Abstract
Dengue fever is considered the most prolific vector-borne disease in the world, with its transmission rate increasing more than eight times in the last two decades. While most cases present mild to moderate symptoms, 5% of patients can develop severe disease. Although the mechanisms are yet not fully comprehended, immune-mediated activation leading to excessive cytokine expression is suggested as a cause of the two main findings in critical patients: increased vascular permeability that may shock and thrombocytopenia, and coagulopathy that can induce hemorrhage. The risk factors of severe disease include previous infection by a different serotype, specific genotypes associated with more efficient replication, certain genetic polymorphisms, and comorbidities such as diabetes, obesity, and cardiovascular disease. The World Health Organization recommends careful monitoring and prompt hospitalization of patients with warning signs or propensity for severe disease to reduce mortality. This review aims to update the diagnosis and management of patients with severe dengue in the intensive care unit.
Keywords: Severe dengue, Intensive care units, Shock, Pathogenesis, Diagnosis, Management
Introduction
Dengue, transmitted by the bite of an infected Aedes spp. mosquito, is the most prevalent vector-borne disease in the world.[1] According to the World Health Organization (WHO), 3.9 billion people in 129 countries are at risk of infection, which is more than half of the world's population.[2] Simulations estimate that over 390 million infections occur yearly, 96 million of which manifest clinically.[3] The reported cases have increased by eight times in the last two decades, with the biggest reported epidemic in history occurring in 2019,[2] with over 56 million cases and 36,000 deaths registered.[4] Severe cases occur in approximately 500,000 people per year, with a mortality rate of 10% among hospitalized patients.[5] However, these deaths could be reduced to less than 1% by implementing early diagnosis and treatment based on warning signs.[6]
Climate changes are predicted to exacerbate the problem and increase the population at risk to 6.1 billion people by 2080 (approximately 60% of the world population).[7] Global warming, which is predicted to increase the Earth's temperature by 2.5–2.9 °C by the end of the century,[8] will cause an increase in transmission in current endemic areas and expand risk areas, leading to an accelerated spread of vectors and expanding all arbovirus infection, such as chikungunya, Zika, and yellow fever.[[9], [10], [11]] Moreover, studies have shown that after being introduced in a specific area, Aedes spp. can genetically mutate to maintain transmission rates in lower temperatures.[12,13]
The Coronavirus disease 2019 (COVID-19) pandemic has presented a new challenge in managing dengue fever in endemic regions. In 2020, the first wave of COVID-19 hit these countries together with a hyperendemic outbreak of dengue, overburdening already crowded healthcare systems.[[14], [15], [16]] Additionally, an overlap of symptoms is common between these two diseases, such as fever, headache, and myalgia, as well as laboratory findings such as thrombocytopenia, leukopenia with lymphopenia, and elevated transaminases, leading to delay and misdiagnosis.[[17], [18], [19], [20], [21]]
Despite the considerable advances in understanding the pathophysiology of the disease, dengue remains a significant challenge to doctors worldwide due to its lack of specific treatment.[22] Therefore, improving the management of dengue and severe dengue in intensive care units (ICUs) is crucial to reduce morbidity and mortality. This paper aims to elucidate the physiology of severe dengue, update the diagnosis and classification of dengue, and review critical patient management.
Pathophysiology
The virus
Dengue virus (DENV) is a Flavivirus belonging to the Flaviviridae family. It has four serotypes (DENV 1–4) with human repercussions and a fifth serotype (DENV5), first described in 2007, restricted to the sylvatic cycle.[23,24] The genome comprised a single-stranded RNA that encodes 10 proteins: 3 structural proteins (membrane; M, envelope; E, and capsid; C) with component function and 7 non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) involved in RNA replication.[1] The mature virion presents an icosahedral outer shell, formed by a lipid bilayer embedded with M and E proteins, and a poorly organized nucleocapsid core, constituted by C proteins, encapsulating the RNA genome.[25,26]
The infection
During the feeding of an infected female mosquito, DENV is inoculated into the skin tissue and introduced to the bloodstream. Once in the skin, the virus infects various immune cells such as macrophages, dendritic cells, and Langerhans cells. These infected cells then migrate to the lymph nodes, where they come into contact with new cells and initiate new infections, leading to viremia (Figure 1).[27]
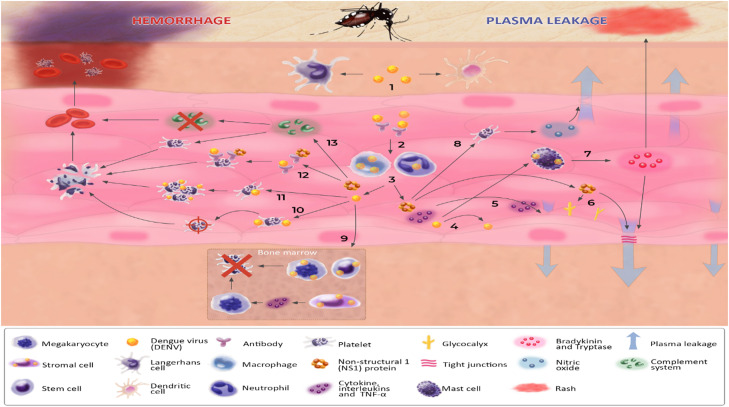
Figure 1.
Pathophysiology of severe dengue. Infection: (1) After inoculating into the skin, the virus infects macrophages, dendritic cells, and Langerhans cells, which migrate to the lymph nodes and start the viremia. (2) Into the bloodstream, DENV and complexes formed between the virus and non-neutralizing immunoglobulin (from previous infections by different serotypes) infect macrophages and neutrophils. (3) Infected cells start releasing virions, non-structural proteins (especially NS1), and pro-inflammatory cytokines. Plasma leakage: (4) DENV directly infects endothelial cells, causing its rupture. (5) Pro-inflammatory cytokines, such as TNF-α and ILs, act in the endothelial cells, increasing permeability. (6) NS1 protein binds to endothelial cells and triggers dysfunction of the EGL by the degradation of the sialic acid and the shedding of heparan sulfate proteoglycans from the EGL, which culminates in the loss of its integrity. (7) DENV infects mast cells, which release tryptases, disrupting the tight junctions, and bradykinins, causing the rash. (8) DENV induces the secretion of IL-1β by platelets, leading to the release of NO, a potent vasodilator. Hemorrhage: (9) DENV infects stem cells, hematopoietic stromal cells, and megakaryocytes in the bone marrow, reducing the production of platelets. (10) Endothelial cells infected by DENV mark platelets throw their rolling process, causing their destruction in the liver. (11) The virus directly infects platelets, activating them and leading to their apoptosis. (12) Immuno-mediated complexes between antibodies and the virus or NS1 cross-react with surface platelet antigens, inducing their destruction. (13) DENV activates the complement system, by the MBL complex, triggering the cleavage of C4 and initiating the lectin pathway. Additionally, the classical complement cascade can be activated by immunocomplex, formed by antibodies linked to antigen proteins. NS1 protein can interact with the complement proteins, promoting the degradation of C4 and halting both the classical and lectin pathways. These mechanisms lead to complement consumption and platelet opsonization and destruction. DENV: Dengue virus; EGL: Endothelial glycocalyx layer; IL: Interleukin; MBL: Mannose-binding lectin; NO: Nitric oxide; NS1: Non-structural 1; TNF: Tumor necrosis factor.
The intrinsic incubation period of DENV – the time between the mosquito bite and the onset of symptoms – is typically 3–10 days.[28] The binding of the E protein with specific receptors leads to the fusion of the viral and host-cell membranes, allowing the virus to enter the cell.
After endocytosis, the viral nucleocapsid is released, and the single-stranded RNA is translated into a polyprotein that is cleaved by non-structural proteins NS2B or NS3. The transcription process then begins at the replication site on the endoplasmic reticulum (ER).[29] Meanwhile, NS1 proteins are produced and released from the host cell, mediating a series of reactions that induce cytokines’ inflammatory response.[30] These findings correspond to the first clinical phase of dengue infection, known as the febrile phase. After this phase, the patient evolves into a critical phase, characterized by increased vascular permeability, before reaching the convalescent phase.
Mechanism of severe infection
Cytokine storm
The antibody-dependent enhancement (ADE) phenomenon is widely regarded as the most significant factor contributing to severe dengue disease. After primary infection, a long-term immunity against the serotype responsible for the infection (homotypic immunity) persists; meanwhile, a short period of cross-protection against another serotype (heterotypic immunity) is present.[29] Subsequently, neutralizing homotypic antibodies circulate for a lifetime, providing long-lasting protection against that primary serotype.[31] However, during a secondary infection for different serotypes, persistent non-neutralizing heterotypic antibodies bind to the new antigens, forming complexes that enhance the entry of DENV through the Fc receptor. This phenomenon aggravates the infection by facilitating the entry of DENV into more host cells.[1,29,32,33] The same phenomenon may occur in vaccinated patients and newborns of previously sensitized mothers.[34]
When a large amount of virus invades the host cells, a strong response from newly infected cells activates multiple inflammatory cascades. Meanwhile, a weak cross-reaction between previously activated T-cells and the new serotype infection induces the production of pro-inflammatory cytokines, such as interferon (INF)-α, IFN-β, and IFN-γ.[35,36] This cytokine release is further aggravated by NS1, which, along with non-neutralizing antibodies, stimulates the release of NF-κB, ultimately resulting in a “cytokine storm.”[23,37]
Vascular permeability
Dengue vascular permeability syndrome, previously known as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), is a complex and multifactorial condition associated with capillary permeability and plasma leakage, potentially leading to severe disease (Figure 1).[29] Several hypotheses have been proposed to explain the mechanism of this permeability. A hypothesis involves direct infection of endothelial cells, causing their apoptosis[38] and cytokine storm, eventually activating several pathways and inducing plasma leakage. Moreover, an immune-mediated mechanism has been proposed involving the formation of complexes between DENV and antibodies. These complexes activate complement, reducing the level of C3 and increasing the levels of C3a and C5a anaphylatoxins: these anaphylatoxins act at the endothelial surface and contribute to vascular leakage.[1,39]
The NS1 protein also contributes to vascular permeability. Each flaviviral NS1 protein interacts with specific tissues – consistent with the respective virus tropism – disrupting the endothelial cells and destroying the barrier that regulates vascular permeability and cell adhesion.[[40], [41], [42]] Although the exact mechanism is still unknown, studies have shown a correlation between TLR4 activation and interleukin (IL)-1β and IL-6 secretion, which may disrupt the tight junction of the endothelial barrier, associated with a direct degradation of the endothelial glycocalyx via heparanase-1 activation.[30,40] Moreover, antibodies against E protein and NS1 could recognize and attack host proteins, such as plasminogen and various coagulation and endothelial cell proteins.[43] The dysfunction of the endothelial barrier results in vascular hyperpermeability and leads to an increased passage of fluids and molecules through the endothelium.[44]
Another mechanism of vascular permeability in dengue involves platelet activation. Recent studies have shown that platelets participate in several inflammatory and immunological processes.[45] DENV induces the production and secretion of IL-1β by platelets, leading to the release of nitric oxide (NO), a potent vasodilator, and increasing endothelial permeability.[46,47] Other factors that may influence vascular permeability in dengue include mast cell activation, platelet-activating factor excretion, the angiopoietin 1 and 2 ratio, bradykinins, and prostaglandins.[48,49]
Thrombocytopenia and coagulopathy
The multifactorial etiology of thrombocytopenia in dengue fever includes early-stage bone marrow megakaryocyte suppression, peripheral destruction of platelets due to antibody-mediated lysis, and increased platelet consumption due to adherence to damaged endothelial cells and subsequent lysis (Figure 1).[50]
DENV suppresses megakaryopoiesis by infecting megakaryocytes and CD34+ hematopoietic stem cells (HSC),[51,52] and causing functional changes in stromal cells. Stroma cells play a crucial role in the bone marrow by releasing cytokines and providing an adequate microenvironment for the proliferation and differentiation of HSC.[51] Additionally, the cross-reactivity of antibodies against DENV proteins and platelet antigens can mediate peripheral platelet destruction. In vitro studies suggest that the recognition of human platelet anti-NS1 immunoglobulins promotes opsonization and subsequent complement-mediated lysis.[53] Elevated levels of anti-DENV immunoglobulin G (IgG) and immunoglobulin M (IgM) in platelet eluates have also been demonstrated in the acute phase of secondary infection. Platelet-associated immunoglobulins are also increased in acute dengue infection compared to the convalescent phase, and their levels are inversely associated with platelet counts.[54] Furthermore, platelet-bound immunoglobulins can induce partial platelet activation, resulting in inefficient aggregation during bleeding.[53] Impaired primary aggregation to adenosine diphosphate (ADP) and the absence of secondary aggregation are also described in studies as qualitative platelet disorders.[55]
Alterations in various elements of the coagulation cascade and fibrinolysis pathways have also been described in dengue fever patients. Slightly prolonged prothrombin time, activated partial thromboplastin time (APTT), and thrombin time are common,[[55], [56], [57]] especially in severe dengue, which may be related to liver damage and coagulation activation leading to the consumption of coagulation factors. A significant increase in tissue factor is also present in the febrile stage.[50] Regarding changes in the fibrinolytic system, levels of tissue plasminogen activator (tPA) and plasminogen activator inhibitor (PAI-1) are slightly elevated in the critical phase, while thrombin activatable fibrinolysis inhibitor levels are reduced. Patients with severe dengue usually have decreased protein S and C levels and significantly increased PAI-1 levels. These findings correlate with worse outcomes, which may reflect the imbalance between procoagulant and fibrinolytic mechanisms.[50] Most patients recover spontaneously from these laboratory abnormalities during the convalescent stage.[50]
Factors associated with severe dengue
Immune status
Following the initial infection for a specific serotype, long-term homotypic and short-term heterotypic antibodies are produced, providing long-lasting immunity against the specific serotype and a brief immunity against all others.[29] However, several non-neutralizing heterotypic antibodies remain. During a second infection by a different serotype, the ADE phenomenon occurs, exacerbating the severity of the disease.[35,58,59] The interval between the first and second infection is an important factor: a longer interval may increase the risk of more severe disease,[60] probably due to a decrease in circulating neutralizing antibodies.[29]
The same phenomenon may occur in infants born to mothers who are previously immune to the DENV and receive protective antibodies from them. This immunity could remain for several months. However, neutralizing maternal antibodies decline after this period, leading to a period of heightened risk for severe disease mediated by ADE.[1,29,61] Similarly, ADE has been proposed as the mechanism behind the increase in hospitalization rates observed among individuals who were immunized before their first dengue infection with the CYD-TDV vaccine (DengvaxiaⓇ, Sanofi Pasteur, Lyon, France).[62,63] As a result, the WHO has recommended using this vaccine only for individuals with confirmed previous infection (seropositive individuals).[64]
Additionally, previous exposure to the Zika virus, a closely related Flavivirus, has been associated with more severe cases of dengue, even during the patient's first episode.[65] Due to the structural similarity between Zika and DENVs, cross-reactive anti-Zika antibodies can trigger mechanisms of severe disease in patients infected with dengue, such as plasma leakage and shock.[66]
Virus strain
Although the clinical presentation of the four serotypes of the DENV may appear similar, they are biologically and phylogenetically distinct. The polyproteins of each serotype have been found to differ by up to 30%, suggesting that they could be considered distinct viruses.[29,67] Furthermore, each serotype exhibits different genotypes, with unique geographic distribution and fluctuations in their prevalence occurring over periods of 2–4 years, likely due to the accumulation of immunity.[61,67] These differences between serotypes and genotypes have a direct impact on the transmission and manifestation of dengue. While all serotypes are capable of causing severe disease, some are more likely to do so than others. For instance, studies have found that the Asian strain of DENV2 has a higher propensity to cause severe disease than the American strain because it replicates more efficiently in human cells and can infect new mosquitos more effectively, resulting in increased transmission.[29,43]
Genetic status
The severity of dengue fever is also influenced by the genetic status of the host. Past studies have shown that individuals of African ancestry are less susceptible to severe dengue fever,[68,69] while recent research has identified several genetic factors related to the severity of the disease. The most extensively studied genetic factor is the human leukocyte antigen (HLA), with HLA class-I alleles being associated with more severe cases and HLA class-II providing a protective effect.[43] Other specific genes, such as polymorphisms in Fcγ receptor II (FcγRII), tumor necrosis factor (TNF)-α, dendritic cell-specific intercellular adhesion molecule 3 (ICAM3)-grabbing non-integrin, Janus kinase 1 (JAK1), cytotoxic T-lymphocyte antigen 4 (CTLA-4), vitamin D receptor, human platelet antigens (HPA), transporters associated with antigen processing, and transforming growth factor (TGF)-β, have also been linked to severe forms of dengue fever.[70]
Host factors
Infants and older adults have a higher risk of developing severe dengue compared to middle-aged adults. Studies have shown that children are five times more likely to develop severe dengue due to their increased susceptibility to vascular permeability,[71,72] while the elderly have an increased risk of severe disease, likely due to the presence of associated chronic conditions.[[73], [74], [75]] In fact, studies have shown that individuals with diabetes mellitus have a four times higher risk of developing severe disease, while those with hypertension and cardiovascular disorders have a two-fold higher risk. Similarly, individuals with renal disease and individuals with asthma have a four-fold and two-fold higher risk of developing severe dengue, respectively.[[73], [74], [75], [76], [77], [78], [79], [80]] Moreover, recent studies have also linked obesity with an increased risk of severe dengue due to its pro-inflammatory status.[81,82]
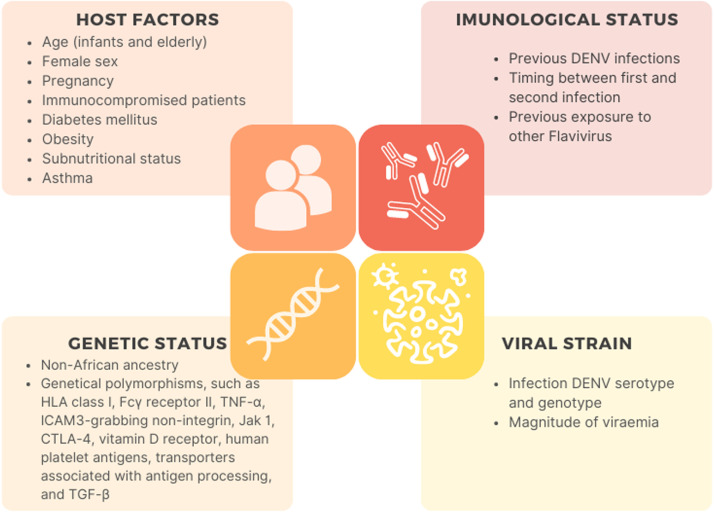
Sex has also been identified as a risk factor for severe disease. Multiple studies indicate that females, including young females, have a greater risk of developing severe dengue and higher mortality rates, although the specific mechanisms responsible for this sex disparity remain unclear.[29,67,72] The most common factors associated with severe disease are summarized in Figure 2.
