Abstract
Sarcopenia and diabetes are two age-related diseases that are common in the elderly population, and have a serious effect on their general health and quality of life. Sarcopenia refers to the progressive loss of muscle mass, strength and function, whereas diabetes is a chronic disease characterized by elevated blood sugar levels. The comorbidity of sarcopenia and diabetes is particularly concerning, as people with diabetes have a higher risk of developing sarcopenia due to the combination of insulin resistance, chronic inflammation and reduced physical activity. In contrast, sarcopenia destroyed blood sugar control and exacerbated the development of people with diabetes, leading to the occurrence of a variety of complications. Fortunately, there are a number of effective treatment strategies for sarcopenia in people with diabetes. Physical exercise and a balanced diet with enough protein and nutrients have been proved to enhance the muscular quality and strength of this population. Additionally, pharmacological therapies and lifestyle changes can optimize blood sugar control, which can prevent further muscle loss and improve overall health outcomes. This review aims to summarize the pathogenesis and comprehensive treatment strategies of sarcopenia in elderly patients with type 2 diabetes, which help healthcare professionals recognize their intimate connection and provide a new vision for the treatment of diabetes and its complications in this population. Through early identification and comprehensive treatment, it is possible to improve the muscle function and general quality of life of elderly with diabetes and sarcopenia.
Keywords: sarcopenia, type 2 diabetes, elderly, pathogenesis, treatment
Graphical Abstract
1. Introduction
Sarcopenia is defined as a disease characterized by progressive loss of skeletal muscle mass and function, which usually occurred in the elderly (1, 2). The loss of skeletal muscle mass and function seriously affect the physical health and quality of life of individuals, leading to difficulties in daily activities and increased risk of falls and fractures, even shortened life expectancy of the elderly (3). Risk factors leading to sarcopenia include age, unbalanced diet and physical inactivity. In addition, inflammation and chronic diseases may accelerate its development (4). In 2016, sarcopenia was officially recognized as a disease with distinctive characteristics by the medical community (5). The prevalence of sarcopenia is approximately 10% in individuals aged 60 and above, whereas it reaches 20% to 30% in those aged 80 and above (6, 7). In view of the aging trend of global population, sarcopenia has emerged as an important health concern. The global population aged 65 years or above is expected to increase from 9% in 2019 to nearly 17% in 2050, which increase the risk of sarcopenia (8).
Diabetes mellitus is a chronic metabolic disease, which is mainly characterized by elevated blood sugar levels (9, 10). More than 60% of individuals aged 65 and above have diabetes, while 50% of the elderly people have prediabetes (11). According to the estimation of the International Diabetes Federation, the number of people with diabetes will reach up to 783 million by 2045 (12). Type 2 diabetes mellitus (T2DM) is the most common type, accounting for more than 90% of people with diabetes (13–15). Persistent hyperglycemia can lead to a range of complications, which have a serious impact on the cardiovascular system, kidneys and eyes, thereby posing serious threats to the overall health (16–19). The complications and severity of diabetes lead to a considerable morbidity and mortality rate, make it one of the most urgent worldwide public health problems (20).
Sarcopenia and diabetes in the elderly are intricately linked and have a negative impact on each other (21). Sayani Das examines the relationship between diabetes and sarcopenia in India’s elderly population revealed that individuals with diabetes had a significantly higher risk of sarcopenia (22). Moreover, recent meta-analyses have revealed that the prevalence of sarcopenia is two to three times greater among patients with diabetes in comparison to those without the condition (23, 24). Osaka et al.’s study further underscored the connection by indicating that sarcopenia elevates the risk of nephropathy in people with diabetes (25). According to Feng et al.’ study, the pooled prevalence of sarcopenia in people with diabetes reached up to 18%, which has become an urgent situation to be prevented early (26). The purpose of this paper is to review the pathogenesis and comprehensive treatment strategies of sarcopenia in patients with T2DM, which aims to help clinicians and nutritionists to identify and intervene at an early stage, ultimately enhancing the overall health and well-being of these patients.
2. Sarcopenia
2.1. Definition and prevalence of sarcopenia
In 1989, the term sarcopenia was initially coined by Rosenberg in 1989 (27, 28). The European Working Group on Sarcopenia in the Elderly (EWGSOP) first defined sarcopenia in 2010 as “age-related, generalized decrease in muscle mass and/or decrease in muscle strength or muscle physiology” (29). A similar agreement was subsequently announced in 2014 by the Asian Sarcopenia Working Group, who also altered the pertinent diagnostic cutoff for the Asian population (30). Sarcopenia was recognized as a disease on a global scale and included in the ICD-10 (M62.84) in 2016 (5). The European Sarcopenia Working Group presented a revised definition of sarcopenia in 2018 and an explanation of muscular function. Sarcopenia was defined as the gradual loss of muscle mass, strength, and muscle function (31). It is difficult to determine the prevalence of sarcopenia consistently since different cutoffs and measurement methods result in dramatically different incidences of the disease across studies. According to a meta-analysis of studies, the prevalence in adults over 60 ranges from 10 to 27 percent (32). The number of sarcopenia patients worldwide will rise year after year as the population ages.
