Abstract
Mucin-specific adhesion of Pseudomonas aeruginosa plays an important role in the initial colonization of this organism in the airways of cystic fibrosis patients. We report here that the flagellar cap protein, FliD, participates in this adhesion process. A polar chromosomal insertional mutation in the P. aeruginosa fliD gene made this organism nonadhesive to mucin in an in vitro mucin adhesion assay. The adhesive phenotype was restored by providing the fliD gene alone on a multicopy plasmid, suggesting involvement of this gene in mucin adhesion of P. aeruginosa. Further supporting this observation, the in vitro competition experiments demonstrated that purified FliD protein inhibited the mucin adhesion of nonpiliated P. aeruginosa PAK-NP, while the same concentrations of PilA and FlaG proteins of P. aeruginosa were ineffective in this function. The regulation of the fliD gene was studied and was found to be unique in that the transcription of the fliD gene was independent of the flagellar sigma factor ς28. Consistent with this finding, no ς28 binding sequence could be identified in the fliD promoter region. The results of the β-galactosidase assays suggest that the fliD gene in P. aeruginosa is regulated by the newly described transcriptional regulator FleQ and the alternate sigma factor ς54 (RpoN).
Pseudomonas aeruginosa is an opportunistic human pathogen that causes lethal infections in compromised individuals and chronic colonization of the lungs of patients with cystic fibrosis, leading to their death. P. aeruginosa has a remarkable ability to persist in the lungs successfully by colonizing respiratory mucus. The molecular mechanisms by which this organism attaches to and colonizes the human airways are poorly understood.
P. aeruginosa has been demonstrated to adhere to intact respiratory epithelial cells in culture (22) as well as to injured respiratory tissue organ culture (32) and injured whole animal trachea (19). Pili present on the surface of P. aeruginosa have been shown to contribute significantly to attachment to the epithelial cells (34). However, P. aeruginosa mutants lacking pili attach to mucin as efficiently as the wild-type strains (20).
Earlier studies from our laboratory have demonstrated an association between the expression of mucin adhesin(s) and the expression of some flagellar genes in P. aeruginosa (24). Mutants defective in the fliF gene (2), which codes for the flagellar membrane and supramembrane ring, and the fliO gene (25), which codes for one of the proteins of the flagellar export apparatus, were nonmotile and nonadhesive, whereas a fliC mutant which is nonmotile and does not make flagellin retains adhesion to mucin (24). These findings suggest that either the mucin adhesin is a structural component of the flagellar apparatus or it utilizes the flagellar export and secretion machinery. Additionally, an alternate sigma factor, RpoN, not only is involved in the transcription of genes specifying bacterial adhesion to mucin and epithelial cells, but also is involved in the expression of flagellar genes (7, 20, 31). Moreover, we have recently identified two regulators, FleR and FleQ, that can potentially work with RpoN to regulate flagellar expression and mucin adhesion (3, 21). However, the specific targets of FleR and FleQ action are still not known. RpoN has also been shown to be important in the regulation of flagellar genes in Pseudomonas putida (8), Caulobacter crescentus (5), Vibrio parahaemolyticus (15), and Campylobacter coli (11).
This report presents evidence that the flagellar cap protein (FliD) is directly involved in mucin adhesion. The nucleotide sequence of the fliD gene of P. aeruginosa was determined, and a chromosomal fliD mutant (PAK-NPD) was constructed by mutation in P. aeruginosa PAK-NP. This mutant was found to be nonmotile and nonadhesive. The motility and adhesion defects of PAK-NPD were complemented by the fliD gene alone, thus suggesting the involvement of FliD in mucin adhesion. The fliD gene product was purified from the Escherichia coli host and was used as a competitor in an in vitro adherence assay. The pure FliD protein specifically inhibited the binding of P. aeruginosa PAK-NP to human respiratory mucins, which indicates direct involvement of this protein in mucin binding. Analysis of the promoter region of the fliD gene suggested that this gene is regulated by the regulator FleQ, which works in concert with RpoN, and is independent of ς28, which controls the fliD genes of many other bacterial species (13).
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
All bacterial strains, plasmid vectors, and their derivatives are shown in Table 1. The bacterial cultures were grown in liquid Luria broth (17), tryptic soy broth (23), or on L agar plates (1.7% agar) with or without antibiotics. The antibiotic concentrations used were as follows: for E. coli, ampicillin at 200 μg/ml and gentamicin at 10 μg/ml; for P. aeruginosa, carbenicillin at 150 μg/ml, gentamicin at 50 μg/ml, tetracycline at 100 μg/ml, and streptomycin at 300 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | hsdR recA lacZYA φ80 lacZ ΔM15 | GIBCO BRL |
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Novagen, Inc., Madison, Wis. |
| P. aeruginosa | ||
| PAK | Wild-type clinical isolate | D. Bradley |
| PAK-NP | PAKpilA::Tcr | 22 |
| PAK-N1G | PAKrpoN::Gmr | 9 |
| PAK-RG | PAKfleR::Gmr | 21 |
| PAK-Q | PAKfleQ::Gmr | 3 |
| MS540 | PAKfliA::Gmr | 28 |
| PAK-D | PAKfliD::Gmr | This study |
| PAK-NPD | PAKpilA::TcrfliD::Gmr | This study |
| Plasmids | ||
| pUC18 | E. coli cloning vector, Ampr | GIBCO BRL |
| pLysS | Plasmid containing the T7 lysozyme gene | Novagen |
| pGEM3Z | Sequencing vector, Ampr, lacZα peptide | Promega, Madison, Wis. |
| pBluescript KS(+) | E. coli cloning vector, Ampr | Stratagene, Inc. |
| pDN19lacΩ | Promoterless lacZ oriV oriT Tetr Strr Ω fragment | 30 |
| pPZ375 | oriV in pGEM3Z | 29 |
| pG10E | pGEM3Z containing a 10-kb EcoRI fragment isolated from the cosmid pRR194 | This study |
| pBS7EA | pBluescript KS(+) containing a 7-kb EcoRI-ApaI insert including the fliDSorf126orf96 operon | This study |
| pBS7EAG | pBS7EA with a gentamicin resistance gene inserted into the EcoRV site in the fliD gene | This study |
| placΩD | pDN19lacΩ with a 495-bp EcoRI-BamHI fragment including the promoter region of the fliD gene | This study |
| pPZ375D | pPZ375 with a complete fliD gene | This study |
| pET15B | Expression vector, T7 promoter, His-tag coding sequence, Ampr, pBR322 origin | Novagen |
| pET15BD | fliD gene inserted as a PCR product into the NdeI-BamHI sites of pET15B | This study |
Enzymes and chemicals.