Figure 2.
Factors associated with severe dengue. CTLA-4: Cytotoxic T-lymphocyte antigen 4; DENV: Dengue virus; HLA: Human leukocyte antigen; JAK1: Janus kinase 1; TGF: Transforming growth factor; TNF: Tumor necrosis factor.
Clinical Features
Clinical and laboratory manifestation
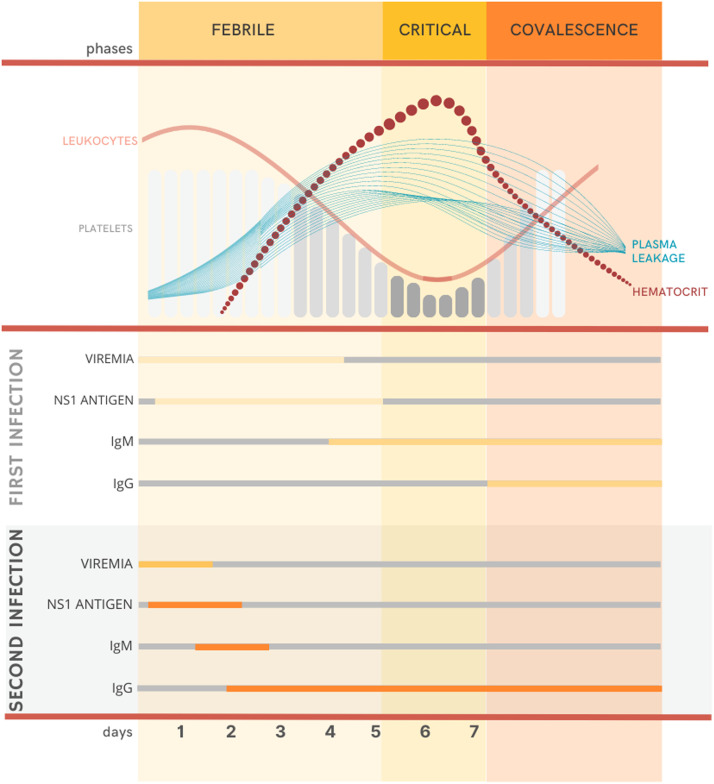
Dengue is a self-limited disease with a wide clinical spectrum ranging from mild to severe manifestations.[1] Approximately 75%–80% of infected people are asymptomatic,[[83], [84], [85]] while 5% can develop severe disease.[86] Classically, the disease progresses in three phases: febrile, critical, and recovery, although these phases are not always well-defined in clinical practice (Figure 3). During the febrile phase, after the incubation period of 3–10 days,[28] patients experience an abrupt onset of fever, headache, myalgia, arthralgia, retro-orbital pain, and anorexia.[87] Nausea, vomiting, sore throat, and pharyngitis may also occur.[88] Abdominal pain and diarrhea are more common in children, although these symptoms may also appear in adolescent and adult patients.[29] During this first phase, leukocytes and platelet levels may drop, and petechiae (small bleeding spots on the skin) and ecchymosis (large subcutaneous bleeding spots) may be present. Generalized morbilliform erythema (blanching with pressure) is also common.[87]
Figure 3.
Clinical phases of dengue infection and the relation with clinical and laboratory finds. IgG: Immunoglobulin G; IgM: Immunoglobulin M; NS1: Non-structural 1.
After 2–5 days, patients experience defervescence, characterized by a sudden drop in fever, and those without vascular permeability issues will start to recover. However, the critical phase begins for those who experience increased vascular permeability, and their clinical status may deteriorate rapidly.[29] Plasma leaks to extravascular space, causing hemoconcentration, characterized by an increase of 20% or more at hematocrit levels,[89] and leukopenia and thrombocytopenia reach the nadir. If the plasma leakage continues, the intravascular volume will reduce, and the patient may progress into shock.[29] There is an increase in the risk of hemorrhage, especially in the gastrointestinal tract.[90] Persistent hypoperfusion may lead to organ impairment, metabolic acidosis, and disseminated intravascular coagulation.[1] In those who recover, this phase lasts for 24–48 h.
During the final stage, convalescence occurs with gradual reabsorption of the extravascular fluid, associated with an improvement in clinical status. Patients experience increased diuresis and appetite,[91] and leukocytes and platelet levels return to regular. Some patients may experience a generalized itchy rash, known as “recovery rash,” due to the liberation of histamine by mast cells.[92] Some patients may persist with symptoms such as headache, myalgia, arthralgia, anorexia, alopecia, and insomnia, for more than two 2 years. Immunological status and gene polymorphisms may be related to these persistent symptoms.[93]
Warning signs and severity classification
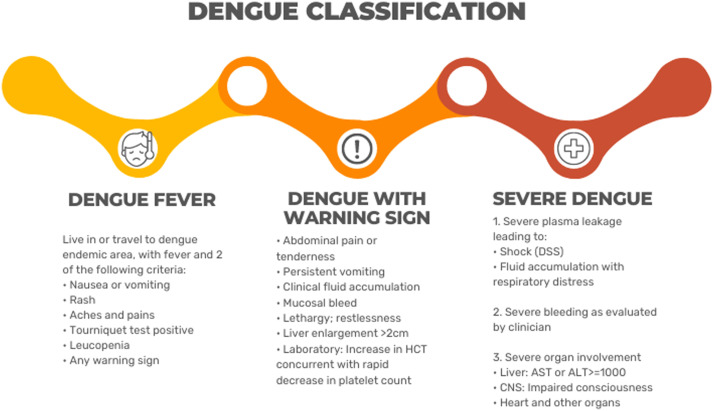
In 2009, the WHO introduced a new classification for dengue cases (Figure 4), replacing the previous terms “Dengue Hemorrhagic Fever” and “Dengue Shock Syndrome” with dengue with or without warning signs and severe dengue.[88] Although the previous terms are still in use, various studies have demonstrated that the new classification better reflects the natural progression of the disease[29] and has a higher sensitivity and specificity in identifying patients at risk for severe dengue.[[94], [95], [96]] Moreover, the new classification facilitates early patient referral to the intensive care unit (ICU), enabling better management of potential deterioration.[91]
Figure 4.
Dengue severity classification according to WHO.[92] ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; DSS: Dengue shock syndrome; WHO: World Health Organization.
The warning signs introduced in the 2009 classification have proven to be a valuable tool for predicting patients with an increased risk of disease progression. Although individual warning signs have low positive predictive value, their combination yields higher accuracy[97] and should be used for close monitoring. Recent studies have identified potential new markers that could be added to this list to improve accuracy. This includes clinical signs in the early stages of the disease (less than seven 7 days), such as altered mental status, tachycardia, pleural effusion, and ascites,[73,74] as well as biomarkers such as elevated total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels and a decrease in albumin levels.[76,98,99]
Severe dengue is defined as severe plasma leakage in a patient, leading to shock – defined as tachycardia, narrowing of the pulse pressure (a difference in systolic and diastolic pressure less than 20 mmHg), delayed capillary filling, and hypotension[29] – and pulmonary fluid accumulation with respiratory distress, severe bleeding, or severe organ involvement, especially the central nervous system (CNS), liver, and heart.[92]
Diagnostic approach
Specific diagnostic
Specific diagnostic tests for dengue depend on the patient's clinical phase (Figure 3). In the initial febrile phase, during the first 5 days of the disease, tests to detect the virus or its antigens are recommended. Detection of NS1 antigen in blood samples by enzyme-linked immunosorbent assay (ELISA) and rapid immunochromatographic (IC) assay are simple and low-cost tests for all clinical sets.[92] With a high specificity (ranging from 90% to 100%), and sensitivity that varies according to the commercially available tests (60%–90%),[100] NS1 antigen can be detected in sera until the ninth day of symptom, during a primary infection, and until the seventh day, during a secondary infection.[101]
Direct nucleic acid amplification in human samples can detect DENV RNA as early as 24–48 h before symptoms[102] through reverse transcriptase-polymerase chain reaction (RT-PCR), real-time RT-PCR, or isothermal amplification methods. These tests retain a high sensitivity (80%–100%) and specificity (99%–100%),[88] but the short window to collect the clinical sample, specific storage needs, and necessary lab infrastructure remain significant barriers, especially in developing countries.[103]
After day 5 of the disease, specific antibodies appear and could be detected by serological tests, even though dengue RNA and antigens could still be present. These tests include hemagglutination inhibition (IH) and ELISA to detect IgM and IgG antibodies.[1,100] The ELISA-based method is more widely used in developing countries due to its relative simplicity and lower cost, associated with high sensitivity (around 90%) and specificity (around 98%).[104] In primary infections, IgM can be detected in the serum sample as early as day 4: its rate increases until day 6 and remains positive for several months. Meanwhile, IgG starts to increase after the recovery phase. In secondary infection, IgM levels could be undetectable, while IgG titers rapidly increase as early as day 3.[92,100] Due to these variations, the WHO recommends the diagnosis of dengue diagnosis can only be confirmed when there is evidence of seroconversion from IgM to IgG or a four-fold increase in IgG titers in paired sera.[88,102]
In endemic areas, where there is transmission of other flaviviruses such as the Zika virus, Chikungunya virus, and yellow fever virus, the differential diagnostic could be challenging because the clinical manifestations are similar and serological tests could cross-react with previous infection or vaccination. In such cases, molecular diagnosis with RT-PCR is recommended if possible.[105] If the only evidence of dengue is a positive anti-DENV IgM test, a plaque reduction neutralization test can be performed to quantify virus-specific antibody titers and confirm the diagnosis. However, this test is rarely available in clinical laboratories.[86,103]
Viral isolation, when DENV is isolated after inoculation of clinical specimens, such as whole blood or tissue, onto cell lines, is the most specific test. However, its applicability is limited by cost – it requires specific laboratories with well-trained personnel – and time – the window period of sample collection is short and dependent on the level of viremia, and the tests require at least 7 days. Therefore, its use is reserved for research purposes.[1,100,104]
Complementary approach
In the overall assessment of patients with suspected or confirmed dengue, the WHO recommends conducting routine laboratory tests.[106] In facilities where it is available, a full blood count should be performed at the first visit and repeated daily, or at least after the third and fifth day of symptoms.[102] Establishing baseline hematocrit levels in the early febrile phase is crucial, as any subsequent rise can suggest plasma leakage.[88] Leukopenia, often accompanied by an increase in atypical lymphocytes, and thrombocytopenia are very common and precede the critical phase.[89,102] Neutropenia may also occur, although it is not typically associated with secondary bacterial infections: prophylactic antibiotics are, therefore, not recommended.[107]
Elevated transaminases and hypoalbuminemia may be present, reflecting liver involvement and predicting potential severe progression.[76,99,108] An increase in serum creatine kinase often occurs and could be a marker of myositis.[109,110] Vascular leakages usually occur preferentially in the pleural and peritoneal space. Therefore, an early assessment using point-of-care ultrasound to detect pleural effusion, gall bladder wall edema, ascites, and pericholecystic fluid collection is recommended.[87,111]
Organ Dysfunctions
Several complications may aggravate patients with dengue fever, including fluid overload, bacterial infections, and organ dysfunctions such as neurological, hepatic, heart, kidney, lung, and hematologic impairment (Supplementary Figure S1).