2.2. Diagnosis process of sarcopenia
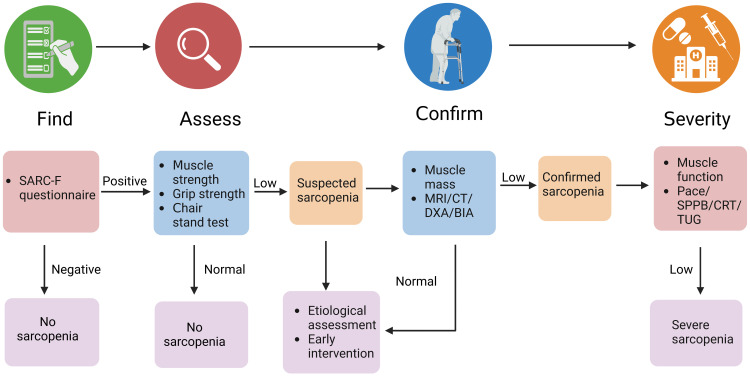
The diagnostic process for sarcopenia was updated by the European Working Group on Sarcopenia in the Elderly as Find-Assess-Confirm-Severity, as illustrates in Figure 1, noting that sarcopenia is considered severe when low muscle strength, low muscle mass and low physical function are all detected (31).
Figure 1.
The diagnostic process for sarcopenia.
2.2.1. SARC-F questionnaire
The European Working Group on Sarcopenia in the Elderly recommends using the SARC-F questionnaire to assess sarcopenia risk. The SARC-F is a simple five-item self-assessment questionnaire created by Morely (33). SARC-F is a rapid and simple screening method for sarcopenia in the elderly, which has been used widely used worldwide (34). Strength, assisted walking, getting up, climbing stairs, and falling are all assessed. A sarcopenia risk is present if the sum of the scores for each of the five items is greater than or equal to 4 points providing that the maximum total score is 10 points (35). However, due to the high specificity and relatively low to moderate sensitivity of SARC-F in predicting sarcopenia, many patients with sarcopenia may go misjudged (36–39). As a result, the SARC-CalF approach has been proposed, which adds a maximum leg circumference based on SARC-F. The results of several researches indicated that this method significantly improves the sensitivity and accuracy of evaluation (40, 41).
2.2.2. Muscle strength
Muscle strength is the maximal force that one or more muscular groups may generate. The European Working Group on Sarcopenia in the Elderly regards muscle strength as the main reference index for sarcopenia. It is recommended to utilize grip strength as an additional measure when assessing muscular strength because it has a relatively positive correlation in other body sections and is simple to use (42, 43). Upper limb muscle grip strength, which can be measured by a manual ergometer, is typically used to assess grip strength in the upper limbs. The chair stand test measures how quickly patients can stand up from the chair without the aid of their arms, and to assess the muscle strength of their lower limbs (31).
2.2.3. Muscle mass
Muscle mass refers to the total amount of skeletal muscle in the body. Computed tomography (CT), magnetic resonance imaging (MRI), dual-energy X-ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA) are common used to estimate the skeletal muscle mass (31). CT and MRI are the current gold standard for assessing muscle mass with high accuracy and reproducibility. Their use is restricted due to their high price, complexity, and high radiation exposure from CT (44). DXA is considered to be one of the ideal methods for determining muscle mass with its quick operation and minimal radiation exposure (45). However, DXA equipment cannot be widely used in hospitals and communities since it is difficult to carry. Instead, due to its simplicity, portability, and affordability, BIA is better suited for extensive hospitals and communities screening and can be used as a less expensive alternative to assess muscle mass (29, 44, 45).
2.2.4. Muscle function
Muscle function is defined as objectively measured exercise-related systemic function. Its testing methods include daily pace assessment, short-physical performance battery (SPPB), the chair rising test (CRT), and stand-up and go test (Time-up and go test, TUG). Daily Gait speed is a quick, secure and reliable sarcopenia test, which can indicate the health status and is related to their survival possibility of the elderly (46). A walking speed of less than 0.8 m/s is suggested by EWGSOP2 as a sign of severe sarcopenia (31). SPPB is a compound test method of muscle function, which is a standard method in both research and clinical application and is widely used (47, 48). Three tests are included in SPPB, with the highest score of 4 points and the full score of 12 points for each individual test. The risk of poor muscle function is indicated by a score of 9 or less.