T4 DNA ligase and all restriction enzymes were purchased from GIBCO-BRL, Inc., Gaithersburg, Md. Pfu DNA polymerase was purchased from Stratagene, La Jolla, Calif. The chemicals were purchased either from Sigma Chemical Co., St. Louis, Mo., or from Amresco, Inc., Solon, Ohio. The Bio-Rad protein assay kit was purchased from Bio-Rad Laboratories, Hercules, Calif.
Electroporations.
Electroporations were performed by a modification of the protocol of Smith and Iglewski (26). The DNA used for the electroporations was prepared by the alkaline lysis procedure (4). For gene replacement experiments involving chromosomal recombinations, the plasmid DNA was linearized by a restriction enzyme and gel purified. About 1 μg of linear DNA fragment was electroporated into the electrocompetent P. aeruginosa cells. For complementation experiments, 50 to 100 ng of supercoiled or covalently closed-circular plasmid DNA was electroporated into the target strains.
β-Galactosidase assay.
Expression of the lacZ gene under the control of the putative fliD promoter region of the fliDSorf126orf96 promoter was measured by β-galactosidase assays as described by Miller (17) with minor modifications. The cells were grown to late log phase (A600 of 0.7 to 1.0), which usually took about 4 to 4.5 h. At this point, the cells were harvested and assayed for β-galactosidase activity. The bacteria containing the lacΩ plasmids were grown in L broth with streptomycin.
PCR amplification.
PCRs were performed in a DNA Thermal Cycler 480 (Perkin-Elmer Cetus). The reactions were performed in 100-μl volumes with Pfu polymerase. Each reaction mixture contained a final concentration of 50 ng of DNA template, 2.5 U of Pfu polymerase, 2.0 mM MgCl2, 0.1 mM deoxynucleoside triphosphates mix, 10% dimethyl sulfoxide, and 0.2 μM primers. Forty cycles were run, each consisting of incubations for 2 min at 95°C, 1 min at 46°C, and 6 min at 72°C. The annealing temperature was kept low because of the low ionic strength of the Pfu reaction buffer, and the extension time increased to 6 min to accommodate the low proofreading capacity of the Pfu polymerase. The primers used for PCRs were purchased from GIBCO BRL. Restriction sites were added to the ends of primers (shown in boldface) to facilitate subsequent cloning of the PCR products. Additional nucleotides were added 5′ to the restriction sites to ensure efficient cleavage. The following primers were used in the PCRs. RER36 and RER40 were used for the PCR amplification of the fliD promoter. RER36 [5′(CCCAAAGAATTCATGGACGTCAGCAATGTC)3′] was located at nucleotide 862 (accession no. L81176); an EcoRI site was added to this primer. RER40 [5′(CCCAAAGGATCCTGTAGCCGTTGATCGTCG)3′] was located between nucleotides 1339 and 1356 (accession no. L81176); a BamHI site was added to this primer. FliD5p (CCCAAAGAATTCAGGAGAAGCAAGATGGCGAAC) was used as a 5′ primer to amplify the complete fliD gene, which was cloned into the vector pPZ375 (29); an EcoRI site was added to this primer, which is shown here in boldface. FliD3p (CCCAAAGGATCCTCAGCTTTTCTTCACAAGGCC) was used as the 3′ primer to amplify the complete fliD gene, which was cloned into the vector pPZ375; a BamHI site was added to this primer, which is shown in boldface. RER39 [5′(CCCAAAAAAAAACATATGGCGAACAGTACGACG)3′] was used as the 5′ primer to amplify the complete fliD gene, which was cloned into the vector pET15B (Novagen, Inc., Madison, Wis.); an NdeI site was added to this primer, which is shown here in boldface.