Overload hydration
During the recovery phase, there is a rapid resorption of the fluid that accumulates in virtual spaces (such as pleura and peritoneum) due to plasma leakage. The discontinuation of fluid supplementation in this phase is necessary to prevent hypervolemia and overload hydration. Excessive fluid administration can cause pulmonary edema or congestive heart failure in the patient.[112]
In addition to the administration of excessive intravenous fluids, other causes of fluid overload include the use of hypotonic rather than isotonic crystalloid solutions, the use of large volumes of crystalloid solutions in cases of unidentified serious bleedings, and inappropriate use of blood components.[88] Patients with comorbid conditions, such as chronic heart failure, lung diseases, and renal dysfunction, are at greater risk of suffering from fluid overload. Therefore, individualized goals and frequent revaluations are crucial to providing these high-risk individuals with better care.
In the recovery phase, if the patient still presents signs of hypervolemia and respiratory distress after stopping intravenous fluids, the administration of diuretics such as furosemide is recommended. Patients that exhibit signs of fluid overload but are still hypotensive should be evaluated to investigate occult bleeding, cardiac impairment, or secondary infection.
Bacterial co-infection
Bacterial co-infection is a relatively uncommon but serious complication in severe cases of dengue. While only around 7% of patients with dengue present concurrent bacteremia, up to 44% of dengue-associated deaths are related to bacterial co-infection.[49,113] Primary bacteremia is the principal site of infection related to dengue fever,[114,115] followed by pneumonia,[114,116] bile duct infection, and urinary tract infection.[115] Additionally, due to its high prevalence in tropical countries, other diseases such as leptospirosis, typhus, malaria, and enteric fever should be considered in the management of patients with severe dengue.
The exact mechanisms behind the association between dengue and bacterial infections are not fully comprehended. However, some hypotheses suggest that vascular rupture and the disintegration of the mucocutaneous barrier, particularly in the gut, may be contributing factors, as most of the bacteria involved in co-infection are typically members of the human microbiota.[113] Another possible cause of bacterial co-infection is severe neutropenia caused by virus-induced bone marrow suppression. However, clinical studies fail to show an increase in infection, antibiotic use, or mortality in those individuals.[107]
Patients with older age, acute renal failure, gastrointestinal bleeding, prolonged fever (more than 5 days), and altered level of conscience have an increased risk of concurrent bacterial infection.[114,117] The diagnosis of co-infection could be challenging due to overlapping symptoms. However, elevated leukocytes, C-reactive protein, and lactate, as well as prolonged aPTT, higher Acute Physiology and Chronic Health Evaluation II (APACHE II) score and Therapeutic Intervention Scoring System, and positive fluid balance are found in patients with bacterial co-infection.[118] In addition, recent studies showed that procalcitonin could be a helpful marker of bacterial co-infection.[119,120] In suspicious cases of sepsis, prompt and appropriate initiation of antibiotic therapy is crucial to reduce morbidity and mortality.[102]
Neurological impairment
DENV is a neurotropic virus that can directly infect the supporting cells of the CNS to cause neural injury. Moreover, some neurological syndromes are related to the inflammatory nature of the disease. ADE can lead to plasma leakage, edematous swelling, and dysregulated cytokine release, resulting in immune-mediated neural damage. The various neurological manifestations related to dengue infection can be categorized into encephalopathy, encephalitis, immune-mediated syndromes, muscle dysfunction, dengue-associated stroke, and neuro-ophthalmic disorders.[[121], [122], [123]]
The pathogenesis of DENV infection in the CNS is still poorly understood. The release of pro-inflammatory cytokines, induced by the virus and NS1 protein, disrupts the blood–brain barrier, facilitating the entry of DENV and other immune mediators into the brain and resulting in neuroinflammation.[121] Additionally, some studies showed that Flavivirus can travel into the CNS through axonal transport from peripheral nerves, carried by immune cells, and, more rarely, transported across the endothelial barrier by intracellular vesicles during transcytosis.[124] Neurological impairment may occur in any clinical phase of the disease. The diagnosis of a neurological disorder associated with dengue infection is challenging because DENV PCR positivity in the cerebrospinal fluid (CSF) is rare – as the sensibility of DENV PCR using CSF is poor – and is linked to the phase of the infection at the time of the CSF collection. Patients with neurological injury are more likely to also present with higher levels of hepatic enzymes, higher hematocrit, and lower platelet counts compared to cases of dengue patients without neurological impairment.[125]
The incidence of encephalopathy, the most common neurological complication of dengue, has been estimated to be between 0.5% and 6.2%.[29] It is characterized by a diminished level of consciousness, cognitive impairment, and seizures. CSF is usually normal, and neuroimaging studies may show cerebral edema.[123,126] Encephalitis might be the most worrisome syndrome among dengue neurological affections. The patient mostly presents with reduced levels of consciousness, headache, fever, nausea and vomiting, seizures, focal neurological deficits, and behavioral symptoms. The CSF profile is usually slightly altered, with slight protein elevations and normal glucose values. CSF lymphocytic pleocytosis is found in 85% of patients.[125] A cranium computed tomography scan may show hyperdense parenchymal foci representing spontaneous microhemorrhages as well as hypodensities in the thalami and basal ganglia, while brain magnetic resonance imaging can identify the exact anatomical areas of involvement: the most commonly affected regions are the basal ganglia, thalamus, temporal lobes, hippocampus, cerebellum, and cerebral white matter, where T2 sequences may demonstrate hyperintensities.[121,127]
Immune-mediated syndromes associated with dengue generally occur after the infection and include acute disseminated encephalomyelitis (ADEM), Guillain–Barré syndrome (GBS), transverse myelitis, acute cerebellitis, and neuritis brachialis.[123] More rarely, isolated cranial nerve palsies may occur, resulting from the direct involvement of cranial nerve nuclei or immune-mediated, and it is often steroid responsive.[121] Ischemic and hemorrhagic strokes also may occur following dengue fever. The pathogenesis may be beyond the commonly observed thrombocytopenia and include cerebral vasculitis. Posterior reversible encephalopathy syndrome is uncommon and possibly related to dysregulated cytokine release phenomena rather than vasogenic disorder as usual.[121]
Currently, there is no curative treatment for dengue fever, including its neurological complications. Supportive measures, which can be defined for the patient, include fever control, hematological monitoring to avoid bleeding, measures to contain cerebral edema, and anti-seizure medications for secondary prophylaxis. There is no evidence to support the use of corticosteroids or antiviral agents to treat encephalopathy or encephalitis, although immune-mediated neurological conditions can respond well to immunomodulators like high doses of corticosteroids or intravenous immunoglobulin (IVIg) therapy.[121] Post-dengue immune-mediated neurological syndromes usually resolve within weeks or a few months.
Hepatic impairment
DENV can directly infect the liver and cause apoptosis of hepatocytes, leading to the classical findings of liver enzyme elevation.[29] The increase of AST and ALT are considered markers of severe dengue.[76,99,108] Several mechanisms are hypothesized as causative of hepatic impairment. DENV can instigate cells apoptosis after entry into hepatocytes and Kupffer cells (mediated by E protein and phagocytosis) via the production of NO and IFN-α or via IL-6 and TNF-α, recruiting CD4+ and CD8+ T lymphocytes, monocytes, and natural killer cells.[128]
A secondary pathway linked to liver injury is ADE, which facilitates Kupffer cell infection and increases cytokine release. Additionally, antibodies against NS1 protein cross-react with the surface antigen of hepatic endothelial cells, inducing their apoptosis and causing liver damage.[129] Besides the injury, hepatocyte apoptosis contributes to reducing viral replication and dissemination. However, if a large number of cells are infected, significant injury may result in hepatic dysfunction and liver failure.[130]
Heart impairment
Cardiac involvement by DENV is considered a rare complication. The spectrum of cardiovascular manifestations in dengue infection is broad, ranging from subtle ST-T changes to fulminant myocarditis.[131] The main pathophysiological factors associated with myocardial impairment include edema from capillary leakage and the presence of circulating myocardial depressant factors such as pro-inflammatory mediators, coronary hypoperfusion, and altered calcium homeostasis.
The most concerning condition of the cardiac impairment of dengue fever is myocarditis. The signs and symptoms may vary from a subclinical rise in cardiac biomarkers and detection of asymptomatic electrocardiogram abnormalities to more severe clinical manifestations such as dyspnea, chest pain, and sudden death. The diagnosis is confirmed by endomyocardial biopsy or cardiac magnetic resonance, but both procedures are not widely available in dengue-endemic areas.[132] Moreover, the pericardium may be affected by dengue infection, and pericardial effusion is commonly observed in severe dengue patients due to systemic plasma leakage.[132] Some case reports of isolated dengue pericarditis have been described in the literature.[133]
Several electrocardiographic (ECG) alterations are associated with dengue infection, such as bradycardia, atrioventricular block, and T-wave and ST-segment abnormalities. The mechanisms of the electrical conditions are still not fully understood. The major hypotheses involve altered autonomic tone, electrolyte and calcium derangements, or subclinical myocarditis. Whether the ECG alterations are clinically relevant in all dengue cases is still theoretical. The presence of bradyarrhythmia in the critical phase of severe dengue, when the patient presents with hypovolemia, is a significant clinical concern because a deterioration in cardiac output will prevent an adequate cardiac response to shock. Therefore, there is a need for careful attention to fluid balance and hemodynamic instability in these patients.[132]
Impaired myocardial function is expected in cases of severe dengue mainly because of the increased vascular permeability and the hypovolemic nature of the shock. However, adequate management of dengue-related hemodynamic instability requires, in addition to vigorous volume infusion, evaluation and treatment of associated ventricular dysfunction.[134]
Kidney impairment
Kidney impairment in patients with dengue fever is mostly mild, ranging from serum electrolyte abnormalities to hematuria and proteinuria. However, patients that develop renal damage may develop more severe complications, such as acute kidney injury (AKI) and hemolytic uremic syndrome.[135] Although not yet fully understood, some mechanisms suggested as causes of AKI in severe dengue are hypotension – leading to hypoperfusion and tubular necrosis – direct injury caused by the virus, indirect injury via the immune system, and rhabdomyolysis.[136,137] Immunohistochemical studies performed on kidney specimens from fatal human cases of dengue infection found viral RNA and observed an accumulation of immune cells and intense acute congestion, suggesting that a combination of high viral load, exacerbated immune response, and increased permeability play a role in renal damage during dengue infection.[138,139]
Furthermore, dengue fever in patients with chronic kidney disease is a particular concern, as these patients have specific difficulties in dealing with the substantial amount of fluid used in the acute phase to treat hemoconcentration and may rapidly evolve to fluid overload. In some cases, hemodialysis is required to re-establish the fluid balance.[140,141] Kidney transplant recipients are also of particular concern because their clinical manifestation may be masked by the use of immunosuppressors, especially calcineurin inhibitors, which limit IL-mediated response.[142] They are more prone to develop severe dengue, and the disease usually has an unfavorable clinical course, marked by higher viral load due to the immunosuppression, susceptibility to present fluid overload, and greater risk for secondary infection during the hospitalization.[143]
Managing renal impairment in patients with dengue requires careful monitoring of fluid administration, with infusion rates and controlled tonicity, to avoid osmolarity disorders and fluid overload. Renal replacement therapy may be necessary, and continuous hemodialysis is recommended, especially in patients in shock.[136,144]
Lung impairment
Pulmonary complications are rare, although upper airway symptoms may occur frequently in the early stages of dengue. Patients with dengue may experience various impairments in the pulmonary system, including pleural effusion, non-cardiogenic pulmonary edema, pneumonitis, acute respiratory distress syndrome, and pulmonary hemorrhage.[[145], [146], [147]] The primary mechanisms associated with pulmonary complications are increased vascular permeability and plasma leakage. Thrombocytopenia and disturbance in the coagulation cascade are responsible for alveolar hemorrhage and hemoptysis.[148,149]
Chest radiography can identify pleural effusion and consolidation, while tomography can identify ground-grass opacities, interlobular septal thickening, and nodules.[146] Lung ultrasound has become an essential tool for managing severe dengue, especially in ICUs, where pleural effusion can be rapidly identified, along with pulmonary edema, characterized as the finding of multiple B lines.[111,150] The treatment for these conditions is unclear based on the literature, and very few case reports are available. However, controlling liquid balance and avoiding fluid overload are crucial approaches to minimizing pleural effusion and pulmonary edema. Similar to the treatment of thrombocytopenic purpura,[151] the use of high-dose corticoids and immunoglobulin has been reported to be successful in literature,[152] although further studies are necessary to establish their effectiveness.
Hematological complications
Hematological complications related to dengue infection are uncommon and include persistent low platelets count and hemophagocytic lymphohistiocytosis (HLH). Persistent thrombocytopenia is a rare complication after dengue infection.[153] In the context of a secondary immune thrombocytopenic purpura, it is defined as the prolonged low platelets count after the recovery phase. The mechanism is still unknown, although persistent antiplatelet IgG may be found, suggesting an immune platelet destruction.[154] Due to its rarity, only case reports can be found in the literature, and successful treatment has been published after the use of corticosteroids and IVIg.[151,[155], [156], [157]]
HLH or macrophage activation syndrome is a potentially fatal hematological complication of dengue infection. Secondary HLH (sHLH) is a hyper-inflammatory state resulting from a strong activation of the immune system caused by conditions such as infection, malignancy, and autoimmune diseases.[158,159] Inappropriate macrophage stimulation in the bone marrow leads to blood cell phagocytosis and an inappropriately elevated release of pro-inflammatory cytokines.[160]
HLH represents a diagnostic challenge in patients with dengue infection due to the potential overlap of many symptoms, such as fever, hepatomegaly, and cytopenia.[161] However, it should be suspected in cases of persistent thrombocytopenia for more than 10 days, persistent fever for more than 7 days, and hyperferritinemia.[162] According to the HLH-2004 guidelines,[158] the diagnosis is made if five of the following eight criteria are fulfilled: fever, splenomegaly, cytopenia (at least two of three lineages in the peripheral blood), hypertriglyceridemia, and/or hypofibrinogenemia, hyperferritinemia, proved hemophagocytosis in bone marrow, spleen, or lymph nodes biopsy, low or absent NK-cell activity, and high levels of soluble IL-2 receptor (sIL-2r).