2.3. Risk factors of sarcopenia
2.3.1. Intrinsic factors
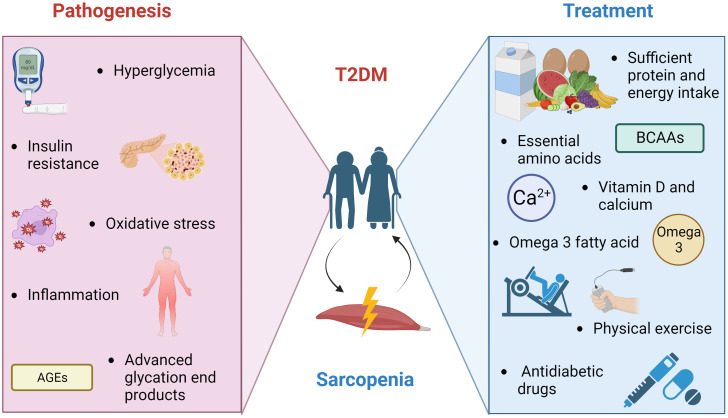
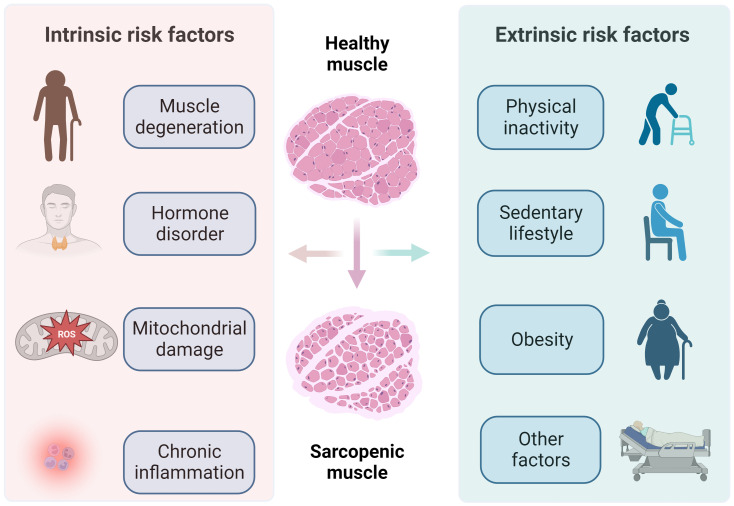
Sarcopenia is a complicated and multifactorial process, which is influenced by both intrinsic and extrinsic factors, as depicted in Figure 2. Age is considered as a key intrinsic risk factor of sarcopenia. Because age not only directly affects muscle quality and function, but also indirectly increases the risk of sarcopenia by bringing some problems such as hormone disorder, mitochondrial damage and chronic inflammation. With the increase of individual age, the metabolic rate of human body gradually decreases. This metabolic leads to the inhibition of protein synthesis, thereby impairing muscle repair and regeneration capabilities (1). Age-related muscle tissue alterations include serious loss of muscle mass, modifications in muscle fiber morphology, deterioration of muscle function, and reduction of muscle contraction time and strength. Consequently, sarcopenia is widely regarded as an age-related disease (49). Furthermore, age also affects the structure and function of skeletal muscle fibers. As age advances, there is a gradual decrease in the quantity and size of muscle fibers with the skeletal muscle, along with an increase in the presence of fat and connective tissue within the muscle matrix, all of which contribute to the degradation of muscle mass (50). Therefore, it can be seen that age is an important factor of sarcopenia, which affects the health and function of muscles in many ways.
Figure 2.
Intrinsic and extrinsic risk factors of sarcopenia.
Moreover, the imbalance of the human endocrine system and the fluctuation of hormone levels caused by aging contribute to an increased risk of sarcopenia by affecting skeletal muscle function. An obvious example is that with adult man ages, the decrease of muscle mass and strength is closely related to steady decline testosterone levels (51). As skeletal muscle undergoes the aging process, the level of reactive oxygen species (ROS) gradually increases, disrupting the body’s ability to maintain oxidative balance. This disruption decreases myogenic differentiation and triggers regenerative errors during the skeletal muscle degeneration (52–55). ROS are mainly produced in mitochondria, which also serve as the primary target of intracellular oxidative stress. High levels of ROS can damage mitochondria, resulting in abnormal mitochondrial function, and potentially contributing to the development of sarcopenia (56–58). In addition, the proliferative and differentiation capabilities of satellite cells decline significantly with age, compromising the regenerative potential of muscle cells. These changes result in fibrosis of aging muscle, thereby considered as a pivotal factor in the initiation of sarcopenia (59–61).
Chronic inflammation related to aging is another important risk factor for sarcopenia (62). The increase of pro-inflammatory cytokine levels and the decrease of anti-inflammatory cytokine levels in vivo are indicators to assess chronic inflammation. Loss of muscle mass and strength is associated with an increase in inflammatory markers in the blood, which reduces lower limb function and physical activity (63, 64). According to several horizontal and longitudinal studies, sarcopenia is associated with high levels of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP). These inflammatory factors are secreted by inflammatory and immune cells, which inhibit muscle synthesis and promote muscle degradation, consequently result in sarcopenia (65–67).
2.3.2. Extrinsic factors
The decline of exercise ability are the main reasons for the loss of muscle mass and strength in the elderly (68). Muscle tissue is highly plastic, which needs constant participation and stimulation under load to maintain the proper metabolism and repair functions. Lack of exercise will result in insufficient muscle stimulation, deceleration of protein synthesis, and decrease of muscle mass (69). Moreover, fear of falling causes psychological burden to the elderly, which makes them unwilling to do exercise and increases the risk of muscle loss. At the same time, physical inactivity can easily lead to obesity in the elderly. The weight of the elderly mostly increases in the form of fat rather than thin tissue, and the occurrence of sarcopenia is usually accompanied by the growth of adipose tissue, which promotes each other (70).
In addition to physical inactivity, it is worthing noting that the elderly who have been engaged in a sedentary lifestyle for a long time face a higher risk of sarcopenia. When sedentary time exceeds 2 hours, even physically active elderly people are prone to sarcopenia, which emphasizes that physical activity itself cannot offset the harmful consequences of long-term inactivity (71). Although muscle mass and muscle fibers will naturally decrease with age, the risk of sarcopenia can be effectively reduced by performing moderate to high-intensity physical activities instead of sitting for a long time (72).
Other extrinsic factors such as muscle disuse caused by physical trauma, chronic stress and long sleep will increase the risk of muscle loss (73–75). In addition, long-term exposure to certain pollutants or toxins will adversely affect muscle health and overall health. Extreme weather conditions or geographical location may affect individuals’ ability to engage in outdoor activities and obtain fresh food, thus increasing the risk of sarcopenia. It must be recognized that the progress of sarcopenia is a dynamic process, and the early symptoms are often difficult to find, which leads to the neglect of this disease. In fact, it is very common for most elderly people to take this problem seriously only when pathological conditions and adverse consequences appear. Therefore, it is very important to pay attention to sarcopenia in the elderly at an early stage.
