, leading to delayed tracheostomy removal.
Author Contributions
Pascal Beuret: Conceptualization, Investigation, Writing-original draft, Visualization, Editing, Validation. Florian Michelin: Investigation, Review, Validation. Audrey Tientcheu, Laurane Chalvet, Benedicte Philippon-Jouve, Jean-Charles Chakarian, Xavier Fabre: Review, Validation.
Acknowledgments
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics Statement
The informed consent was obtained from the patient for publish this case report.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Managing Editor: Jingling Bao
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Dres M., Demoule A. Diaphragm dysfunction during weaning from mechanical ventilation: an underestimated phenomenon with clinical implications. Crit Care. 2018;22(1):73. doi: 10.1186/s13054-018-1992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haaksma M.E., Smit J.M., Boussuges A., Demoule A., Dres M., Ferrari G., et al. EXpert consensus On Diaphragm UltraSonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit Care. 2022;26(1):99. doi: 10.1186/s13054-022-03975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber A.F., Bertoni M., Coiffard B., Fard S., Wong J., Reid W.D., et al. Abdominal muscle use during spontaneous breathing and cough in patients who are mechanically ventilated: a Bi-center Ultrasound Study. Chest. 2021;160(4):1316–1325. doi: 10.1016/j.chest.2021.05.053. [DOI] [PubMed] [Google Scholar]
- 4.Shi Z.H., de Vries H., de Grooth H.J., Jonkman A.H., Zhang Y., Haaksma M., et al. Changes in respiratory muscle thickness during mechanical ventilation: focus on expiratory muscles. Anesthesiology. 2021;134(5):748–759. doi: 10.1097/ALN.0000000000003736. [DOI] [PubMed] [Google Scholar]
- 5.Thille A.W., Boissier F., Muller M., Levrat A., Bourdin G., Rosselli S., et al. Role of ICU-acquired weakness on extubation outcome among patients at high risk of reintubation. Crit Care. 2020;24(1):86. doi: 10.1186/s13054-020-2807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan J., Zhang X., Song J. Predictive power of extubation failure diagnosed by cough strength: a systematic review and meta-analysis. Crit Care. 2021;25(1):357. doi: 10.1186/s13054-021-03781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C.T., Yu C.J. Conventional weaning parameters do not predict extubation outcome in intubated subjects requiring prolonged mechanical ventilation. Respir Care. 2013;58(8):1307–1314. doi: 10.4187/respcare.01773. [DOI] [PubMed] [Google Scholar]
- 8.Ceriana P., Carlucci A., Navalesi P., Rampulla C., Delmastro M., Piaggi G., et al. Weaning from tracheotomy in long-term mechanically ventilated patients: feasibility of a decisional flowchart and clinical outcome. Intensive Care Med. 2003;29(5):845–848. doi: 10.1007/s00134-003-1689-z. [DOI] [PubMed] [Google Scholar]
- 9.Stelfox H.T., Crimi C., Berra L., Noto A., Schmidt U., Bigatello L.M., et al. Determinants of tracheostomy decannulation: an international survey. Crit Care. 2008;12(1):R26. doi: 10.1186/cc6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estenne M., Pinet C., De Troyer A. Abdominal muscle strength in patients with tetraplegia. Am J Respir Crit Care Med. 2000;161(3 Pt 1):707–712. doi: 10.1164/ajrccm.161.3.9906020. [DOI] [PubMed] [Google Scholar]
- 11.Park J.H., Kang S.W., Lee S.C., Choi W.A., Kim D.H. How respiratory muscle strength correlates with cough capacity in patients with respiratory muscle weakness. Yonsei Med J. 2010;51(3):392–397. doi: 10.3349/ymj.2010.51.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trebbia G., Lacombe M., Fermanian C., Falaize L., Lejaille M., Louis A., et al. Cough determinants in patients with neuromuscular disease. Respir Physiol Neurobiol. 2005;146(2–3):291–300. doi: 10.1016/j.resp.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Vivier E., Muller M., Putegnat J.B., Steyer J., Barrau S., Boissier F., et al. Inability of diaphragm ultrasound to predict extubation failure: a Multicenter Study. Chest. 2019;155(6):1131–1139. doi: 10.1016/j.chest.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Jung B., Moury P.H., Mahul M., de Jong A., Galia F., Prades A., et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016;42(5):853–861. doi: 10.1007/s00134-015-4125-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.