The best treatment for HLH in dengue patients is still unknown since only case reports are found in the literature. However, similar treatments applying to sHLH caused by other infectious agents have shown improved outcomes.[162] In mild cases, the addition of low doses of corticosteroids can be beneficial. However, in moderate and severe cases, high doses of dexamethasone (10 mg/m2 divided over 12 h periods) or methylprednisolone (2 mg/(kg·day)) are mandatory and must be promptly initiated. Adjunctive therapy with IVIg (1.6 g/kg in split doses over 2–3 days) may be considered due to the anti-inflammatory and antiviral potential of IVIg.[163]
Management
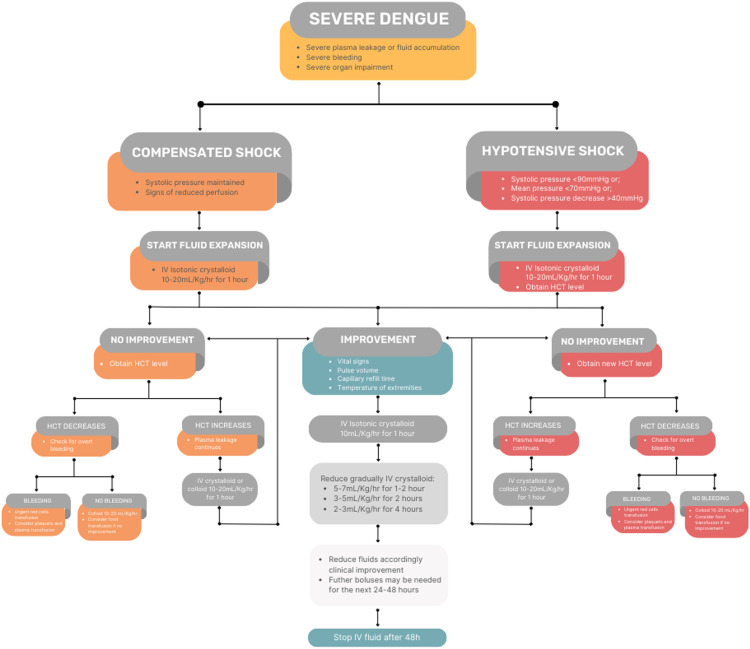
None of the several medications studied as potential treatments for dengue have demonstrated efficacy in reducing symptoms and complications. Therefore, treating general symptoms and predicting critical complications are the main management of severe dengue (Figure 5). Most patients will not require hospitalization, although it is mandatory to recognize those who will require hospitalization early.
Figure 5.
Management of severe dengue, according to WHO.[101]
WHO: World Health Organization.
General management
The cornerstone for the management of dengue is supportive care and early recognition of warning signs. During the febrile phase, treating symptoms with rest, antipyretic, and hydration is sufficient. Every patient must be instructed regarding the warning signs and the critical phase that could follow defervescence. Patients with risk factors for severe disease must be closely followed. Acetaminophen or metamizole are preferable drugs for temperature control, considering their safety profile. Non-steroidal anti-inflammatories and acetylsalicylic acid must be avoided due to their increased risk of gastrointestinal bleeding and Reye's syndrome.[164]
Due to the inflammatory aspect of the disease, the use of corticosteroids in dengue infection was always considered in the management of severe cases.[165] Some articles in the 1970s and 1980s found some reduction in mortality, but since then, several studies have failed to demonstrate the benefit of using corticosteroids in dengue infection, even in more severe cases.[166,167] A 2014 Cochrane review[168] showed no significant reduction in progression to severe disease, bleeding, severe thrombocytopenia, and mortality. Therefore, the WHO contraindicates the use of corticosteroids in dengue infections.[164] More randomized control trials are needed to better address this issue.
Management of plasma leakage and shock
Plasma leakage is the main mechanism of dengue shock and probably the greatest concern in severe dengue. Around the time of defervescence, the patient may experience worsening symptoms and increased capillary permeability.[102] Volume restitution is needed to prevent or reverse the hypovolemic shock. Oral rehydration may be sufficient in patients without warning signs and who tolerate oral fluid intake. However, patients who do not tolerate oral fluid intake, experience a persistent increase in hematocrit despite oral hydration, or present warning signs should receive intravenous fluids.
According to the WHO Recommendations for Clinical Management of Dengue,[102] patients with severe dengue should be promptly admitted to ICUs and receive fluid expansion. No benefit or distinction in clinical improvement was found with the use of colloid infusions over crystalloid; therefore, initial fluid expansion with crystalloid is suitable.[[169], [170], [171]] The intravenous colloid solution is warranted in intractable shock resistant to first resuscitation.[169,170] Fluid resuscitation should be guided by clinical parameters to improve central and peripheral circulation, characterized as a decrease of tachycardia, improvement of blood pressure, or pulse volume, return of capillary refill time to less than 3 s, improvements in the level of consciousness (more alert or less restless), urine output ≥0.5 mL/(kg·h), and decreasing metabolic acidosis.[88] Hematocrit measurements may help to assess fluid responsiveness. When hematocrit declines without clinical improvement or maintenance of hypovolemic shock, occult bleeding must be investigated. Details about the management could be found in Figure 5.
The first 24–48 h of plasma leakage is critical, and the patient may develop recurrent shock during this period. The unique dynamism of this condition requires close medical observation and sequential evaluations to guarantee the proper recovery of hemodynamic stability. Intravenous fluids should then be weaned gradually after the improvement of the perfusion parameters. The infusion rate of fluid administration should be progressively reduced over the 24–48 h following the plasma leakage. With proper treatment, most patients will recover in a few days.
Management of thrombocytopenia and hemorrhage
Bleeding manifestations are common in dengue, affecting approximately 20%–60% of hospitalized patients.[[172], [173], [174]] While mucocutaneous bleeding is often mild, severe hemorrhage in the gastrointestinal, gynecological, and pulmonary systems may occur, leading to fatal outcomes.[88,175,176] Interestingly, several studies have reported a weak correlation of thrombocytopenia[[177], [178], [179]] and coagulation abnormalities[180,181] with the incidence and severity of bleeding. Furthermore, the duration of shock is one of the main risk factors for severe hemorrhage in patients with severe dengue.[178] Efforts to closely monitor hematocrit and vigorous intravenous therapy may be crucial to reduce blood product usage and shorten hospital stay.[182]
Prophylactic platelet transfusion
As thrombocytopenia is fairly common, the benefit of prophylactic platelet transfusion has been a matter of interest in several researches.[176,183] However, randomized controlled trials (RCTs) evaluating the role of transfusion for non-bleeding or mildly bleeding patients with platelet counts below 20,000/mm³[176] and 30,000/mm³[183] have failed to show a significant reduction in progression to severe bleeding. Conversely, transfusion-related adverse events, such as circulatory overload and allergic reactions, have been reported.[176,183]
Studies evaluating post-transfusion platelet increment (PPI) found that non-responders were individuals with lower baseline platelet counts (<10,000/mm³).[183] The authors hypothesized that immune-mediated destruction may be more pronounced in patients with lower platelet counts. Therefore, those more prone to receive platelet concentrates may also be more likely to be poor responders and not benefit from transfusion.[183] These findings are consistent with a retrospective pediatric study of complicated DSS patients[182] and a large observational study in adults, including severe dengue patients,[184] which also demonstrated no association between platelet transfusion and reduction of bleeding risk.
Currently, there is no evidence supporting prophylactic platelet transfusion. However, more studies with sufficient power are needed to evaluate transfusion benefits in preventing severe bleeding at lower thresholds, especially in patients with severe dengue.[176]
Transfusion and other agents for bleeding patients
Currently, there is no RCT evaluating the benefit of platelet transfusion for patients with significant bleeding manifestations.[185] Additionally, immune-mediated platelet lysis may destroy donor platelets.[183] As a result, WHO suggests providing only fresh packed red blood cells (RBC) and whole blood transfusion support for patients with significant hemorrhage.[88] However, considering that the contribution of immune-mediated platelet destruction may vary among individuals and that about half of the patients are PPI responders,[183] it seems reasonable to consider platelet transfusion in cases of persistent severe life-threatening bleeding with thrombocytopenia.[50,87,184,186,187]
Physicians should also be aware that because thrombocytopenia may not be the only cause of bleeding, it is important to evaluate and correct coagulation abnormalities with fresh frozen plasma (10 mL/kg), cryoprecipitate (1 unit per each 10 kg), and vitamin K.[187] There is currently insufficient evidence to support the routine use of other agents such as recombinant activated factor VII (rFVIIa), IVIg, and anti-D globulin.[185]
Specific and local measures to control bleeding may also be useful, such as anterior nasal packing with hemostatic sponges for epistaxis and hormonal methods for menorrhagia.[50,188] Unnecessary invasive procedures should be avoided. It is important to note that plasma leakage leads to elevated hematocrit levels above the baseline value. Therefore, physicians should always suspect internal bleeding in cases of persistent or worsening shock despite hematocrit decrease.[102,187] Providing RBC transfusion is also necessary to maintain adequate tissue oxygenation.
Special Population
Pregnant women
Pregnancy is a well-known risk factor for severe dengue,[73] as the physiological changes that occur during gestation may often mask the clinical and laboratory features of dengue infection, leading to misdiagnosis.[102] Widened pulse pressure, hemodilution due to blood volume expansion with a decrease of hematocrit, leukocytosis with associated lymphopenia, and gestational thrombocytopenia are some of the systemic alterations that may confuse diagnosis.[189] Pathological conditions such as hyperemesis, eclampsia or preeclampsia, and HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome are common causes of delayed management.[190]
Pregnant women have a 3.4 times higher risk of developing severe dengue (odds ratio [OR]= 3.38; 95% confidence interval [CI]: 2.10 to 5.42),[191] which is associated with an increased risk of maternal mortality (OR= 4.14; 95% CI: 1.17 to 14.73),[192] miscarriage (OR= 3.51; 95% CI: 1.15 to 10.77),[193] stillbirth (OR: 2.71; 95% CI: 1.44 to 5.10),[192] and neonatal deaths (OR= 3.03; 95% CI: 1.17 to 7.83).[192] Other adverse pregnancy outcomes, such as preterm birth (OR= 2.4; 95% CI: 1.3 to 4.4),[194] low birth weight (OR= 2.1; 95% CI: 1.1 to 4.0),[194] and congenital malformation of the brain (OR= 4.5; 95% CI: 1.7 to 11.3),[195] have also been reported. The principal mechanism underlying these complications is thought to be plasma leakage, which affects the placenta, causing local inflammatory responses such as deciduitis, choriodeciduitis, intervillositis, and multifocal necrotizing villitis.
The management of dengue in pregnant women is similar to that in the general population, with some special considerations. The WHO recommends in-hospital monitoring for all pregnant women, especially those close to full-term. Due to physiological hemodilution, baseline hematocrit should be established during the first days of the disease. The growing gravid uterus may narrow the tolerance of liquid accumulation: fluid overload should, therefore, be avoided.[102]
Elective delivery should be postponed,[190] and the use of tocolytics for uterine inhibition, although not a consensus, should be considered, especially in thrombocytopenic women.[196] If delivery is inevitable, platelets transfusion should be administered, and intravenous oxytocin analogs are recommended to stimulate uterine contraction and reduce post-partum hemorrhage.[190] Although information about the best mode of delivery is sparse, most guidelines recommend avoiding cesarean section.[189] Finally, newborns should be closely monitored for signs of dengue due to the risk of vertical transmission.[102] Despite studies suggesting a potential transmission through mother's milk,[197] there is no recommendation for suppressing breastfeeding.[164]
Older adults
Managing dengue in elderly patients could be challenging due to immunological senescence, resulting in atypical clinical presentations. Studies have shown that elderly patients with dengue are more likely to present with isolated fever episodes, headaches, rashes, nausea, vomiting, and mental confusion,[[198], [199], [200]] as well as leukopenia, higher hematocrit, lower albumin, and experience nadir platelet counts.[199] Older adults are also more likely to develop severe dengue because they cumulate three risk factors: immunological impairment due to senescence, higher probability of acquiring secondary infection, and increased prevalence of chronic diseases.[201]
In addition to being more susceptible to severe dengue, elderly patients are also more prone to experiencing complications, such as pleural effusion, mucosal bleeding, gastrointestinal bleeding, hematuria, liver involvement, and acute renal failure.[200,[202], [203], [204]] These patients are more likely to be admitted to ICUs, have longer hospital stays, and acquire hospital-related infections, especially pneumonia and urinary tract infections.[199,200] The fatality rate is high, reaching seven-fold risk or higher in some studies.[200,203,204]
Management with judicious fluid therapy is imperative, and non-invasive monitoring with echocardiography and inferior vena cava ultrasound is recommended.[201] Cardiogenic shock was found to be an important aggravation of severe disease among elderly patients. Therefore, the use of inotropic agents must be considered.[201] Regularly reviewing the need for indwelling medical devices is also recommended to avoid hospital-acquired infections.[91] Reconciliation of previous medications could be challenging due to polypharmacy (see below).
Hypertensive patients
Hypotension could be misinterpreted in patients with chronic hypertension. Therefore, hypotensive must be considered when mean arterial pressure falls by 40 mmHg from baseline. When evaluating shock, heart rate should not be relied upon in patients taking β-blockers and calcium channel blockers as these agents can decrease or increase heart rate, respectively. Moreover, diuretic agents must be discontinued, and the use of other antihypertensives should be closely monitored and promptly suspended if any signs of plasma leakage and shock are identified.[102]
Diabetic patients
Like other infections, dengue fever can precipitate diabetic ketoacidosis and hyperosmolar hyperglycemia in diabetic patients. Additionally, hyperglycemia can also cause osmotic diuresis, which can aggravate hypovolemia. Therefore, elevated glycemia must be monitored and treated. Oral hypoglycemic agents can cause lactic acidosis, and vomiting may reduce their absorption. Thus, these agents must be discontinued during severe dengue.[102]
Patients on anticoagulants and antiplatelets
The management of anticoagulation therapy in dengue infection is a subject of debate among clinicians as there is no consensus on whether to suspend or continue it. However, weighing the potential risk of suspension against the risk of bleeding is essential. For patients at high risk of a thromboembolic event, it is recommended to suspend anticoagulation therapy during the critical phase of dengue, when the platelet count is less than 100,000/µL. In these cases, unfractionated heparin may be introduced, but close monitoring for bleeding is necessary. Anticoagulation therapy can be resumed once the patient stabilizes and the platelet count increases above 50,000/µL.[205,206]
Regarding antiplatelet agents, only one retrospective cohort study assessed the safety of continuing vs. discontinuing therapy. The study found no increase in bleeding in the first set or adverse cardiac or cerebrovascular event in the second.[207] Balancing risk–benefit on an individual basis for each patient is mandatory.[208,209]
Patients on statins
Statins are drugs that inhibit the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase and have been shown to have an immunomodulatory effect and reduce cytokine expression in non-infective diseases. Some in vitro and animal models studies have demonstrated that statins directly reduce viral shedding and inflammation.[210,211] However, clinical data in humans failed to confirm these findings.[212] Even though one RCT has demonstrated the safety and tolerance of statin use during dengue infection,[212] clinicians recommend suspending statin use during dengue infection due to its common adverse event of increasing transaminases and creatine kinase.[213]
Future Perspectives
Several antiviral candidates targeting different viruses have been proposed, including nucleoside inhibitors, non-nucleoside inhibitors, and flexible nucleoside analogs known as “fleximes.” These tools have been used to target classical viral proteins such as NS3 protease, NS3 helicase, NS4B, and NS5, as well as other targets like NS1, E protein, and viral capsid.[214,215] Despite efforts to develop effective drugs, few have advanced to the clinical trial stage, and so there is no clear short-term prospect for the availability of a promising antiviral.