3. Pathogenesis of interaction between sarcopenia and T2DM
3.1. Insulin resistance and muscle mass
Insulin resistance is considered as the main pathophysiological basis of T2DM, which plays an important role in the occurrence and development of sarcopenia (76–78). Insulin is very important to control metabolism, and its main function is to promote protein synthesis, muscle growth and the absorption and utilization of glucose by muscle tissue (79). Insulin resistance occurs when the sensitivity and reactivity of insulin receptors decrease. As a result, blood sugar fails to effectively enter muscle tissue. This leads to insufficient nutrition supply of muscle tissue, which leads to muscle atrophy and reduction (80). Skeletal muscle can absorb 75% of blood sugar through insulin signaling system (81). Therefore, muscle cells with insulin signaling system problems will not be able to effectively use the glucose in the blood stream, leading to accelerated protein decomposition and accelerated muscle loss (82–84). Low muscle mass may make blood sugar control disorder, further aggravating the development of diabetes (78).
3.2. Hyperglycemia
Hyperglycemia is a significant risk factor leading to the loss of muscle mass and function during aging (85), which affect the development and progress of sarcopenia through various mechanisms. Firstly, hyperglycemia leads to an increase in the formation of advanced glycation end products, which accumulate in muscle tissue, destroy muscle contraction and endothelial function, and finally worsen muscle function (86). Secondly, the oxidative stress and inflammatory reaction caused by hyperglycemia will lead to muscle cell damage and cell death (87, 88). In addition, the increase of blood sugar level declines the responsiveness of muscle cells to insulin, which reduces the absorption and utilization of glucose and amino acids by these cells. Muscle tissue cannot get enough nutritional support, leading to the aggravation of sarcopenia (89). Lastly, hyperglycemia affects the energy metabolism of muscle cells by destroying the balance between glycogen production and decomposition. This disorder leads to the decrease of glycogen storage in muscle cells, which leads to the insufficient energy supply of these cells and accelerates the onset of sarcopenia (90). In a word, hyperglycemia poses many threats to muscle health in the process of aging, affecting the structure and function of muscle tissue.
3.3. Oxidative stress and inflammatory reaction
Muscle atrophy in people with diabetes mainly comes from oxidative stress and inflammation. Chronic inflammation, oxidative stress, insulin resistance, the formation of advanced glycation end products and hyperglycemia are all linked to cellular redox imbalance (88, 91). This factors together hinder the metabolism of protein in skeletal muscle, leading to increased oxidative damage of DNA, lipids and protein (92). The increase of lipid metabolites in patients with T2DM can lead to excessive production of ROS. This overproduction can be alleviated by antioxidant enzymes, which play a vital role in regulating muscle regeneration, inducing angiogenesis and reducing muscle fibrosis. Furthermore, the high level of ROS will reduce muscle fiber size and muscle mass, destroy muscle homeostasis, interfere with muscle production, and thus aggravate muscle atrophy (93). Inflammatory response is a complex and important biological mechanism, which responds to pathogens and injury stimuli by activating immune cells and releasing inflammatory mediators, thus protecting the body against injury (94). However, persistent chronic inflammation may represent a potential pathological condition that damages muscle tissue and promote the development and progress of sarcopenia (95).
3.4. Sarcopenic obesity
Sarcopenic obesity (SO) is defined as the co-existence of obesity and sarcopenia in a consensus achieved by the European Society for Clinical Nutrition and Metabolism and the European Association for the Study of Obesity (ESPEN-EASO) in 2022 (96). The prevalence of sarcopenic obesity is high among the elderly, with about one in every ten people suffering from it (97). A large number of studies have shown that SO can increase more negative effects than sarcopenia or obesity alone, including a significant increase in the probability of falling, disability (98). Obesity exists in both people with sarcopenia and diabetes. A study shows that more than half of diabetics are obese at the same time (99). Therefore, the coexistence of SO and T2D has become a common health problem. Studies have shown that the prevalence of SO in diabetic patients is as high as 27%, and it is related to serious adverse consequences (100). Obesity and T2D are one of the main causes of the dysfunction of skeletal muscle stem cell regeneration, which is manifested by the loss of muscle content and the progressive decline of glucose and lipid metabolism of skeletal muscle, thus accelerating the process of diabetes and entering a vicious circle (101). Therefore, we should pay attention to the early screening and identification of SO in people with diabetes, and choose appropriate intervention measures to reduce the incidence and various adverse outcomes in this population.
4. Comprehensive treatment strategies
4.1. Control of blood sugar level
Diabetes is a chronic disease characterized by elevated blood sugar level, which can lead to the occurrence of various complications. Among these, muscle loss, known as sarcopenia, is a common complication influenced by high blood sugar. The decrease of mitochondrial activity induced by hyperglycemia aggravates insulin resistance and impairs energy metabolism (102). Furthermore, the inflammatory responses triggered by hyperglycemia will further lead to insulin resistance and muscle atrophy by inhibiting insulin signaling (103). Therefore, blood sugar control is crucial for people with diabetes and sarcopenia. Effective management of blood sugar level can slow down the rate of muscle loss and reduce protein decomposition. In well-controlled people with diabetes, stable insulin level contributes to the synthesis of muscle protein and promotes muscle quality and strength. This can not only prevent the onset of sarcopenia, but also accelerate muscle growth and repair, and ultimately enhance the overall muscle quality and body function. Skeletal muscle, as the most important exercise organ of the human body, assist in storing protein, regulating glucose metabolism and stabilizing blood sugar level, contributing to overall health of the body (81, 104, 105).