Plasmid constructions.
pG10E was constructed by cloning a 10-kb EcoRI fragment which was excised from the cosmid pRR194 (21) into the EcoRI site of pGEM3Z (Promega, Inc., Madison, Wis.). A 7-kb EcoRI-ApaI fragment was isolated from pG10E and inserted into the EcoRI and ApaI sites of pBluescript KS(+), to give pBS7EA. This construct contained the complete fliDSorf126orf96 operon. The plasmid pBS7EA was partially digested with EcoRV (two EcoRV sites), and a gentamicin resistance gene cassette was inserted selectively in the EcoRV site present in the fliD gene, leading to the construction of pBS7EAG. This construct was utilized to generate a chromosomal mutation in the P. aeruginosa fliD gene by marker exchange. The plasmid used for complementation of the fliD mutation, pPZ375D, was obtained by cloning a 1.4-kb PCR fragment carrying the complete fliD gene into pPZ375. The fliD expression construct, pET15BD, was constructed by cloning a 1.4-kb PCR fragment containing the complete fliD gene into the NdeI and BamHI sites of the expression vector pET15B. The promoter fusion construct placΩD was the result of cloning of a 495-bp EcoRI-BamHI fragment containing the putative fliD promoter region into the EcoRI and BamHI sites of the promoter probe vector pDN19lacΩ (30).
DNA sequencing.
DNA sequencing was performed according to the Taq dyedeoxy terminator and dye primer cycle sequencing protocols developed by Applied Biosystems (Perkin-Elmer Corp., Foster City, Calif.). Fluorescence-labeled dideoxynucleotides and primers were used, respectively. The labeled extension products were analyzed with an Applied Biosystems model 373A DNA sequencer. Double-stranded sequences were aligned and assembled by programs in the Sequencher software package (Gene Codes Corp., Ann Arbor, Mich.).
Motility assay.
Bacterial strains were grown overnight at 37°C on fresh L agar plates with or without antibiotics. The cells were then transferred with a sterile toothpick to 0.3% agar plates with or without antibiotics. These plates were incubated at 37°C for 16 h, and motility was assessed qualitatively by examining the circular swarm formed by the growing bacterial cells.
Adhesion assay.
Human tracheobronchial mucins were prepared from sputum of a patient with chronic bronchitis by ultracentrifugation, as described previously (35). The bacterial strains were grown in Trypticase soy broth (BBL Microbiology Systems) overnight at 37°C, and the inoculum was adjusted by spectrophotometer to between 1 × 107 and 2 × 107 CFU/ml. Strains containing plasmids which coded for antibiotic resistance were grown in broth containing carbenicillin (150 μg/ml). Microtiter plates were coated with mucins at a concentration of 50 μg/ml (33). Bacteria were added to the wells, and the plates were incubated at 37°C for 30 min. The plates were washed 15 times in a manually operated microtiter plate washer, and the bacteria bound to the wells were desorbed with Triton X-100 and plated for enumeration. Each strain was tested a minimum of three times. The results are mean values derived from these experiments.
Expression and purification of FliD.
The complete fliD coding sequence was inserted as a 1.4-kb PCR product into the NdeI-BamHI sites of the plasmid pET15B. The resulting plasmid, pET15BD, was introduced into E. coli BL21(pLysS) (Novagen), which contains the T7 polymerase gene on the chromosome under the control of the lacUV5 promoter. Bacterial cultures were grown to A550s of 0.4 to 0.5, and the T7 promoter was induced by the addition of a 2.0 mM final concentration of isopropyl-β-d-thiogalactopyranoside (IPTG). The cultures were grown for an additional 4 h and then harvested. These pellets were resuspended in binding buffer (5 mM imidazole, 0.5 M NaCl, 20 mM Tris-HCl [pH 7.9]). The cell lysate was prepared by disrupting the cells in a French pressure cell at 16,000 lb/in2. The cell debris was removed by centrifugation at 15,000 × g for 30 min. A small disposable column containing 2.5 ml of chelating Sepharose Fast Flow resin (Pharmacia Biotech, Inc., Piscataway, N.J.) was packed. The column was charged with 50 mM NiSO4 according to the pET instruction manual provided by Novagen, Inc. Further steps in the purification of His-FliD were performed according to the pET instruction manual. The His-FliD protein was finally eluted with elution buffer (1 M imidazole, 0.4 M NaCl, 20 mM Tris-HCl [pH 7.9]). The protein was dialyzed against 100 volumes of phosphate-buffered saline (PBS) with three changes. The dialyzed purified His-FliD protein was stored at 4°C.
Thrombin cleavage of His-FliD protein.
The 6× His tag was removed from the His-FliD fusion protein by using the thrombin cleavage capture kit (Novagen). Fifty micrograms of the fusion protein was incubated at room temperature with 0.5 U of biotinylated thrombin and thrombin cleavage buffer in a total reaction volume of 150 μl. The incubation was done for 4 h with continuous mixing of the reaction components. The uncleaved His-FliD fusion protein was removed from the reaction mixture by allowing it to bind to the His tag affinity resin at room temperature for 90 min. The supernatant contained essentially the pure FliD protein.
Bio-Rad protein assay.
Proteins were quantitated by using the Bio-Rad protein assay kit. The Bio-Rad protein assay is based on the color change of Coomassie brilliant blue G-250 dye in response to various concentrations of protein. The manufacturer’s instructions were followed to perform this assay.
Nucleotide sequence accession number.
The nucleotide sequence consisting of 3,925 nucleotides containing the complete sequences of flaG, fliD, fliS, orf126, and orf96 has been submitted to GenBank (accession no. L81176).
RESULTS
Nucleotide sequence of the chromosomal region containing fliD and fliS.