Given the lack of an effective antiviral, the search for a vaccine is becoming increasingly necessary to prevent dengue infection and severe disease. Two tetravalent dengue vaccines have been approved for use in different countries: CYD-TDV, developed by Sanofi Pasteur, and TAK-003, developed by Takeda.
The CYD-TDV, the first licensed dengue vaccine, is a recombinant tetravalent vaccine that uses the attenuated Yellow-Fever virus 17D strain as the replication backbone. The vaccine composition replaces the PrM and E protein of Yellow-Fever virus 17D with the PrM and E protein of the different serotypes of DENV. The overall vaccine efficacy (VE) ranges from 56.5% to 60.8%, with specific protection of over 70% for DENV3 and DENV4: the VE for DENV1 and DENV2 is only 40%–50%. Clinical trials have shown that the vaccine is 65.6% effective in children aged over 9 years and 44.6% effective in children aged under 9 years.[216] This age-dependent efficacy is likely due to previous exposure to natural infection, as seropositive volunteers had higher titers of neutralizing antibodies detectable by PRNT50 than baseline seronegative volunteers.[217]
Regarding hospitalization, vaccinated volunteers aged 5 years and younger were five times more likely to be hospitalized than the placebo control group. Additionally, a 5-year follow-up study of pre-vaccination dengue-seronegative volunteers aged 2–16 years found a higher risk of hospitalization compared to the control group (hazard ratio [HR]: 1.75; 95% CI: 1.14 to 2.70). This risk was also higher in dengue-seronegative participants aged 9–16 years (HR: 1.41, 95% CI: 0.74 to 2.68).[218] Due to the excess risk of hospitalization among seronegative trial participants, the WHO Global Advisory Committee on Vaccine Safety concluded that individuals not infected with the DENV should not be vaccinated with CYD-TDV.[64] These results limit the use of the CYD-TDV vaccine in pediatric populations, a group at high risk of severe disease.[216,219]
Another vaccine recently licensed for commercial use is TAK-003, a live-attenuated tetravalent vaccine based on a recombinant DNA chimeric virus associated with a live-attenuated DENV2 virus. Phase 3 clinical trials involving volunteers aged 4–16 years showed that the VE also depends on dengue serostatus, serotype, and age. Additionally, the VE dropped for a long time. Twelve months after two doses, the overall VE was 80.9% (95% CI: 75.2 to 85.3), decreasing to 73.3% (95% CI: 66.5 to 78.8) at the end of 18 months of follow-up, 72.7% (95% CI: 67.1 to 77.3) at 24 months, and 62% (95% CI: 56.6 to 66.7) at 36 months of follow-up.[219,220]
Regarding protection against hospitalization, the overall efficacy of the TAK-003 vaccine was 95.4% at 12 months. This efficacy remained high at 18 months (90.4%). However, although statistically inconclusive, the hospitalization rate due to DENV3 in previous dengue-seronegative individuals was higher than that of the control group at both 18-month and 36-month follow-up periods.[219,220]
Other vaccines are being developed, some of them in the clinical trial stages. It is still difficult to state the impact that the approved vaccines may have in endemic countries. However, even with limitations, reducing the severity of the disease and, consequently, hospitalization may already be a great advance. Challenges regarding different vaccine efficacies in different serotypes may pose a greater challenge in countries that have implemented large-scale use, making genomic surveillance essential for the success of the strategy.
Author Contributions
Alexandre Mestre Tejo: Conceptualization, Methodology, Project administration, Visualization, Writing original draft, Writing review & editing. Debora Toshie Hamasaki: Writing original draft. Letícia Mattos Menezes: Writing original draft. Yeh-Li Ho: Writing original draft, Writing review & editing.
Acknowledgments
We would like to thank Danilo Sanefuji (danilo.sanefuji@gmail.com) for the wonderful artwork in this paper and Andréa Machado de Almeida Mattos (andreamattosufmg@gmail.com), associate-professor of Universidade Federal de Minas Gerais, for English language editing and reviewing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics Statement
Not applicable.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Managing Editor: Jingling Bao
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jointm.2023.07.007.
Declaration of generative AI and AI-Assisted technologies in the writing process
During the preparation of this work, the authors used ChatGPT by OpenAI in order to improve readability and language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Appendix. Supplementary materials
Data availability
The data sets generated during and/or analyzed during the present study are available from the corresponding author upon reasonable request.
References
- 1.Harapan H., Michie A., Sasmono R.T., Imrie A. Dengue: a minireview. Viruses. 2020;12(8):829. doi: 10.3390/v12080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2022. Dengue and severe dengue.https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue Available from: [Google Scholar]
- 3.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Quam M., Zhang T., Sang S. Global burden for dengue and the evolving pattern in the past 30 years. J Travel Med. 2021;28(8):taab146. doi: 10.1093/jtm/taab146. [pii] [DOI] [PubMed] [Google Scholar]
- 5.Salles T.S., da Encarnação Sá-Guimarães T., de Alvarenga E., Guimarães-Ribeiro V., de Meneses M., de Castro-Salles P.F., et al. History, epidemiology and diagnostics of dengue in the American and Brazilian contexts: a review. Parasit Vectors. 2018;11(1):264. doi: 10.1186/s13071-018-2830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam P.K., Tam D.T., Diet T.V., Tam C.T., Tien N.T., Kieu N.T., et al. Clinical characteristics of dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis. 2013;57(11):1577–1586. doi: 10.1093/cid/cit594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messina J.P., Brady O.J., Golding N., Kraemer M., Wint G., Ray S.E., et al. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019;4(9):1508–1515. doi: 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson M.C., Stanberry L.R. Climate change and vectorborne diseases. N Engl J Med. 2022;387(21):1969–1978. doi: 10.1056/NEJMra2200092. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer M., Reiner R.C., Jr, Brady O.J., Messina J.P., Gilbert M., Pigott D.M., et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4(5):854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwamura T., Guzman-Holst A., Murray K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat Commun. 2020;11(1):2130. doi: 10.1038/s41467-020-16010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diseases T.L.I. Twin threats: climate change and zoonoses. Lancet Infect Dis. 2023;23(1):1. doi: 10.1016/S1473-3099(22)00817-9. [DOI] [PubMed] [Google Scholar]
- 12.Vega-Rua A., Zouache K., Caro V., Diancourt L., Delaunay P., Grandadam M., et al. High efficiency of temperate Aedes albopictus to transmit chikungunya and dengue viruses in the Southeast of France. PLoS ONE. 2013;8(3):e59716. doi: 10.1371/journal.pone.0059716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercier A., Obadia T., Carraretto D., Velo E., Gabiane G., Bino S., et al. Impact of temperature on dengue and chikungunya transmission by the mosquito Aedes albopictus. Sci Rep. 2022;12(1):6973. doi: 10.1038/s41598-022-10977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabiu A.T., Mohan A., Çavdaroğlu S., Xenophontos E., Costa A., Tsagkaris C., et al. Dengue and COVID-19: a double burden to Brazil. J Med Virol. 2021;93(7):4092–4093. doi: 10.1002/jmv.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panda P.K., Sharawat I.K. COVID-19 and/with dengue infection: a curse in an overburdened healthcare system. Trop Doct. 2021;51(1):106–108. doi: 10.1177/0049475520975945. [DOI] [PubMed] [Google Scholar]
- 16.Harapan H., Ryan M., Yohan B., Abidin R.S., Nainu F., Rakib A., et al. Covid-19 and dengue: double punches for dengue-endemic countries in Asia. Rev Med Virol. 2021;31(2):e2161. doi: 10.1002/rmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malavige G.N., Jeewandara C., Ogg G.S. Dengue and COVID-19: two sides of the same coin. J Biomed Sci. 2022;29(1):48. doi: 10.1186/s12929-022-00833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prapty C., Rahmat R., Araf Y., Shounak S.K., Noor-A-Afrin RTI, et al. SARS-CoV-2 and dengue virus co-infection: epidemiology, pathogenesis, diagnosis, treatment, and management. Rev Med Virol. 2023;33(1):e2340. doi: 10.1002/rmv.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masyeni S., Santoso M.S., Widyaningsih P.D., Asmara D.W., Nainu F., Harapan H., et al. Serological cross-reaction and coinfection of dengue and COVID-19 in Asia: experience from Indonesia. Int J Infect Dis. 2021;102:152–154. doi: 10.1016/j.ijid.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Qushayri A.E., Kamel A., Reda A., Ghozy S. Does dengue and COVID-19 co-infection have worse outcomes? A systematic review of current evidence. Rev Med Virol. 2022;32(5):e2339. doi: 10.1002/rmv.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsheten T., Clements A., Gray D.J., Adhikary R.K., Wangdi K. Clinical features and outcomes of COVID-19 and dengue co-infection: a systematic review. BMC Infect Dis. 2021;21(1):729. doi: 10.1186/s12879-021-06409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srisawat N., Thisyakorn U., Ismail Z., Rafiq K., Gubler D.J. World Dengue Day: a call for action. PLoS Negl Trop Dis. 2022;16(8) doi: 10.1371/journal.pntd.0010586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S.K., Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol. 2021;67(10):687–702. doi: 10.1139/cjm-2020-0572. [DOI] [PubMed] [Google Scholar]
- 24.Mustafa M.S., Rasotgi V., Jain S., Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India. 2015;71(1):67–70. doi: 10.1016/j.mjafi.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uno N., Ross T.M. Dengue virus and the host innate immune response. Emerg Microbes Infect. 2018;7(1):167. doi: 10.1038/s41426-018-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn R.J., Zhang W., Rossmann M.G., Pletnev S.V., Corver J., Lenches E., et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108(5):717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khetarpal N., Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016 doi: 10.1155/2016/6803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan M., Johansson M.A. The incubation periods of dengue viruses. PLoS ONE. 2012;7(11):e50972. doi: 10.1371/journal.pone.0050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman M.G., Gubler D.J., Izquierdo A., Martinez E., Halstead S.B. Dengue infection. Nat Rev Dis Primers. 2016;2:16055. doi: 10.1038/nrdp.2016.55. [DOI] [PubMed] [Google Scholar]
- 30.Chen H.R., Lai Y.C., Yeh T.M. Dengue virus non-structural protein 1: a pathogenic factor, therapeutic target, and vaccine candidate. J Biomed Sci. 2018;25(1):58. doi: 10.1186/s12929-018-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SABIN A.B. The dengue group of viruses and its family relationships. Bacteriol Rev. 1950;14(3):225–232. doi: 10.1128/br.14.3.193-258.1950. [DOI] [PubMed] [Google Scholar]
- 32.Halstead S.B., O'Rourke E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halstead S.B. Immune enhancement of viral infection. Prog Allergy. 1982;31:301–364. [PubMed] [Google Scholar]
- 34.Rey F.A., Stiasny K., Vaney M.C., Dellarole M., Heinz F.X. The bright and the dark side of human antibody responses to flaviviruses: lessons for vaccine design. EMBO Rep. 2018;19(2):206–224. doi: 10.15252/embr.201745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothman A.L. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11(8):532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 36.Kuczera D., Assolini J.P., Tomiotto-Pellissier F., Pavanelli W.R., Silveira G.F. Highlights for dengue immunopathogenesis: antibody-dependent enhancement, cytokine storm, and beyond. J Interferon Cytokine Res. 2018;38(2):69–80. doi: 10.1089/jir.2017.0037. [DOI] [PubMed] [Google Scholar]
- 37.Alayli F., Scholle F. Dengue virus NS1 enhances viral replication and pro-inflammatory cytokine production in human dendritic cells. Virology. 2016;496:227–236. doi: 10.1016/j.virol.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meuren L.M., Prestes E.B., Papa M.P., de Carvalho L., Mustafá Y.M., da Costa L.S., et al. Infection of endothelial cells by dengue virus induces ROS production by different sources affecting virus replication, cellular activation, death and vascular permeability. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.810376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekaran S.D., Ismail A.A., Thergarajan G., Chandramathi S., Rahman S., Mani R.R., et al. Host immune response against DENV and ZIKV infections. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.975222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puerta-Guardo H., Glasner D.R., Espinosa D.A., Biering S.B., Patana M., Ratnasiri K., et al. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep. 2019;26(6) doi: 10.1016/j.celrep.2019.01.036. 1598-613.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo N., Roodsari S.Z., Tin N.L., Wong M.P., Biering S.B., Harris E. Molecular determinants of tissue specificity of flavivirus nonstructural protein 1 interaction with endothelial cells. J Virol. 2022;96(19) doi: 10.1128/jvi.00661-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beatty P.R., Puerta-Guardo H., Killingbeck S.S., Glasner D.R., Hopkins K., Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med. 2015;7(304):304ra141. doi: 10.1126/scitranslmed.aaa3787. [DOI] [PubMed] [Google Scholar]