4.2. Nutrition intervention
Compared with the general population, people with diabetes face a higher risk of malnutrition and are more prone to sarcopenia. Nutritional therapies have the potential to prevent or even reverse sarcopenia and frailty (106). Two elderly patients with malignant hematological diseases were studied by Matsunaga et al., and the effectiveness of rehabilitation treatment on sarcopenia was reported (107). Chan et al.’s study noted that expanding nutritional counseling for people with diabetes and effectively reduce the incidence of sarcopenia (108). Several studies demonstrate that diet is no obvious evident benefit for elderly with diabetes and sarcopenia (102, 103, 109). However, the majorities of studies have consistently shown the positive effects of diet and nutrition intervention in the prevention and control of sarcopenia (110–119). Moreover, for many elderly people who cannot exercise, nutritional intervention is still the most promising treatment and prevention strategy (120, 121). Therefore, it is crucial to emphasize the importance of a balanced diet, comprehensive nutrition and vigilant monitoring of diabetes and malnutrition in the elderly. The comprehensive overview of the relationship between nutritional intervention and sarcopenia is shown in Table 1.
Table 1.
Studies on the effect of nutritional intervention on sarcopenia in elderly people with diabetes.
| Nutrition intervention | Study design | Target population | Age (years old) | Research objectives | Results | References |
|---|---|---|---|---|---|---|
| Protein intake | Cross-sectional | T2DM patients | 65.0 ± 7.7 | Physical function outcomes | Positively associated | (110) |
| Protein intake | Cross-sectional | T2DM patients | ≥31 | Muscle loss | Negatively affected | (111) |
| Nutrition counseling | Cross-sectional | T2DM patients | 45-90 | Sarcopenia | Negatively associated | (108) |
| Mediterranean Diet | Cross-sectional | Overweight or Obese T2DM patients | 71.2 ± 8.2 | Gait speed | Positively associated | (112) |
| Energy intake | Cross-sectional | T2DM patients | ≥65 | The presence of sarcopenia | Negatively associated | (113) |
| Vitamin D intake | Prospective cohort study | T2DM patients | ≥65 | Muscle loss | Negatively associated | (114) |
| Omega-3 fatty acids intake | Cross-sectional | T2DM patients | 74.2 ± 5.7 | Sarcopenia | Negatively associated | (115) |
| Malnutrition | Cross-sectional | T2DM patients | 76.6 ± 6.27 | The prevalence of sarcopenia | Negatively associated | (116) |
| Resistance training and nutritional interventions | Prospective cohort study. | Frail T2DM patients | 79 ± 5.6 | SPPB, maximal strength and power output | Positively improved | (117) |
| Nutrition intervention | Randomized controlled trial | Obese and overweight patients with diabetic foot ulcers | 30-70 | Body composition | No significant correlation | (102) |
| Vitamin D intake | Randomized controlled trial | Obese T2DM patients | 30-60 | Body composition | No significant correlation | (103) |
| Branched chain amino acids | Longitudinal study | T2DM patients | 56.6 ± 10.6 | Skeletal muscle loss | Negatively associated | (118) |
| Serum vitamin D level | Prospective observational cohort study | Men | 59.1 ± 10.5 | Diabetes | No significant correlation | (109) |
| Calorie-restricted diet and recreational sports training | prospective study | T2DM patients | 60.0 ± 6.0 | Expression of skeletal muscle gene | Downregulates the expression of atrophy-associated myokines and increases the expression of anti-inflammatory gene IL-15 | (119) |
4.2.1. Protein
Protein intake is beneficial to the elderly people with both sarcopenia and T2DM. Increasing protein’s habitual intake can not only effectively improve blood sugar control and maintain muscle mass, but also promote weight control, reduce inflammation and increase insulin sensitivity (122). Protein is a key basic element of muscle function and an indispensable nutrient for muscle synthesis. Wu et al. revealed that the increase of protein intake was related to the increase of grip strength and the decrease of prevalence of sarcopenia (123). Many studies have consistently confirmed that adequate intake of protein can not only stimulate the synthesis of protein in muscle, but also reduce the degradation process of protein and improve the effective utilization of nutrients in muscle (124–126). In addition, proper protein intake has a positive effect on blood sugar regulation, which helps to reduce the risk of diabetes. Protein intake can directly promote postprandial insulin secretion, which may be beneficial to the blood sugar regulation of elderly T2D patients (127). Studies have pointed out that the elderly with sarcopenia should consume 1.6g/kg of protein every day, and the ESPEN expert group and the PROT-AGE research group also suggested that people with chronic diseases (such as diabetes) should consume about 1.2-1.5g/kg/day of protein (128–130). Therefore, higher protein intake should be encouraged for the elderly people with sarcopenia and T2DM.