The nucleotide sequence of a 3.9-kb fragment of P. aeruginosa DNA was determined on both DNA strands. This region contained four open reading frames (ORFs) which appeared to comprise a single operon. The location of these ORFs relative to other flagellar genes is shown in Fig. 1. The first ORF consisted of 1,437 nucleotides, which is predicted to contain a gene that codes for a polypeptide consisting of 478 amino acids (molecular mass, 52.6 kDa). The deduced amino acid sequence of this ORF was compared to known protein sequences in the GenBank, PI, and SWISS-PROT databases by the BLAST program (1). This ORF had strong homology to the fliD genes of other bacteria (Fig. 2) and was therefore called the fliD gene of P. aeruginosa. As shown in Fig. 2, the P. aeruginosa FliD (flagellar cap protein) was homologous to the FliDs of other bacteria throughout the ORF, except for a stretch of 12 amino acids (amino acids 196 to 207) which were found in the P. aeruginosa FliD but were absent from all the other cap proteins to which it was compared. The functional significance of these amino acids remains to be explored.
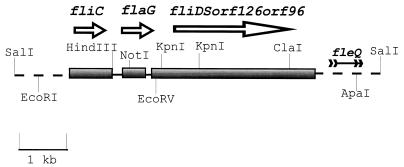
FIG. 1.
Map of the fliDSorf126orf96 region showing the gene arrangement in P. aeruginosa. The map is drawn approximately to scale. The shaded rectangles show the coding regions. The arrows beside the shaded rectangles indicate the direction of transcription. The EcoRV site used for insertional inactivation of the fliD gene is shown.
FIG. 2.
Computer-generated alignment (Prettybox program [Richard Westerman, Purdue University]) of FliD of P. aeruginosa with homologous FliD proteins of other organisms. Dark shading shows identity of amino acids, while the two shades of gray show degrees of similarity (based on the GCG Comparison Table [Genetics Computer Group, University of Wisconsin, Madison]). Psa, P. aeruginosa; Eco, E. coli; Salty, S. typhimurium; Bacsu, B. subtilis; Vibpa, V. parahaemolyticus.
The second ORF consisted of 399 nucleotides, which is predicted to contain a gene that codes for a polypeptide consisting of 132 amino acids (molecular mass, 14.5 kDa). This gene has a strong homology to fliS genes of other bacteria, and it very likely represents the P. aeruginosa fliS homolog. The third ORF consisted of 381 nucleotides, which is predicted to contain a gene that codes for a polypeptide consisting of 126 amino acids (molecular mass, 13.9 kDa). This ORF is also homologous with the fliS genes of other bacteria, including that of P. aeruginosa. In other bacterial species, these genes are arranged as an operon, consisting of fliDST genes (6, 10). In P. aeruginosa, the fliS gene has apparently undergone a duplication, and the two homologs have evolved separately. The P. aeruginosa fliS and orf126 share 38.5% identity and 59% similarity at the amino acid level. Whether the fliT gene is found at another chromosomal location and whether the product of the orf126 gene performs the same function in P. aeruginosa as FliT does in other bacteria are not known at present. The fourth ORF (orf96) consisting of 288 nucleotides had no apparent homology to any known proteins in the database.
Upstream of the fliDSorf126orf96 operon (Fig. 1), an ORF was identified which consisted of 384 nucleotides and was predicted to contain a gene that codes for a polypeptide consisting of 128 amino acids (molecular mass, 14 kDa). This ORF was homologous to the flaG genes of Vibrio anguillarum (16), V. parahaemolyticus (15), and Bacillus subtilis (6) and was therefore called the flaG gene.
Construction of a fliD mutant and its complementation.
In order to examine the possible function of FliD, a chromosomal fliD mutant was constructed in P. aeruginosa PAK-NP by gene replacement. The P. aeruginosa fliD gene located on a 7.0-kb EcoRI-ApaI fragment was inactivated by inserting a gentamicin resistance gene cassette into an EcoRV site in the fliD gene (Fig. 1). The insertionally inactivated fliD gene on the plasmid was electroporated into PAK-NP, where it replaced the corresponding chromosomal copy of the fliD gene by double reciprocal recombination, giving rise to a fliD mutant strain, PAK-NPD. The replacement of the wild-type fliD in PAK-NPD was confirmed by Southern blot analysis (data not shown). Another fliD mutant, PAK-D, was constructed in P. aeruginosa PAK by the same strategy (data not shown). Since this mutant is sensitive to tetracycline, it was used in the promoter fusion experiments which required the use of a plasmid carrying tetracycline resistance.
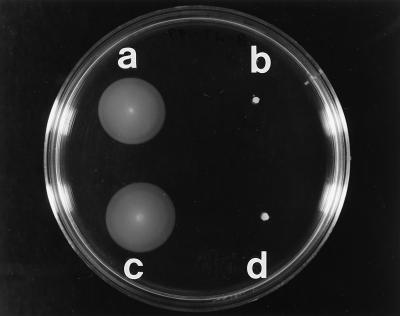
Since the fliD gene was located close to fleQ, which has been shown to be involved in motility and mucin adhesion in P. aeruginosa (3), we tested this mutant in motility and mucin adhesion assays. These results showed that the fliD mutant PAK-NPD was nonmotile (Fig. 3) and nonadhesive (Fig. 4). In order to confirm that the nonmotile and nonadhesive phenotype of PAK-NPD was indeed a result of inactivation of the fliD gene and was not due to polar effects on downstream genes, this gene was cloned as a 1.4-kb EcoRI-BamHI fragment on a multicopy plasmid, and this construct (pPZ375D) was introduced into PAK-NPD by electroporation. Motility (Fig. 3) and mucin adhesion (Fig. 4) functions were restored in PAK-NPD by a fliD gene provided on a plasmid without the need for the downstream genes of the putative operon, while the vector did not complement the fliD mutation.
FIG. 3.