- 43.Bhatt P., Sabeena S.P., Varma M., Arunkumar G. Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol. 2021;78(1):17–32. doi: 10.1007/s00284-020-02284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puerta-Guardo H., Glasner D.R., Harris E. Dengue Virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016;12(7) doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dib P., Quirino-Teixeira A.C., Merij L.B., Pinheiro M., Rozini S.V., Andrade F.B., et al. Innate immune receptors in platelets and platelet-leukocyte interactions. J Leukoc Biol. 2020;108(4):1157–1182. doi: 10.1002/JLB.4MR0620-701R. [DOI] [PubMed] [Google Scholar]
- 46.Quirino-Teixeira A.C., Andrade F.B., Pinheiro M., Rozini S.V., Hottz E.D. Platelets in dengue infection: more than a numbers game. Platelets. 2022;33(2):176–183. doi: 10.1080/09537104.2021.1921722. [DOI] [PubMed] [Google Scholar]
- 47.Pinheiro M., Rozini S.V., Quirino-Teixeira A.C., Barbosa-Lima G., Lopes J.F., Sacramento C.Q., et al. Dengue induces iNOS expression and nitric oxide synthesis in platelets through IL-1R. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1029213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malavige G.N., Ogg G.S. Pathogenesis of vascular leak in dengue virus infection. Immunology. 2017;151(3):261–269. doi: 10.1111/imm.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguilar-Briseño J.A., Moser J., IA Rodenhuis-Zybert. Understanding immunopathology of severe dengue: lessons learnt from sepsis. Curr Opin Virol. 2020;43:41–49. doi: 10.1016/j.coviro.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Chuansumrit A., Chaiyaratana W. Hemostatic derangement in dengue hemorrhagic fever. Thromb Res. 2014;133(1):10–16. doi: 10.1016/j.thromres.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 51.Clark K.B., Noisakran S., Onlamoon N., Hsiao H.M., Roback J., Villinger F., et al. Multiploid CD61+ cells are the pre-dominant cell lineage infected during acute dengue virus infection in bone marrow. PLoS ONE. 2012;7(12):e52902. doi: 10.1371/journal.pone.0052902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogt M.B., Lahon A., Arya R.P., Spencer Clinton J.L., Rico-Hesse R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Negl Trop Dis. 2019;13(11) doi: 10.1371/journal.pntd.0007837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun D.S., King C.C., Huang H.S., Shih Y.L., Lee C.C., Tsai W.J., et al. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost. 2007;5(11):2291–2299. doi: 10.1111/j.1538-7836.2007.02754.x. [DOI] [PubMed] [Google Scholar]
- 54.Saito M., Oishi K., Inoue S., Dimaano E.M., Alera M.T., Robles A.M., et al. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin Exp Immunol. 2004;138(2):299–303. doi: 10.1111/j.1365-2249.2004.02626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srichaikul T., Nimmannitya S., Sripaisarn T., Kamolsilpa M., Pulgate C. Platelet function during the acute phase of dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 1989;20(1):19–25. [PubMed] [Google Scholar]
- 56.Adane T., Getawa S. Coagulation abnormalities in dengue fever infection: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(8) doi: 10.1371/journal.pntd.0009666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wills B.A., Oragui E.E., Stephens A.C., Daramola O.A., Dung N.M., Loan H.T., et al. Coagulation abnormalities in dengue hemorrhagic Fever: serial investigations in 167 Vietnamese children with dengue shock syndrome. Clin Infect Dis. 2002;35(3):277–285. doi: 10.1086/341410. [DOI] [PubMed] [Google Scholar]
- 58.Katzelnick L.C., Gresh L., Halloran M.E., Mercado J.C., Kuan G., Gordon A., et al. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358(6365):929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrone S.R., Lok S.M. Structural perspectives of antibody-dependent enhancement of infection of dengue virus. Curr Opin Virol. 2019;36:1–8. doi: 10.1016/j.coviro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Alvarez M., Rodriguez-Roche R., Bernardo L., Vázquez S., Morier L., Gonzalez D., et al. Dengue hemorrhagic Fever caused by sequential dengue 1-3 virus infections over a long time interval: Havana epidemic, 2001–2002. Am J Trop Med Hyg. 2006;75(6):1113–1117. [PubMed] [Google Scholar]
- 61.Guzman M.G., Harris E. Dengue. Lancet. 2015;385(9966):453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 62.van Leur S.W., Heunis T., Munnur D., Sanyal S. Pathogenesis and virulence of flavivirus infections. Virulence. 2021;12(1):2814–2838. doi: 10.1080/21505594.2021.1996059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadinegoro S.R., Arredondo-García J.L., Capeding M.R., Deseda C., Chotpitayasunondh T., Dietze R., et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373(13):1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 64.WHO. W. H. O . 2018. Dengue vaccines: WHO position paper – September 2018.https://www.who.int/publications-detail-redirect/who-wer9335-457-476 Available from: [Google Scholar]
- 65.Katzelnick L.C., Narvaez C., Arguello S., Lopez Mercado B., Collado D., Ampie O., et al. Zika virus infection enhances future risk of severe dengue disease. Science. 2020;369(6507):1123–1128. doi: 10.1126/science.abb6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valencia B.M., Sigera P.C., Weeratunga P., Tedla N., Fernando D., Rajapakse S., et al. Effect of prior Zika and dengue virus exposure on the severity of a subsequent dengue infection in adults. Sci Rep. 2022;12(1):17225. doi: 10.1038/s41598-022-22231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitehorn J., Simmons C.P. The pathogenesis of dengue. Vaccine. 2011;29(42):7221–7228. doi: 10.1016/j.vaccine.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Bravo J.R., Guzmán M.G., Kouri G.P. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) Trans R Soc Trop Med Hyg. 1987;81(5):816–820. doi: 10.1016/0035-9203(87)90041-1. [DOI] [PubMed] [Google Scholar]
- 69.Halstead S.B., Streit T.G., Lafontant J.G., Putvatana R., Russell K., Sun W., et al. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65(3):180–183. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 70.Harapan H., Fajar J.K., Wahyuniati N., Anand J.R., Nambaru L., Jamil K.F. Non-HLA gene polymorphisms and their implications on dengue virus infection. Egypt J Med Hum Genet. 2013;14:1–11. doi: 10.1016/j.ejmhg.2012.08.003. [DOI] [Google Scholar]
- 71.Guzmán M.G., Kouri G., Bravo J., Valdes L., Vazquez S., Halstead S.B. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis. 2002;6(2):118–124. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 72.Anders K.L., Nguyet N.M., Chau N.V., Hung N.T., Thuy T.T., Lien le B., et al. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg. 2011;84(1):127–134. doi: 10.4269/ajtmh.2011.10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan K., Chen Y., Zhong M., Lin Y., Liu L. Risk and predictive factors for severe dengue infection: a systematic review and meta-analysis. PLoS ONE. 2022;17(4) doi: 10.1371/journal.pone.0267186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chagas G., Rangel A.R., Noronha L.M., Veloso F., Kassar S.B., Oliveira M., et al. Risk factors for mortality in patients with dengue: a systematic review and meta-analysis. Trop Med Int Health. 2022;27(8):656–668. doi: 10.1111/tmi.13797. [DOI] [PubMed] [Google Scholar]
- 75.Rathore A.P., Farouk F.S., St John A.L. Risk factors and biomarkers of severe dengue. Curr Opin Virol. 2020;43:1–8. doi: 10.1016/j.coviro.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Sangkaew S., Ming D., Boonyasiri A., Honeyford K., Kalayanarooj S., Yacoub S., et al. Risk predictors of progression to severe disease during the febrile phase of dengue: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(7):1014–1026. doi: 10.1016/S1473-3099(20)30601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsheten T., Clements A., Gray D.J., Adhikary R.K., Furuya-Kanamori L., Wangdi K. Clinical predictors of severe dengue: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10(1):123. doi: 10.1186/s40249-021-00908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kien N.D., El-Qushayri A.E., Ahmed A.M., Safi A., Mageed S.A., Mehyar S.M., et al. Correction to: association of allergic symptoms with dengue infection and severity: a systematic review and meta-analysis. Virol Sin. 2020;35(1):124. doi: 10.1007/s12250-019-00180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pang J., Hsu J.P., Yeo T.W., Leo Y.S., Lye D.C. Diabetes, cardiac disorders and asthma as risk factors for severe organ involvement among adult dengue patients: a matched case-control study. Sci Rep. 2017;7:39872. doi: 10.1038/srep39872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee I.K., Hsieh C.J., Lee C.T., Liu J.W. Diabetic patients suffering dengue are at risk for development of dengue shock syndrome/severe dengue: emphasizing the impacts of co-existing comorbidity(ies) and glycemic control on dengue severity. J Microbiol Immunol Infect. 2020;53(1):69–78. doi: 10.1016/j.jmii.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Sekaran S.D., Liew Z.M., Yam H.C., Raju C.S. The association between diabetes and obesity with dengue infections. Diabetol Metab Syndr. 2022;14(1):101. doi: 10.1186/s13098-022-00870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallagher P., Chan K.R., Rivino L., Yacoub S. The association of obesity and severe dengue: possible pathophysiological mechanisms. J Infect. 2020;81(1):10–16. doi: 10.1016/j.jinf.2020.04.039. [DOI] [PubMed] [Google Scholar]
- 83.Grange L., Simon-Loriere E., Sakuntabhai A., Gresh L., Paul R., Harris E. Epidemiological risk factors associated with high global frequency of inapparent dengue virus infections. Front Immunol. 2014;5:280. doi: 10.3389/fimmu.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kyaw A.K., Ngwe Tun M.M., Naing S.T., Htwe T.T., Mar T.T., Khaing T.M., et al. Inapparent dengue virus infection among students in Mandalay. Myanmar. Trans R Soc Trop Med Hyg. 2020;114(1):57–61. doi: 10.1093/trstmh/trz071. [DOI] [PubMed] [Google Scholar]
- 85.Manning J.E., Chea S., Parker D.M., Bohl J.A., Lay S., Mateja A., et al. Development of inapparent dengue associated with increased antibody levels to Aedes Aegypti salivary proteins: a longitudinal dengue cohort in Cambodia. J Infect Dis. 2022;226(8):1327–1337. doi: 10.1093/infdis/jiab541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong J.M., Adams L.E., Durbin A.P., Muñoz-Jordán J.L., Poehling K.A., Sánchez-González L.M., et al. Dengue: a growing problem with new interventions. Pediatrics. 2022;149(6) doi: 10.1542/peds.2021-055522. [pii] [DOI] [PubMed] [Google Scholar]
- 87.Kularatne S.A., Dalugama C. Dengue infection: global importance, immunopathology and management. Clin Med. 2022;22(1):9–13. doi: 10.7861/clinmed.2021-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.2009. Dengue: guidelines for diagnosis, treatment, prevention and control: new edition. Geneva. [PubMed] [Google Scholar]
- 89.Wilder-Smith A., Ooi E.E., Horstick O., Wills B. Dengue. Lancet. 2019;393(10169):350–363. doi: 10.1016/S0140-6736(18)32560-1. [DOI] [PubMed] [Google Scholar]
- 90.Chawla P., Yadav A., Chawla V. Clinical implications and treatment of dengue. Asian Pac J Trop Med. 2014;7(3):169–178. doi: 10.1016/S1995-7645(14)60016-X. [DOI] [PubMed] [Google Scholar]
- 91.Lee T.H., Lee L.K., Lye D.C., Leo Y.S. Current management of severe dengue infection. Expert Rev Anti Infect Ther. 2017;15(1):67–78. doi: 10.1080/14787210.2017.1248405. [DOI] [PubMed] [Google Scholar]
- 92.Muller D.A., Depelsenaire A.C., Young P.R. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl_2):S89–89S95. doi: 10.1093/infdis/jiw649. [DOI] [PubMed] [Google Scholar]
- 93.García G., González N., Pérez A.B., Sierra B., Aguirre E., Rizo D., et al. Long-term persistence of clinical symptoms in dengue-infected persons and its association with immunological disorders. Int J Infect Dis. 2011;15(1):e38–e43. doi: 10.1016/j.ijid.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Horstick O., Jaenisch T., Martinez E., Kroeger A., See L., Farrar J., et al. Comparing the usefulness of the 1997 and 2009 WHO dengue case classification: a systematic literature review. Am J Trop Med Hyg. 2014;91(3):621–634. doi: 10.4269/ajtmh.13-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ajlan B.A., Alafif M.M., Alawi M.M., Akbar N.A., Aldigs E.K., Madani T.A. Assessment of the new World Health Organization's dengue classification for predicting severity of illness and level of healthcare required. PLoS Negl Trop Dis. 2019;13(8) doi: 10.1371/journal.pntd.0007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prasad D., Kumar C., Jain A., Kumar R. Accuracy and applicability of the revised WHO classification (2009) of dengue in children seen at a tertiary healthcare facility in northern India. Infection. 2013;41(4):775–782. doi: 10.1007/s15010-013-0405-3. [DOI] [PubMed] [Google Scholar]
- 97.Thein T.L., Gan V.C., Lye D.C., Yung C.F., Leo Y.S. Utilities and limitations of the World Health Organization 2009 warning signs for adult dengue severity. PLoS Negl Trop Dis. 2013;7(1):e2023. doi: 10.1371/journal.pntd.0002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huy B.V., Toàn N.V. Prognostic indicators associated with progresses of severe dengue. PLoS ONE. 2022;17(1) doi: 10.1371/journal.pone.0262096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Md Sani S.S., Han W.H., Bujang M.A., Ding H.J., Ng K.L., Amir Shariffuddin M.A. Evaluation of creatine kinase and liver enzymes in identification of severe dengue. BMC Infect Dis. 2017;17(1):505. doi: 10.1186/s12879-017-2601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parkash O., Shueb R.H. Diagnosis of dengue infection using conventional and biosensor based techniques. Viruses. 2015;7(10):5410–5427. doi: 10.3390/v7102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kok B.H., Lim H.T., Lim C.P., Lai N.S., Leow C.Y., Leow C.H. Dengue virus infection - a review of pathogenesis, vaccines, diagnosis and therapy. Virus Res. 2023;324 doi: 10.1016/j.virusres.2022.199018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.World Health Organization & UNICEF/UNDP/World Bank/WHO. Special Programme for Research and Training in Tropical Diseases. Handbook for clinical management of dengue. Switzerland: World Health Organization; 2012. https://www.apps.who.int/iris/handle/10665/76887Geneva.