4.2.2. Essential amino acids
Amino acids are the basic units of muscle protein, and the impact of protein consumption on the rate of muscle synthesis mainly focus on essential amino acids (131, 132). These essential amino acids play a vital role in both muscle synthesis and maintenance, demonstrating a linear relationship with muscle protein synthesis within a specific dosage range (132). In particular, leucine stands out because of its remarkable stimulating effect (133–135). Long-term continuous intake of essential amino acids rich in leucine is related to significant enhancement of skeletal muscle mass, strength, and walking speed in the elderly (136). Rieu et al. studied the completely balanced diet of 20 healthy male subjects with or without leucine, and found that leucine is effective in promoting the synthesis of muscle protein and counteracting the influence of sarcopenia (137). A study emphasizes that leucine can act as insulin secretagogue to effectively improve postprandial blood glucose control and promote the synthesis of muscle protein (138). Therefore, ensuring adequate dietary intake of protein and essential amino acids has become a key factor to maintain overall health and function.
4.2.3. Vitamin D and calcium
Vitamin D play an important role in maintaining muscle health and has a direct impact on muscle development. Vitamin D is a fat-soluble nutrient that strengthens bone health by promoting the absorption of essential minerals such as calcium and phosphorus. A paired case-control study by Yang et al. showed that vitamin D deficiency was associated with an increased risk of sarcopenia (139). Compared with young women with sufficient vitamin D level, young women with insufficient vitamin D level showed a decrease in muscle mass, and at the same time, the fat penetration in muscle increased by about 24%, further highlighting the far-reaching influence of vitamin D on muscle strength (140). In addition, compared with people with sufficient vitamin D, the grip strength of the elderly with low serum vitamin D level is significantly reduced (141). Visser et al. conducted a longitudinal aging study and found that vitamin D deficiency is not only associated with the weakening of muscle function and the onset of sarcopenia, but also increases the risk of osteoporosis and fracture (142). Individuals with sarcopenia consume lesser essential nutrients such as calcium, iron and zinc than the general population, further aggravating the problems related to muscle health (143). Encouragingly, studies involving elderly women show that continuous supplementation of vitamin D and calcium can significantly improve skeletal muscle performance and reduce the incidence of falls (144). These comprehensive studies emphasize the potential benefits of vitamin D and calcium supplements in promoting muscle health, especially in the elderly and individuals with chronic diseases.
4.2.4. Omega-3 fatty acids
A growing body of evidences emphasize the potential role of omega-3 fatty acids in preserving and regulating the quality and function of skeletal muscle. Robinson et al.’ study on diet and its relationship to grip strength in community-dwelling older men and women revealed a positive correlation between eating fatty fish and increasing grip strength (145). Dos Reis et al.’s study revealed that the intake of omega-3 fatty acids is negatively correlated with the incidence of sarcopenia, and a favorable relationship with the skeletal muscle mass index of limbs was also proved (146). A systematic meta-analysis conducted by Bird et al. further supported these findings, emphasizing the positive effects of supplementation of omega-3 long-chain polyunsaturated fatty acids on muscle quality and strength (147). A study showed that omega-3 fatty acid supplements are also expected to reduce inflammation (148). Rats fed with a combination of omega-3 polyunsaturated fatty acids derived from fish oil and wheat oligopeptides showed significant potential to prevent age-related muscle loss and reduced oxidative stress and inflammation in skeletal muscle (149). The comprehensive findings of these studies demonstrate that omega-3 fatty acids have positive and multifaceted potential in muscle protection and inflammation reduction, and are expected to become a potential nutritional support strategy for elderly patients with sarcopenia.
4.3. Physical exercise
Physical exercise is considered as a safe and efficient intervention measures to prevent and treat diabetes mellitus complicated with sarcopenia. Participating in any form of physical activity can significantly improve sarcopenia in the elderly, which has been supported by a lot of research (116, 150–157), as shown in Table 2. On a global scale, physical exercise occupies a central position as the main prevention and treatment method of sarcopenia (31). Studies from different countries have consistently emphasized the far-reaching advantages of regular physical activity in maintaining muscle quality and function (158–162). Studies have emphasized the dual benefits of aerobic exercise and anaerobic exercise in reducing insulin resistance. Aerobic exercise can especially enhance insulin sensitivity, while resistance training and strength training can also significantly improve insulin resistance (163, 164). For those elderly who struggle with sarcopenia, physical exercise, with special emphasis on resistance training, is considered as an effective strategy to combat the decline of activity ability related to age-related chronic diseases (165–168). When combined with nutritional supplements, resistance training can maximize its intervention (169, 170). According to international guidelines, it is recommended that patients with T2DM participate in resistance training at least twice a week (171). Therefore, it is imperative to incorporate resistance training into the exercise guide tailored for the elderly.
Table 2.
Studies on the effect of physical exercise on sarcopenia in elderly people with diabetes.