Soft L agar (0.3%) plate showing the motility phenotype of different P. aeruginosa strains. (a) pilA mutant PAK-NP. (b) pilA fliD mutant PAK-NPD. (c) PAK-NPD containing pPZ375D. (d) PAK-NPD containing the multicopy vector pPZ375.
FIG. 4.
Adhesion of pilA and fliD mutants of P. aeruginosa PAK to mucin. PAK-NP, pilA mutant of PAK; PAK-NPD, pilA fliD mutant of PAK; PAK-NPD (375D), PAK-NPD complemented with the complete fliD gene on a multicopy plasmid vector, pPZ375; PAK-NPD (375), PAK-NPD with the vector pPZ375. Differences shown by asterisks are significant (∗ versus ∗∗, P < 0.001) by Student’s t test.
Overexpression of the fliD gene and purification of the FliD fusion protein.
The fliD gene was overexpressed under the control of an inducible T7 promoter on a plasmid in E. coli. The complete fliD coding sequence was inserted as a PCR product into the NdeI-BamHI sites of the plasmid pET15B. This resulted in an in-frame fusion of six histidine codons to the initiation codon of fliD. The resulting plasmid, pET15BD, and the vector control plasmid, pET15BD, were introduced into E. coli BL21(DE3), which has the T7 polymerase gene inserted into the chromosome. Bacterial cultures were grown and induced as explained in Materials and Methods. The induced and uninduced whole-cell extracts of E. coli BL21(DE3) containing pET15B (vector alone) or pET15BD (vector plus fliD) were analyzed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (10% polyacrylamide) (14) (Fig. 5). A new band representing the FliD fusion protein (His-FliD) (indicated by an arrow) was observed at the expected location (Fig. 5, lane 3).
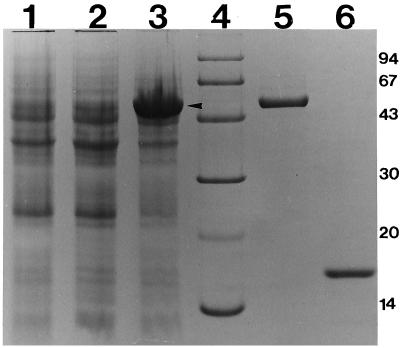
FIG. 5.
Overexpression and purification of FliD. FliD was overexpressed in E. coli host BL21 with the expression vector pET15B (Novagen, Inc.). Lanes: 1, BL21(pET15B) vector control induced with 2 mM IPTG for 4 h at 37°C; 2, BL21(pET15BD) vector with FliD insert uninduced; 3, BL21(pET15BD) vector with FliD insert induced with 2 mM IPTG for 4 h at 37°C; 4, Pharmacia low-molecular-mass (kilodaltons) markers; 5, approximately 400 ng of purified His-FliD protein; 6, approximately 400 ng of purified His-FlaG protein.
The His-FliD protein was purified from the cell lysates of E. coli BL21(DE3) carrying pET15BD as described in Materials and Methods. A small aliquot of the purified His-FliD was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide) (Fig. 5, lane 5). A single band was observed which migrated at the same location as the induced fliD gene product in the whole-cell extracts of E. coli BL21(DE3) carrying pET15BD (indicated by an arrow in Fig. 5). The 6× His tag attached to the purified FliD fusion protein was removed by thrombin cleavage as explained in Materials and Methods. The purified FliD protein without the 6× His tag was analyzed again by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide) to ascertain the purity of the FliD protein. The cleavage presented a single band with mobility slightly faster than that of FliD carrying the N-terminal His tag (data not shown). The protein concentration of this purified preparation of the FliD protein was determined by using the Bio-Rad protein assay kit as described in Materials and Methods.
The flaG gene was overexpressed by the same expression system as that used for the fliD gene (data not shown). Subsequently, the FlaG protein was purified in the same manner as the FliD protein (Fig. 5, lane 6).
Effect of the FliD protein on mucin adhesion of strain PAK-NP.
The purified FliD protein without the 6× His tag, was utilized in the in vitro competition assays to test whether it could compete with P. aeruginosa PAK-NP cells for binding to mucin. Two P. aeruginosa control proteins were used in this assay: purified pili (PilA protein) and purified FlaG protein. Based on our previous observations, it is clear that neither pilA (20) nor flaG (data not shown) is involved in flagellar formation or adhesion to mucin. P. aeruginosa pili were purified by the method of Frost and Paranchych (7a), while P. aeruginosa FlaG protein was expressed and purified exactly the same way as the FliD protein. Equimolar (1.83 μM) concentrations of purified proteins and their dilutions were allowed to bind to mucin for 30 min at 37°C, and the excess, unbound protein was washed away. The bacterial adhesion assay was then performed as explained in Materials and Methods.
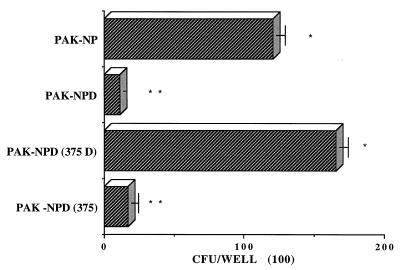
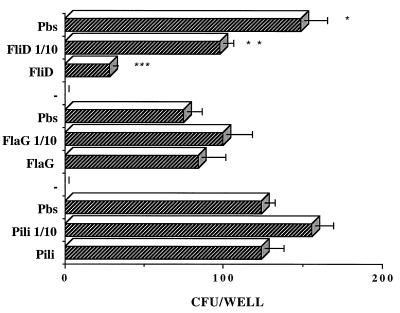
As shown in Fig. 6, only FliD protein inhibited P. aeruginosa PAK-NP binding to mucin. At the same molar concentration, the two control proteins PilA and FlaG did not inhibit the binding of P. aeruginosa PAK-NP to mucin.