- 103.Raafat N., Blacksell S.D., Maude R.J. A review of dengue diagnostics and implications for surveillance and control. Trans R Soc Trop Med Hyg. 2019;113(11):653–660. doi: 10.1093/trstmh/trz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peeling R.W., Artsob H., Pelegrino J.L., Buchy P., Cardosa M.J., Devi S., et al. Evaluation of diagnostic tests: dengue. Nat Rev Microbiol. 2010;8(12 Suppl):S30–S38. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 105.Martins I.C., Ricardo R.C., Santos NC. Dengue, West Nile, and Zika Viruses: potential novel antiviral biologics drugs currently at discovery and preclinical development stages. Pharmaceutics. 2022;14(11):2535. doi: 10.3390/pharmaceutics14112535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Organización Panamericana de la Salud. Síntesis de evidencia: directrices para el diagnóstico y el tratamiento del dengue, el chikunguña y el zika en la Región de las Américas [Evidence synthesis: guidelines for diagnosis and treatment of dengue, chikungunya, and zika in the Region of the AmericasSíntese de evidências: diretrizes para o diagnóstico e o tratamento da dengue, chikungunya e zika na Região das Américas] Rev Panam Salud Publica. 2022 Jul 20;46:e82. doi: 10.26633/RPSP.2022.82. Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thein T.L., Lye D.C., Leo Y.S., Wong J.G., Hao Y., Wilder-Smith A. Severe neutropenia in dengue patients: prevalence and significance. Am J Trop Med Hyg. 2014;90(6):984–987. doi: 10.4269/ajtmh.14-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fernando S., Wijewickrama A., Gomes L., Punchihewa C.T., Madusanka S.D., Dissanayake H., et al. Patterns and causes of liver involvement in acute dengue infection. BMC Infect Dis. 2016;16:319. doi: 10.1186/s12879-016-1656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tejo A.M., Dias N.B., Tedesco W.L.D., Jr. Rabdomiólise maciça associada a miosite por dengue. Braz J Infect Dis. 2021;25 doi: 10.1016/j.bjid.2020.101202. [DOI] [Google Scholar]
- 110.Gulia M., Dalal P., Gupta M., Kaur D. Concurrent Guillain-Barré syndrome and myositis complicating dengue fever. BMJ Case Rep. 2020;13(2) doi: 10.1136/bcr-2019-232940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dewan N., Zuluaga D., Osorio L., Krienke M.E., Bakker C., Kirsch J. Ultrasound in dengue: a scoping review. Am J Trop Med Hyg. 2021;104(3):826–835. doi: 10.4269/ajtmh.20-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lum L., Ng C.J., Khoo E.M. Managing dengue fever in primary care: a practical approach. Malays Fam Physician. 2014;9(2):2–10. [PMC free article] [PubMed] [Google Scholar]
- 113.Trunfio M., Savoldi A., Viganò O., d'Arminio Monforte A. Bacterial coinfections in dengue virus disease: what we know and what is still obscure about an emerging concern. Infection. 2017;45(1):1–10. doi: 10.1007/s15010-016-0927-6. [DOI] [PubMed] [Google Scholar]
- 114.Lee J.C., Cia C.T., Lee N.Y., Ko N.Y., Chen P.L., Ko W.C. Causes of death among dengue patients causes of death among hospitalized adults with dengue fever in Tainan, 2015: emphasis on cardiac events and bacterial infections. J Microbiol Immunol Infect. 2022;55(2):207–214. doi: 10.1016/j.jmii.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 115.See K.C., Phua J., Yip H.S., Yeo L.L., Lim T.K. Identification of concurrent bacterial infection in adult patients with dengue. Am J Trop Med Hyg. 2013;89(4):804–810. doi: 10.4269/ajtmh.13-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nagassar R.P., Bridgelal-Nagassar R.J., McMorris N., Roye-Green K.J. Staphylococcus aureus pneumonia and dengue virus co-infection and review of implications of coinfection. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thein T.L., Ng E.L., Yeang M.S., Leo Y.S., Lye D.C. Risk factors for concurrent bacteremia in adult patients with dengue. J Microbiol Immunol Infect. 2017;50(3):314–320. doi: 10.1016/j.jmii.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 118.Chen C.M., Chan K.S., Cheng K.C., Chou W., Chao H.C., Yeh C.Y., et al. The exploration of risk factors of concurrent bacteraemia in patients critically ill with severe dengue. J Med Microbiol. 2016;65(12):1505–1511. doi: 10.1099/jmm.0.000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sunil T., Alexandra B., Tanu M., Ali M.M., Neha A., Navin B. Bacterial sepsis in dengue fever - a paediatric perspective. Trop Doct. 2021;51(3):371–375. doi: 10.1177/0049475521993608. [DOI] [PubMed] [Google Scholar]
- 120.Chen C.M., Chan K.S., Chao H.C., Lai C.C. Diagnostic performance of procalcitonin for bacteremia in patients with severe dengue infection in the intensive care unit. J Infect. 2016;73(1):93–95. doi: 10.1016/j.jinf.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 121.Trivedi S., Chakravarty A. Neurological complications of dengue fever. Curr Neurol Neurosci Rep. 2022;22(8):515–529. doi: 10.1007/s11910-022-01213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carod-Artal F.J., Wichmann O., Farrar J., Gascón J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906–919. doi: 10.1016/S1474-4422(13)70150-9. [DOI] [PubMed] [Google Scholar]
- 123.Carod-Artal F.J. Neurological manifestations of dengue viral infection. Res Rep Trop Med. 2014;5:95–104. doi: 10.2147/RRTM.S55372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Vries L., Harding A.T. Mechanisms of neuroinvasion and neuropathogenesis by pathologic flaviviruses. Viruses. 2023;15(2):261. doi: 10.3390/v15020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mehta V.K., Verma R., Jain A., Sharma N., Mahdi A.A. A study of dengue encephalitis with laboratory and clinical parameters in Tertiary Center of North India. J Family Med Prim Care. 2021;10(11):4041–4046. doi: 10.4103/jfmpc.jfmpc_632_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li G.H., Ning Z.J., Liu Y.M., Li X.H. Neurological manifestations of dengue infection. Front Cell Infect Microbiol. 2017;7:449. doi: 10.3389/fcimb.2017.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lnu P., Sehgal V., Bhalla Sehgal L., Gulati N., Kapila S. The spectrum of MRI findings in dengue encephalitis. Cureus. 2022;14(9):e29048. doi: 10.7759/cureus.29048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chia P.Y., Thein T.L., Ong S., Lye D.C., Leo Y.S. Severe dengue and liver involvement: an overview and review of the literature. Expert Rev Anti Infect Ther. 2020;18(3):181–189. doi: 10.1080/14787210.2020.1720652. [DOI] [PubMed] [Google Scholar]
- 129.Lin C.F., Wan S.W., Chen M.C., Lin S.C., Cheng C.C., Chiu S.C., et al. Liver injury caused by antibodies against dengue virus nonstructural protein 1 in a murine model. Lab Invest. 2008;88(10):1079–1089. doi: 10.1038/labinvest.2008.70. [DOI] [PubMed] [Google Scholar]
- 130.Dissanayake H.A., Seneviratne S.L. Liver involvement in dengue viral infections. Rev Med Virol. 2018;28(2) doi: 10.1002/rmv.1971. [DOI] [PubMed] [Google Scholar]
- 131.Rahim A., Hameed A., Ishaq U., Malik J., Zaidi S., Khurshid H., et al. Cardiovascular sequelae of dengue fever: a systematic review. Expert Rev Cardiovasc Ther. 2022;20(6):465–479. doi: 10.1080/14779072.2022.2082945. [DOI] [PubMed] [Google Scholar]
- 132.Yacoub S., Wertheim H., Simmons C.P., Screaton G., Wills B. Cardiovascular manifestations of the emerging dengue pandemic. Nat Rev Cardiol. 2014;11(6):335–345. doi: 10.1038/nrcardio.2014.40. [DOI] [PubMed] [Google Scholar]
- 133.Tayeb B., Piot C., Roubille F. Acute pericarditis after dengue fever. Ann Cardiol Angeiol. 2011;60(4):240–242. doi: 10.1016/j.ancard.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 134.Pesaro A.E., D'Amico E., Aranha L.F. Dengue: cardiac manifestations and implications in antithrombotic treatment. Arq Bras Cardiol. 2007;89(2):e12–e15. doi: 10.1590/s0066-782x2007001400015. [DOI] [PubMed] [Google Scholar]
- 135.Burdmann E.A. Flaviviruses and kidney diseases. Adv Chronic Kidney Dis. 2019;26(3):198–206. doi: 10.1053/j.ackd.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 136.Bignardi P.R., Pinto G.R., Boscarioli M., Lima R., Delfino V. Acute kidney injury associated with dengue virus infection: a review. J Bras Nefrol. 2022;44(2):232–237. doi: 10.1590/2175-8239-JBN-2021-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Masset C., Le Turnier P., Bressollette-Bodin C., Renaudin K., Raffi F., Dantal J. Virus-associated nephropathies: a narrative review. Int J Mol Sci. 2022;23(19):12014. doi: 10.3390/ijms231912014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pagliari C., Simões Quaresma J.A., Kanashiro-Galo L., de Carvalho L.V., Vitoria W.O., da Silva W.L., et al. Human kidney damage in fatal dengue hemorrhagic fever results of glomeruli injury mainly induced by IL17. J Clin Virol. 2016;75:16–20. doi: 10.1016/j.jcv.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 139.Nunes P., Rioja L., Coelho J., Salomão N.G., Rabelo K., José C.C., et al. Renal injury in DENV-4 fatal cases: viremia, immune response and cytokine profile. Pathogens. 2019;8(4):223. doi: 10.3390/pathogens8040223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daher E.F., Barros E., Silva Junior G.B. Balieiro; São Paulo: 2019. Nefrologia tropical. [Google Scholar]
- 141.Gurugama P., Jayarajah U., Wanigasuriya K., Wijewickrama A., Perera J., Seneviratne S.L. Renal manifestations of dengue virus infections. J Clin Virol. 2018;101:1–6. doi: 10.1016/j.jcv.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 142.Delfino V., Mazzali M. Dengue in kidney transplanted patients: additions to the puzzle. J Bras Nefrol. 2022;44(1):6–8. doi: 10.1590/2175-8239-JBN-2022-E003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Prasad N., Novak J.E., Patel M.R. Kidney diseases associated with parvovirus B19, Hanta, Ebola, and Dengue virus infection: a brief review. Adv Chronic Kidney Dis. 2019;26(3):207–219. doi: 10.1053/j.ackd.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 144.Vachvanichsanong P., Thisyakorn U., Thisyakorn C. Dengue hemorrhagic fever and the kidney. Arch Virol. 2016;161(4):771–778. doi: 10.1007/s00705-015-2727-1. [DOI] [PubMed] [Google Scholar]
- 145.Mehmood A., Afzal M.W., Ahmad M., Mufti M., Malik J., Zaidi S. Respiratory sequelae of dengue fever. Trop Doct. 2023;53(2):237–240. doi: 10.1177/00494755221127355. [DOI] [PubMed] [Google Scholar]
- 146.Marchiori E., Hochhegger B., Zanetti G. Pulmonary manifestations of dengue. J Bras Pneumol. 2020;46(1) doi: 10.1590/1806-3713/e20190246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Preeprem N., Phumeetham S. Paediatric dengue shock syndrome and acute respiratory failure: a single-centre retrospective study. BMJ Paediatr Open. 2022;6(1) doi: 10.1136/bmjpo-2022-001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de Almeida R.R., Paim B., de Oliveira S.A., Jr Souza AS, Gomes A., Escuissato D.L., et al. Dengue hemorrhagic fever: a state-of-the-art review focused in pulmonary involvement. Lung. 2017;195(4):389–395. doi: 10.1007/s00408-017-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.von Ranke F.M., Zanetti G., Hochhegger B., Marchiori E. Infectious diseases causing diffuse alveolar hemorrhage in immunocompetent patients: a state-of-the-art review. Lung. 2013;191(1):9–18. doi: 10.1007/s00408-012-9431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koyama H., Chierakul W., Charunwatthana P., Sanguanwongse N., Phonrat B., Silachamroon U., et al. Lung ultrasound findings of patients with dengue infection: a prospective observational study. Am J Trop Med Hyg. 2021;105(3):766–770. doi: 10.4269/ajtmh.20-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tejo A.M., Dias N.B., Inácio M.V.S., Del Tedesco W.L.D., Jr Púrpura trombocitopênica imune associada a infecção pelo vírus da dengue. 12°Congr Paul Infectologia. 2021;25 doi: 10.1016/j.bjid.2020.101211. [DOI] [Google Scholar]
- 152.Aguiar L.A., Oliveira-Scussel A., Menezes J.C., Idaló P.B., LÉG F., Zago L., et al. Pulmonary hemorrhage in dengue: differential diagnosis with acute viral respiratory syndromes including COVID-19. Rev Inst Med Trop Sao Paulo. 2022;64:e13. doi: 10.1590/S1678-9946202264013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kohli U., Saharan S., Lodha R., Kabra S.K. Persistent thrombocytopenia following dengue shock syndrome. Indian J Pediatr. 2008;75(1):82–83. doi: 10.1007/s12098-008-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thadchanamoorthy V., Dayasiri K. Dengue hemorrhagic fever as a rare cause of chronic immune thrombocytopenic purpura-a pediatric case report. Trop Med Health. 2020;48:59. doi: 10.1186/s41182-020-00248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boo Y.L., Lim S.Y., HS P'ng, Liam C., Huan N.C. Persistent thrombocytopenia following dengue fever: what should we do. Malays Fam Physician. 2019;14(3):71–73. [PMC free article] [PubMed] [Google Scholar]
- 156.Verma S.P., Hamide A., Wadhwa J., Sivamani K. Corticosteroid responsive prolonged thrombocytopenia in a case of dengue fever. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-200249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kumar P., Charaniya R., Ghosh A., Sahoo R. Intravenous immunoglobulin responsive persistent thrombocytopenia after dengue haemorrhagic fever. J Clin Diagn Res. 2016;10(4):OD10–OD11. doi: 10.7860/JCDR/2016/17770.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Henter J.I., Horne A., Aricó M., Egeler R.M., Filipovich A.H., Imashuku S., et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 159.Ramos-Casals M., Brito-Zerón P., López-Guillermo A., Khamashta M.A., Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 160.Karras A., Hermine O. Hemophagocytic syndrome. Rev Med Interne. 2002;23(9):768–778. doi: 10.1016/s0248-8663(02)00673-2. [DOI] [PubMed] [Google Scholar]
- 161.Rosado F.G., Kim A.S. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139(6):713–727. doi: 10.1309/AJCP4ZDKJ4ICOUAT. [DOI] [PubMed] [Google Scholar]
- 162.Wan Jamaludin W.F., Periyasamy P., Wan Mat W.R., Abdul Wahid S.F. Dengue infection associated hemophagocytic syndrome: therapeutic interventions and outcome. J Clin Virol. 2015;69:91–95. doi: 10.1016/j.jcv.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 163.Hines M.R., von Bahr Greenwood T., Beutel G., Beutel K., Hays J.A., Horne A., et al. Consensus-based guidelines for the recognition, diagnosis, and management of hemophagocytic lymphohistiocytosis in critically ill children and adults. Crit Care Med. 2022;50(5):860–872. doi: 10.1097/CCM.0000000000005361. [DOI] [PubMed] [Google Scholar]