| Physical exercise intervention | Duration | Target population | Age (years old) | Research objectives | Results | References |
|---|---|---|---|---|---|---|
| Physical exercise | / | T2DM patients | 76.6 ± 6.27 | The prevalence of sarcopenia | Negatively associated | (116) |
| Short-term acute moderate-intensity resistance exercise | 24h | Older T2DM patients | / | Blood glucose | Blood glucose decreased | (150) |
| Progressive Sandbag Exercise Training | 12 weeks | T2DM and possible sarcopenia patients | >50 | Muscle strength | Significantly improved | (151) |
| Circuit resistance training | 10 weeks | T2DM patients | >65 | Physical and metabolic function | Effective | (152) |
| Muscle power training | 12 weeks | T2DM patients | 70.5 ± 7.8 | Functional capacity, body balance and lower limb muscle strength | Significantly improved | (153) |
| Resistance training | 12 weeks | T2DM patients | 69.7 ± 6.9 | Lower limb strength and muscle mass | Significantly improved | (154) |
| Resistance training | 12 weeks | T2DM patients | >65 | Muscle function | Significantly improved | (155) |
| Low-volume walking high-intensity interval training (HIIT) | 12 weeks | Older women with T2DM | / | Physical capacity | Significantly improved | (156) |
4.4. Selection of antidiabetic drugs
The effect of antidiabetic drugs on sarcopenia is still ongoing research topic, with certain drugs demonstrating unclear effects. Some antidiabetic drugs, particularly long-term use of sulfonylurea drugs, such as glinide, gliclazide and glibedi, have been associated with muscle loss or injury. These drugs play a role by inhibiting KATP channel to stimulate insulin secretion (172). According to a large number of clinical reports, this mechanism is related to rapid skeletal muscle atrophy (173–175). On the contrary, some antidiabetic drugs show promise in maintaining muscle quality. In a study by Lee et al., it was observed that elderly men with diabetes experienced rapid skeletal muscle loss, but those who used insulin sensitizers such as metformin or thiazolidinedione lost significantly less weight than those who did not receive such treatment. This indicates that insulin sensitizer may weaken muscle loss (176). Some other studies have also shown that insulin administration can increase the skeletal muscle mass or gait speed of patients with diabetes, which may slow down sarcopenia in patients with T2DM (177–179). However, it is impossible to overlook the weight gain caused by insulin use (180). In conclusion, the effect of antidiabetic drugs on sarcopenia varies with specific drugs and individual conditions. Further clinical research is needed to fully clarify the complex relationship between antidiabetic drugs and sarcopenia.
5. Conclusion
In this study, the pathogenesis of sarcopenia in people with diabetes and comprehensive treatment strategies were summarized. There is a complex interrelationship between the two diseases. Type 2 diabetes leads to the progress of sarcopenia, and conversely, sarcopenia exacerbates the severity of diabetes. Lifestyle choice, age-related metabolic changes, elevated blood sugar levels and insulin resistance all contribute to sarcopenia in patients with T2DM. Therefore, healthcare professionals need to pay more attention to the early prevention of muscle loss in elderly diabetes patients.
Effective prevention and treatment of sarcopenia complicated by diabetes requires dietary intervention rich in protein, essential amino acids, vitamin D, calcium and other important nutrients (including omega-3 fatty acids). In addition, it is very necessary to advocate moderate exercise and physical exercise among patients with T2DM and sarcopenia. Considering all kinds of exercise types and intensities, it is very important to customize these activities according to the needs of the elderly. Equally important is the management of blood sugar level and the improvement of insulin resistance. When prescribing antidiabetic drugs, clinicians should include sarcopenia in the evaluation of its pharmacological effects. However, there is no specific drug for sarcopenia at present, which emphasizes the importance of active prevention.
Age-related sarcopenia has become an urgent global health challenge as the population ages. Diabetes is still one of the most common chronic diseases among the elderly. The complicated interaction and common pathogenic mechanism between these two diseases have a far-reaching impact on the health of the elderly. Health care professionals must give priority to the early detection, screening and diagnosis of sarcopenia in people with diabetes, and formulate comprehensive and reasonable preventive measures in time, and ultimately promote the well-being of the elderly population.
Author contributions
YH: Writing – original draft, Conceptualization, Visualization, Writing – review & editing. JX: Writing – review & editing. BW: Writing – review & editing. SD: Writing – review & editing. RS: Writing – review & editing. WZ: Supervision, Writing – review & editing. ST: Supervision, Writing – review & editing. BH: Supervision, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work is supported by “The 14th Five-Year Plan” Application Characteristic Discipline of Hunan Province (Pharmaceutical Science) with Changsha Medical University, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet (2019) 393(10191):2636–46. doi: 10.1016/s0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 2. Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care (2010) 13(1):1–7. doi: 10.1097/MCO.0b013e328333c1c1 [DOI] [PubMed] [Google Scholar]
- 3. Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, et al. Sarcopenia - Molecular mechanisms and open questions. Ageing Res Rev (2021) 65:101200. doi: 10.1016/j.arr.2020.101200 [DOI] [PubMed] [Google Scholar]
- 4. Yuan S, Larsson SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533 [DOI] [PubMed] [Google Scholar]