FIG. 6.
Effect of FliD protein on P. aeruginosa binding to mucin. The mucin adhesion of P. aeruginosa PAK-NP in PBS (Pbs) is compared to mucin adhesion in the presence of different amounts of P. aeruginosa proteins. Two control P. aeruginosa proteins, FlaG and PilA, were used at the same molar concentrations as FliD to test for any nonspecific effects of a protein. Differences shown by asterisks are significant (∗ versus ∗∗, P < 0.01; ∗ versus ∗∗∗, P < 0.001) by Student’s t test.
Regulation of the fliD gene.
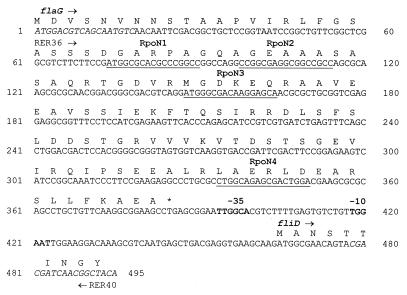
The fliD upstream sequence was visually analyzed for the presence of consensus ς28 (TAAA-N15-GCCGATAA), ς54 (YTGGYAYR-N3-YYTGCW), and ς70 (TTGACA-N17-TATAAT) recognition sites. Figure 7 shows the 495-bp sequence, including the fliD promoter. No ς28 binding site could be detected in this region. However, four putative ς54 binding sites were located at nucleotides 74, 98, 148, and 334. Finally, a sequence homologous to the ς70 promoter was identified at nucleotides 418 (−10 box) and 394 (−35 box), (Fig. 7).
FIG. 7.
Promoter region of fliDSorf126orf96 operon. The promoter region of fliDSorf126orf96 used for β-galactosidase assays included the complete coding sequence of the flaG gene and extended into the coding region of fliD. The primers used for the β-galactosidase experiments, RER36 and RER40, are shown. In the upstream sequence, the four putative RpoN binding sites (YTGGYAYR-N3-YYTGCW) are underlined and the −10 and −35 elements of the ς70 binding site (TTGACA-N17-TATAAT) are in boldface.
To understand the regulation of the fliD gene, the fliD promoter (495-bp sequence shown in Fig. 7) was fused with the promoterless lacZ gene, and the activity of the fliD promoter was measured in a number of P. aeruginosa strains. Table 2 shows the results of these β-galactosidase assays. The β-galactosidase activity in the wild-type PAK was 10 times that of the pDN19lacΩ vector control, demonstrating the existence of a promoter in the fliD upstream sequence. This promoter was insensitive to the transcriptional regulator FleR and the flagellar sigma factor ς28, since the fleR and the ς28 (fliA) mutants had β-galactosidase activities as high as that of the wild-type PAK (Table 2). Both rpoN (PAK-N1G) and fleQ (PAK-Q) mutants had significantly reduced activity of the fliD promoter, indicating that the transcription from the fliD promoter requires ς54 (product of the rpoN gene) and the transcriptional activator FleQ. However, the fliD promoter still had a basal activity in the absence of either ς54 or FleQ, suggesting a dual regulation, perhaps involving ς70, of the fliD promoter.
TABLE 2.
Control of the fliD promoter in P. aeruginosa
| Host strain | Genetic background | Mean β-galactosidase activity (Miller units) ± SD
|
|
|---|---|---|---|
| Vector alone | fliD promoter | ||
| PAK | Wild type | 112 ± 15 | 1,154 ± 9 |
| PAK-N1G | rpoN mutant | 102 ± 13 | 166 ± 8 |
| PAK-Q | fleQ mutant | 65 ± 16 | 331 ± 4 |
| PAK-RG | fleR mutant | 87 ± 1 | 1,107 ± 48 |
| MS540 | fliA mutant | 112 ± 34 | 1,798 ± 73 |
DISCUSSION
The adhesion of P. aeruginosa to mucin is one of the earlier steps in the process of P. aeruginosa colonization of the human airways. In pursuit of a specific adhesin responsible for this interaction, we have discovered that a structural component of the flagellum, FliD, exhibits a direct association with mucin. A polar insertional mutation in the P. aeruginosa fliD gene abolished the motility and mucin adhesion functions of the organism. Both the motility and the mucin adhesion functions were restored when the P. aeruginosa fliD gene alone was provided on a multicopy plasmid. In vitro competition assays demonstrated that the purified FliD protein was capable of specifically inhibiting the association of P. aeruginosa cells with mucin.