- 164.de la Salud O.P. Evidence synthesis: guidelines for diagnosis and treatment of dengue, chikungunya, and zika in the Region of the AmericasSíntese de evidências: diretrizes para o diagnóstico e o tratamento da dengue, chikungunya e zika na Região das Américas. Rev Panam Salud Publica. 2022;46:e82. doi: 10.26633/RPSP.2022.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bandara S., Herath H. Effectiveness of corticosteroid in the treatment of dengue - a systemic review. Heliyon. 2018;4(9):e00816. doi: 10.1016/j.heliyon.2018.e00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rajapakse S. Intravenous immunoglobulins in the treatment of dengue illness. Trans R Soc Trop Med Hyg. 2009;103(9):867–870. doi: 10.1016/j.trstmh.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 167.Rajapakse S., Rodrigo C., Maduranga S., Rajapakse A.C. Corticosteroids in the treatment of dengue shock syndrome. Infect Drug Resist. 2014;7:137–143. doi: 10.2147/IDR.S55380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang F., Kramer C.V. Corticosteroids for dengue infection. Cochrane Database Syst Rev. 2014;2014(7) doi: 10.1002/14651858.CD003488.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ngo N.T., Cao X.T., Kneen R., Wills B., Nguyen V.M., Nguyen T.Q., et al. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis. 2001;32(2):204–213. doi: 10.1086/318479. [DOI] [PubMed] [Google Scholar]
- 170.Dung N.M., Day N.P., Tam D.T., Loan H.T., Chau H.T., Minh L.N., et al. Fluid replacement in dengue shock syndrome: a randomized, double-blind comparison of four intravenous-fluid regimens. Clin Infect Dis. 1999;29(4):787–794. doi: 10.1086/520435. [DOI] [PubMed] [Google Scholar]
- 171.Wills B.A., Nguyen M.D., Ha T.L., Dong T.H., Tran T.N., Le T.T., et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353(9):877–889. doi: 10.1056/NEJMoa044057. [DOI] [PubMed] [Google Scholar]
- 172.Thomas L., Brouste Y., Najioullah F., Hochedez P., Hatchuel Y., Moravie V., et al. Prospective and descriptive study of adult dengue cases in an emergency department, in Martinique. Med Mal Infect. 2010;40(8):480–489. doi: 10.1016/j.medmal.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 173.Laul A., Laul P., Merugumala V., Pathak R., Miglani U., Saxena P. Clinical profiles of dengue infection during an outbreak in Northern India. J Trop Med. 2016;2016 doi: 10.1155/2016/5917934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rai S., Chakravarti A., Matlani M., Bhalla P., Aggarwal V., Singh N., et al. Clinico-laboratory findings of patients during dengue outbreak from a tertiary care hospital in Delhi. Trop Doct. 2008;38(3):175–177. doi: 10.1258/td.2007.070229. [DOI] [PubMed] [Google Scholar]
- 175.Woon Y.L., Hor C.P., Hussin N., Zakaria A., Goh P.P., Cheah W.K. A two-year review on epidemiology and clinical characteristics of dengue deaths in Malaysia, 2013-2014. PLoS Negl Trop Dis. 2016;10(5) doi: 10.1371/journal.pntd.0004575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lye D.C., Archuleta S., Syed-Omar S.F., Low J.G., Oh H.M., Wei Y., et al. Prophylactic platelet transfusion plus supportive care versus supportive care alone in adults with dengue and thrombocytopenia: a multicentre, open-label, randomised, superiority trial. Lancet. 2017;389(10079):1611–1618. doi: 10.1016/S0140-6736(17)30269-6. [DOI] [PubMed] [Google Scholar]
- 177.Lye D.C., Lee V.J., Sun Y., Leo Y.S. Lack of efficacy of prophylactic platelet transfusion for severe thrombocytopenia in adults with acute uncomplicated dengue infection. Clin Infect Dis. 2009;48(9):1262–1265. doi: 10.1086/597773. [DOI] [PubMed] [Google Scholar]
- 178.Lum L.C., Goh A.Y., Chan P.W., El-Amin A.L., Lam S.K. Risk factors for hemorrhage in severe dengue infections. J Pediatr. 2002;140(5):629–631. doi: 10.1067/mpd.2002.123665. [DOI] [PubMed] [Google Scholar]
- 179.Pothapregada S., Kamalakannan B., Thulasingam M. Role of platelet transfusion in children with bleeding in dengue fever. J Vector Borne Dis. 2015;52(4):304–308. [PubMed] [Google Scholar]
- 180.Krishnamurti C., Kalayanarooj S., Cutting M.A., Peat R.A., Rothwell S.W., Reid T.J., et al. Mechanisms of hemorrhage in dengue without circulatory collapse. Am J Trop Med Hyg. 2001;65(6):840–847. doi: 10.4269/ajtmh.2001.65.840. [DOI] [PubMed] [Google Scholar]
- 181.Mairuhu A.T., Mac Gillavry M.R., Setiati T.E., Soemantri A., ten Cate H., Brandjes D.P., et al. Is clinical outcome of dengue-virus infections influenced by coagulation and fibrinolysis? A critical review of the evidence. Lancet Infect Dis. 2003;3(1):33–41. doi: 10.1016/s1473-3099(03)00487-0. [DOI] [PubMed] [Google Scholar]
- 182.Lum L.C., Mel-A A., Goh A.Y., Chan P.W., Lam S.K. Preventive transfusion in dengue shock syndrome-is it necessary. J Pediatr. 2003;143(5):682–684. doi: 10.1067/s0022-3476(03)00503-1. [DOI] [PubMed] [Google Scholar]
- 183.Khan Assir M.Z., Kamran U., Ahmad H.I., Bashir S., Mansoor H., Anees S.B., et al. Effectiveness of platelet transfusion in dengue Fever: a randomized controlled trial. Transfus Med Hemother. 2013;40(5):362–368. doi: 10.1159/000354837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee T.H., Wong J.G., Leo Y.S., Thein T.L., Ng E.L., Lee L.K., et al. Potential harm of prophylactic platelet transfusion in adult dengue patients. PLoS Negl Trop Dis. 2016;10(3) doi: 10.1371/journal.pntd.0004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rajapakse S., de Silva N.L., Weeratunga P., Rodrigo C., Fernando S.D. Prophylactic and therapeutic interventions for bleeding in dengue: a systematic review. Trans R Soc Trop Med Hyg. 2017;111(10):433–439. doi: 10.1093/trstmh/trx079. [DOI] [PubMed] [Google Scholar]
- 186.Tayal A., Kabra S.K., Lodha R. Management of Dengue: an updated review. Indian J Pediatr. 2023;90(2):168–177. doi: 10.1007/s12098-022-04394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brasil. Ministério da Saúde Secretaria de Vigilância em Saúde. Departamento de Vigilância das. Dengue : Diagnóstico e Manejo Clínico : Adulto e Criança. 2016 [Google Scholar]
- 188.Tangnararatchakit K., Chuansumrit A., Chaiyaratana W., Lertwongrath S., Gajaseeni N., Udomchaisakul R., et al. Excessive menstrual bleeding in adolescents with dengue infection. Pediatr Infect Dis J. 2010;29(1):92–93. doi: 10.1097/INF.0b013e3181bf5406. [DOI] [PubMed] [Google Scholar]
- 189.Chong V., Tan J., Arasoo V. Dengue in pregnancy: a southeast asian perspective. Trop Med Infect Dis. 2023;8(2):86. doi: 10.3390/tropicalmed8020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Howard-Jones A.R., Pham D., Sparks R., Maddocks S., Dwyer D.E., Kok J., et al. Arthropod-borne flaviviruses in pregnancy. Microorganisms. 2023;11(2):433. doi: 10.3390/microorganisms11020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Machado C.R., Machado E.S., Rohloff R.D., Azevedo M., Campos D.P., de Oliveira R.B., et al. Is pregnancy associated with severe dengue? A review of data from the Rio de Janeiro surveillance information system. PLoS Negl Trop Dis. 2013;7(5):e2217. doi: 10.1371/journal.pntd.0002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rathore S.S., Oberoi S., Hilliard J., Raja R., Ahmed N.K., Vishwakarma Y., et al. Maternal and foetal-neonatal outcomes of dengue virus infection during pregnancy. Trop Med Int Health. 2022;27(7):619–629. doi: 10.1111/tmi.13783. [DOI] [PubMed] [Google Scholar]
- 193.Paixão E.S., Teixeira M.G., Costa M., Rodrigues L.C. Dengue during pregnancy and adverse fetal outcomes: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):857–865. doi: 10.1016/S1473-3099(16)00088-8. [DOI] [PubMed] [Google Scholar]
- 194.Paixão E.S., Campbell O.M., Teixeira M.G., Costa M.C., Harron K., Barreto M.L., et al. Dengue during pregnancy and live birth outcomes: a cohort of linked data from Brazil. BMJ Open. 2019;9(7) doi: 10.1136/bmjopen-2018-023529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Paixão E.S., Teixeira M.G., Costa M., Barreto M.L., Rodrigues L.C. Symptomatic dengue during pregnancy and congenital neurologic malformations. Emerg Infect Dis. 2018;24(9):1748–1750. doi: 10.3201/eid2409.170361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Escobar M.F., Mora B.L., Cedano J.A., Loaiza S., Rosso F. Comprehensive treatment in severe dengue during preterm and term labor: could tocolysis be useful. J Matern Fetal Neonatal Med. 2020;33(14):2445–2450. doi: 10.1080/14767058.2018.1554044. [DOI] [PubMed] [Google Scholar]
- 197.Desgraupes S., Hubert M., Gessain A., Ceccaldi P.E., Vidy A. Mother-to-child transmission of arboviruses during breastfeeding: from epidemiology to cellular mechanisms. Viruses. 2021;13(7):1312. doi: 10.3390/v13071312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee C.C., Hsu H.C., Chang C.M., Hong M.Y., Ko W.C. Atypical presentations of dengue disease in the elderly visiting the ED. Am J Emerg Med. 2013;31(5):783–787. doi: 10.1016/j.ajem.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 199.Ng W.Y., Ngim C.F., Chow K.Y., Goh S., Zaid M., Dhanoa A. Clinical manifestations, laboratory profile and outcomes of dengue virus infection in hospitalised older patients. Trans R Soc Trop Med Hyg. 2022;116(6):545–554. doi: 10.1093/trstmh/trab168. [DOI] [PubMed] [Google Scholar]
- 200.Rowe E.K., Leo Y.S., Wong J.G., Thein T.L., Gan V.C., Lee L.K., et al. Challenges in dengue fever in the elderly: atypical presentation and risk of severe dengue and hospital-acquired infection [corrected] PLoS Negl Trop Dis. 2014;8(4):e2777. doi: 10.1371/journal.pntd.0002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lin R.J., Lee T.H., Leo Y.S. Dengue in the elderly: a review. Expert Rev Anti Infect Ther. 2017;15(8):729–735. doi: 10.1080/14787210.2017.1358610. [DOI] [PubMed] [Google Scholar]
- 202.Kuo H.J., Lee I.K., Liu J.W. Analyses of clinical and laboratory characteristics of dengue adults at their hospital presentations based on the World Health Organization clinical-phase framework: emphasizing risk of severe dengue in the elderly. J Microbiol Immunol Infect. 2018;51(6):740–748. doi: 10.1016/j.jmii.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 203.Hökerberg Y., Kohn F., Souza T.S., Passos S. Clinical profile of dengue in the elderly using surveillance data from two epidemics. Rev Soc Bras Med Trop. 2022;55:e0290. doi: 10.1590/0037-8682-0290-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee I.K., Liu J.W., Yang K.D. Clinical and laboratory characteristics and risk factors for fatality in elderly patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 2008;79(2):149–153. doi: 10.4269/ajtmh.2008.79.149. [DOI] [PubMed] [Google Scholar]
- 205.Lum L.H., Chan M., Leo Y.S. Strategy in managing anticoagulation therapy following prosthetic heart valve replacement in a patient with dengue fever. Int J Cardiol. 2015;199:432–434. doi: 10.1016/j.ijcard.2015.07.098. [DOI] [PubMed] [Google Scholar]
- 206.Gamakaranage C., Rodrigo C., Samarawickrama S., Wijayaratne D., Jayawardane M., Karunanayake P., et al. Dengue hemorrhagic fever and severe thrombocytopenia in a patient on mandatory anticoagulation: balancing two life threatening conditions: a case report. BMC Infect Dis. 2012;12:272. doi: 10.1186/1471-2334-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chia P.Y., Htun H.L., Leo Y.S., Lye D.C. Safety of temporary interruption of antiplatelet therapy in dengue fever with thrombocytopenia. J Infect. 2021;82(2):270–275. doi: 10.1016/j.jinf.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 208.Gupta K., Shah D., Naik D., Chandan A., Bhavsar N., Shah B. Dengue fever after coronary artery bypass grafting with ventricular dysfunction. Asian Cardiovasc Thorac Ann. 2021;29(3):220–222. doi: 10.1177/0218492320963957. [DOI] [PubMed] [Google Scholar]
- 209.Ehelepola N., Athurupana A., Bowatte P., Dissanayake W.P. Continuation of dual antiplatelet therapy in a patient with a coronary artery stent with dengue hemorrhagic fever: a clinical conundrum. Am J Trop Med Hyg. 2020;102(1):17–19. doi: 10.4269/ajtmh.19-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Martínez-Gutierrez M., Castellanos J.E., Gallego-Gómez J.C. Statins reduce dengue virus production via decreased virion assembly. Intervirology. 2011;54(4):202–216. doi: 10.1159/000321892. [DOI] [PubMed] [Google Scholar]
- 211.Martinez-Gutierrez M., Correa-Londoño L.A., Castellanos J.E., Gallego-Gómez J.C., Osorio J.E. Lovastatin delays infection and increases survival rates in AG129 mice infected with dengue virus serotype 2. PLoS ONE. 2014;9(2):e87412. doi: 10.1371/journal.pone.0087412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Whitehorn J., Nguyen C., Khanh L.P., Kien D., Quyen N., Tran N., et al. Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2016;62(4):468–476. doi: 10.1093/cid/civ949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chia P.Y., Htun H.L., Ling W.P., Leo Y.S., Yeo T.W., Lye D. Hyperlipidemia, statin use and dengue severity. Sci Rep. 2018;8(1):17147. doi: 10.1038/s41598-018-35334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bifani A.M., Chan K., Borrenberghs D., Tan M., Phoo W.W., Watanabe S., et al. Therapeutics for flaviviral infections. Antiviral Res. 2023;210 doi: 10.1016/j.antiviral.2022.105517. [DOI] [PubMed] [Google Scholar]
- 215.Obi J.O., Gutiérrez-Barbosa H., Chua J.V., Deredge D.J. Current trends and limitations in dengue antiviral research. Trop Med Infect Dis. 2021;6(4):180. doi: 10.3390/tropicalmed6040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pintado Silva J., Fernandez-Sesma A. Challenges on the development of a dengue vaccine: a comprehensive review of the state of the art. J Gen Virol. 2023;104(3) doi: 10.1099/jgv.0.001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Villar L., Dayan G.H., Arredondo-García J.L., Rivera D.M., Cunha R., Deseda C., et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372(2):113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 218.Sridhar S., Luedtke A., Langevin E., Zhu M., Bonaparte M., Machabert T., et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379(4):327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 219.Hou J., Ye W., Chen J. Current development and challenges of tetravalent live-attenuated dengue vaccines. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.840104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Biswal S., Reynales H., Saez-Llorens X., Lopez P., Borja-Tabora C., Kosalaraksa P., et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med. 2019;381(21):2009–2019. doi: 10.1056/NEJMoa1903869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the present study are available from the corresponding author upon reasonable request.