The fliD genes of E. coli and Salmonella typhimurium encode the filament cap protein, also called the hook-associated protein 2 (HAP2), and form an operon, fliDST (10, 12). The role of FliD in these organisms is to facilitate the polymerization of endogenous flagellin at the tips of the growing flagellar filaments. However, there are conflicting reports regarding the role played by the fliS and fliT genes in flagellar formation (6, 10, 36). The fliS gene has been implicated as a chaperone involved in the export of flagellin (36), while fliT apparently has no effect on flagellar formation. All three genes of the fliDST operon have been shown to negatively regulate the export of FlgM, the flagellum-specific anti-sigma factor (37). fliD mutants of E. coli and S. typhimurium secrete excess amounts of FlgM into the culture medium. Our studies show that the gene arrangement of the fliDST operon in P. aeruginosa is quite different. The fliT gene seems to be absent from this operon, and instead there is a duplication of the fliS gene. The fact that the fliD gene alone could complement the motility and mucin adhesion defects of the P. aeruginosa fliD mutant suggests that the fliS and fliT genes are not important for these two functions. Whether there is excess excretion of FlgM in the P. aeruginosa fliD mutant as in the fliD mutants of E. coli and S. typhimurium remains to be tested. However, by analogy, we expect a similar phenotype of this mutant. The adhesion-negative phenotype of the P. aeruginosa fliD mutant is probably not due to excess export of FlgM (lower intracellular concentration of FlgM and hence upregulation of class 2 and class 3 genes [e.g., fliC]), since our earlier reports have shown that the flagellin (fliC)-negative mutant is adhesive to mucin (24).
The alignment of the deduced amino acid sequence of the P. aeruginosa fliD gene with those of the other FliD proteins shows that the structure of this protein is conserved through the entire ORF. However, it is interesting that a stretch of 12 amino acids (amino acids 196 to 207) was absent from the other FliD proteins. The significance of this stretch of amino acids is not clear at present.
Our studies indicate that the regulation of the fliD gene in P. aeruginosa is quite different from that in other organisms. It has been suggested that the fliD gene of S. typhimurium belongs to class 3A, since it is dually regulated by ς28 (fliA) and the master regulator FlhD (13). However, our analysis of the sequence upstream of the fliD gene revealed that there is no ς28 binding sequence present in this region. Consistent with these data, the promoter studies showed that the transcription of the fliD gene was independent of ς28. Furthermore, we have previously shown that a ς28-deficient P. aeruginosa fliA mutant still adheres to mucin (24); if the fliD gene were ς28 regulated, then one would have expected a loss of adhesion in the fliA mutant. The promoter studies also demonstrated the requirement of the transcriptional regulator, FleQ, and the sigma factor RpoN. The transcriptional regulator FleQ belongs to the group of transcriptional activators which work in concert with RpoN and was previously shown to be involved in the regulation of motility and mucin adhesion in P. aeruginosa (3). Upstream of the fliD gene, we detected several putative ς54 binding sites and a strong ς70 promoter as well. The precise promoter assignment still awaits determinations based on the mapping of the transcriptional start site for this gene. Given the observed basal-level expression of fliD-lacZ fusions in fleQ and rpoN mutant backgrounds, it is conceivable that fliD is transcribed by two species of RNA polymerase, one containing ς54 and the other containing ς70. FleQ could be directly regulating fliD gene expression in conjunction with RpoN. Alternatively, FleQ could regulate the expression of another regulator which may be involved in transcription of fliD with either ς54 or ς70. This dual regulation would imply that the same gene is expressed under different conditions, responding to different needs of the bacterial cell, such as motility and adherence.
How does a flagellar cap protein function in adherence? One possible scenario involves an initial interaction of the flagellar tip with mucin. In fact, scanning electron photomicrographs of the surface of CF epithelia suggest that this might be the case in reality (27). This fragile interaction can be then strengthened by further attachment with additional FliD proteins located in the outer membrane, synthesized as a consequence of flagellar breakage following the initial binding step. Alternatively, other signals provided by the host may direct the synthesis of additional FliD exclusively for function in mucin adherence. This could explain the existence of two regulatory mechanisms for fliD expression, as implied by our analysis of the fliD gene promoter.
Interestingly, FliD has been implicated in virulence of Proteus mirabilis. A fliD mutant of P. mirabilis was shown to be attenuated in the colonization of the urinary tract and in virulence in a mouse model of ascending urinary tract infection (18). Further studies are geared towards finding the role for P. aeruginosa fliD in vivo. In summary, we postulate that the flagellar cap protein FliD is directly involved in the binding of P. aeruginosa to mucin. These findings will allow us to answer questions pertaining to the structure and role of this protein in the colonization process. Identification of the precise mucin binding site and finding the receptor that is recognized by this adhesin are among the challenges of the future. This information would prove useful in the development of new approaches to the prevention of colonization of the respiratory tract by P. aeruginosa.
ACKNOWLEDGMENTS
We acknowledge the Interdisciplinary Center for Biotechnology Research (ICBR) computer facilities of the University of Florida for use of the VAX computers for DNA sequence analyses. This work was supported by NIH grants HL33622 (R.R.) and AI32624 (S.L.).
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. Cloning and characterization of Pseudomonas aeruginosa fliF, necessary for flagellar assembly and bacterial adherence to mucin. Infect Immun. 1996;64:2130–2136. doi: 10.1128/iai.64.6.2130-2136.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. A transcriptional activator, FleQ, regulates mucin adhesion and flagellar gene expression in Pseudomonas aeruginosa in a cascade manner. J Bacteriol. 1997;179:5574–5581. doi: 10.1128/jb.179.17.5574-5581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brun Y V, Shapiro L. A temporally controlled sigma-factor is required for polar morphogenesis and normal cell division in Caulobacter. Genes Dev. 1992;6:2395–2408. doi: 10.1101/gad.6.12a.2395. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Helmann J D. The Bacillus subtilis ςD-dependent operon encoding the flagellar proteins FliD, FliS, and FliT. J Bacteriol. 1994;176:3093–3101. doi: 10.1128/jb.176.11.3093-3101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi E, Mehl T, Nunn D, Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Frost L S, Paranchych W. Composition and molecular weight of pili purified from Pseudomonas aeruginosa K. J Bacteriol. 1977;131:259–269. doi: 10.1128/jb.131.1.259-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inouye S, Kimoto M, Nakazawa A, Nakazawa T. Presence of flagella in Pseudomonas putida is dependent on the ntrA (rpoN) gene. Mol Gen Genet. 1990;221:295–298. [Google Scholar]
- 9.Ishimoto K S, Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci USA. 1989;86:1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawagishi I, Muller V, Williams A W, Irikura V M, Macnab R M. Subdivision of flagellar region III of the Escherichia coli and Salmonella typhimurium chromosomes and identification of two additional flagellar genes. J Gen Microbiol. 1992;138:1051–1065. doi: 10.1099/00221287-138-6-1051. [DOI] [PubMed] [Google Scholar]
- 11.Kinsella N, Guerry P, Cooney J, Trust T J. The flgE gene of Campylobacter coli is under the control of the alternative sigma factor ς54. J Bacteriol. 1997;179:4647–4653. doi: 10.1128/jb.179.15.4647-4653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutsukake K, Ohya Y, Yamaguchi S, Iino T. Operon structure of flagellar genes in Salmonella typhimurium. Mol Gen Genet. 1988;214:11–15. doi: 10.1007/BF00340172. [DOI] [PubMed] [Google Scholar]
- 13.Kutsukake K, Ide N. Transcriptional analysis of the flgK and fliD operons of Salmonella typhimurium which encode flagellar hook-associated proteins. Mol Gen Genet. 1995;247:275–281. doi: 10.1007/BF00293195. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.McCarter L L. Genetic and molecular characterization of the polar flagellum of Vibrio parahaemolyticus. J Bacteriol. 1995;177:1595–1609. doi: 10.1128/jb.177.6.1595-1609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGee K, Hörstedt P, Milton D L. Identification and characterization of additional flagellin genes from Vibrio anguillarum. J Bacteriol. 1996;178:5188–5198. doi: 10.1128/jb.178.17.5188-5198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 18.Mobley H L T, Belas R, Lockatell V, Chippendale G, Trifillis A L, Johnson D E, Warren J W. Construction of a flagellum-negative mutant of Proteus mirabilis: effect of internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1996;64:5332–5340. doi: 10.1128/iai.64.12.5332-5340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Paranchych et al.
- 19.Ramphal R, Pyle M. Adherence of mucoid and nonmucoid Pseudomonas aeruginosa to acid-injured tracheal epithelium. Infect Immun. 1983;41:345–351. doi: 10.1128/iai.41.1.345-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramphal R, Koo L, Ishimoto K S, Totten P A, Lara J C, Lory S. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infect Immun. 1991;59:1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchings B W, Almira E C, Lory S, Ramphal R. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect Immun. 1995;63:4868–4876. doi: 10.1128/iai.63.12.4868-4876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saiman L, Ishimoto K, Lory S, Prince A. The effect of piliation and exoproduct expression on the adherence of Pseudomonas aeruginosa to respiratory epithelial monolayers. J Infect Dis. 1990;161:541–548. doi: 10.1093/infdis/161.3.541. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Simpson D A, Ramphal R, Lory S. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992;60:3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson D A, Ramphal R, Lory S. Characterization of Pseudomonas aeruginosa fliO, a gene involved in flagellar biosynthesis and adherence. Infect Immun. 1995;63:2950–2957. doi: 10.1128/iai.63.8.2950-2957.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith A W, Iglewski B H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989;17:10509. doi: 10.1093/nar/17.24.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 28.Starnbach M N, Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992;6:459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 29.Temple L, Sage A, Christie G E, Phibbs P V., Jr Two genes for carbohydrate catabolism are divergently transcribed from a region of DNA containing the hexC locus in Pseudomonas aeruginosa PAO1. J Bacteriol. 1994;176:4700–4709. doi: 10.1128/jb.176.15.4700-4709.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Totten P A, Lory S. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J Bacteriol. 1990;172:7188–7199. doi: 10.1128/jb.172.12.7188-7199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Totten P A, Lara J C, Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990;172:389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsang K W, Rutman A, Tanaka E, Lund V, Dewar A, Cole P J, Wilson R. Interactions of Pseudomonas aeruginosa with human respiratory mucosa in vitro. Eur Respir J. 1994;7:1746–1753. doi: 10.1183/09031936.94.07101746. [DOI] [PubMed] [Google Scholar]
- 33.Vishwanath S, Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984;45:197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods D E, Straus D C, Johanson W G, Jr, Berry V K, Bass J A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980;29:1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodward H, Horsey B, Bhavanandan V P, Davidson E A. Isolation, purification, and properties of respiratory glycoproteins. Biochemistry. 1982;21:694–701. doi: 10.1021/bi00533a017. [DOI] [PubMed] [Google Scholar]
- 36.Yokoseki T, Kutsukake K, Ohnishi K, Iino T. Functional analysis of the flagellar genes in the fliD operon of Salmonella typhimurium. Microbiology. 1995;141:1715–1722. doi: 10.1099/13500872-141-7-1715. [DOI] [PubMed] [Google Scholar]
- 37.Yokoseki T, Iino T, Kutsukake K. Negative regulation by FliD, FliS, and FliT of the export of the flagellum-specific anti-sigma factor, FlgM, in Salmonella typhimurium. J Bacteriol. 1996;178:899–901. doi: 10.1128/jb.178.3.899-901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]