- 5. Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle (2016) 7(5):512–4. doi: 10.1002/jcsm.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord (2017) 16:21. doi: 10.1186/s40200-017-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volpato S, Bianchi L, Cherubini A, Landi F, Maggio M, Savino E, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci (2014) 69(4):438–46. doi: 10.1093/gerona/glt149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Almohaisen N, Gittins M, Todd C, Sremanakova J, Sowerbutts AM, Aldossari A, et al. Prevalence of undernutrition, frailty and sarcopenia in community-dwelling people aged 50 years and above: systematic review and meta-analysis. Nutrients (2022) 14(8):1537. doi: 10.3390/nu14081537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract (2014) 103(2):137–49. doi: 10.1016/j.diabres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 10. Yang YY, Chen Z, Yang XD, Deng RR, Shi LX, Yao LY, et al. Piperazine ferulate prevents high-glucose-induced filtration barrier injury of glomerular endothelial cells. Exp Ther Med (2021) 22(4):1175. doi: 10.3892/etm.2021.10607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association Professional Practice Committee . 13. Older Adults: Standards of Medical Care in Diabetes-2022. Diabetes Care. Diabetes Care (2022) 45(Suppl 1):S195–S207. doi: 10.2337/dc22-S013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magliano DJ, Boyko EJ, committee IDFDAtes . IDF Diabetes Atlas. Idf diabetes atlas Vol. 2021. . Brussels: International Diabetes Federation© International Diabetes Federation; (2021). [Google Scholar]
- 13. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol (2018) 14(2):88–98. doi: 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- 14. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 15. Kerner W, Brückel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes (2014) 122(7):384–6. doi: 10.1055/s-0034-1366278 [DOI] [PubMed] [Google Scholar]
- 16. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj (2000) 321(7258):405–12. doi: 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang YY, Shi LX, Li JH, Yao LY, Xiang DX. Piperazine ferulate ameliorates the development of diabetic nephropathy by regulating endothelial nitric oxide synthase. Mol Med Rep (2019) 19(3):2245–53. doi: 10.3892/mmr.2019.9875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mi W, Xia Y, Bian Y. Meta-analysis of the association between aldose reductase gene (CA)n microsatellite variants and risk of diabetic retinopathy. Exp Ther Med (2019) 18(6):4499–509. doi: 10.3892/etm.2019.8086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Tan S, Liu M, Li J. LncRNA TINCR is downregulated in diabetic cardiomyopathy and relates to cardiomyocyte apoptosis. Scand Cardiovasc J (2018) 52(6):335–9. doi: 10.1080/14017431.2018.1546896 [DOI] [PubMed] [Google Scholar]
- 20. Jiang H, Xia C, Lin J, Garalleh HAL, Alalawi A, Pugazhendhi A. Carbon nanomaterials: A growing tool for the diagnosis and treatment of diabetes mellitus. Environ Res (2023) 221:115250. doi: 10.1016/j.envres.2023.115250 [DOI] [PubMed] [Google Scholar]
- 21. Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes (2019) 12:1057–72. doi: 10.2147/dmso.S186600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Das S. The impact of diabetes on sarcopenia in community-dwelling older adults in India: key findings from the longitudinal ageing study in India (LASI). Diabetes Epidemiol Manage (2023) 12:100158. doi: 10.1016/j.deman.2023.100158 [DOI] [Google Scholar]
- 23. Chung SM, Moon JS, Chang MC. Prevalence of sarcopenia and its association with diabetes: A meta-analysis of community-dwelling Asian population. Front Med (Lausanne) (2021) 8:681232. doi: 10.3389/fmed.2021.681232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiao YS, Chai YH, Gong HJ, Zhuldyz Z, Stehouwer CDA, Zhou JB, et al. The association between diabetes mellitus and risk of Sarcopenia: accumulated evidences from observational studies. Front Endocrinol (Lausanne) (2021) 12:782391. doi: 10.3389/fendo.2021.782391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Osaka T, Fukui M. IDF2022-0754 Sarcopenia is a risk factor for progression of diabetic nephropathy in type 2 diabetes. Diabetes Res Clin Pract (2023) 197:110390. doi: 10.1016/j.diabres.2023.110390 [DOI] [Google Scholar]
- 26. Feng L, Gao Q, Hu K, Wu M, Wang Z, Chen F, et al. Prevalence and risk factors of sarcopenia in patients with diabetes: A meta-analysis. J Clin Endocrinol Metab (2022) 107(5):1470–83. doi: 10.1210/clinem/dgab884 [DOI] [PubMed] [Google Scholar]
- 27. Rosenberg IH. Summary comments. Am J Clin Nutr (1989) 50(5):1231–3. doi: 10.1093/ajcn/50.5.1231 [DOI] [Google Scholar]
- 28. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr (1997) 127(5 Suppl):990s–1s. doi: 10.1093/jn/127.5.990S [DOI] [PubMed] [Google Scholar]
- 29. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing (2010) 39(4):412–23. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc (2014) 15(2):95–101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 31. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing (2019) 48(4):601. doi: 10.1093/ageing/afz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle (2022) 13(1):86–99. doi: 10.1002/jcsm.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc (2013) 14(8):531–2. doi: 10.1016/j.jamda.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 34. Bauer J, Morley JE, Schols A, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia: A time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle (2019) 10(5):956–61. doi: 10.1002/jcsm.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle (2016) 7(1):28–36. doi: 10.1002/jcsm.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc (2014) 15(9):630–4. doi: 10.1016/j.jamda.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 37. Bahat G, Yilmaz O, Kılıç C, Oren MM, Karan MA. Performance of SARC-F in regard to sarcopenia definitions, muscle mass and functional measures. J Nutr Health Aging (2018) 22(8):898–903. doi: 10.1007/s12603-018-1067-8 [DOI] [PubMed] [Google Scholar]
- 38. Yang M, Hu X, Xie L, Zhang L, Zhou J, Lin J, et al. SARC-F for sarcopenia screening in community-dwelling older adults: Are 3 items enough? Med (Baltimore) (2018) 97(30):e11726. doi: 10.1097/md.0000000000011726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ida S, Murata K, Nakadachi D, Ishihara Y, Imataka K, Uchida A, et al. Development of a Japanese version of the SARC-F for diabetic patients: an examination of reliability and validity. Aging Clin Exp Res (2017) 29(5):935–42. doi: 10.1007/s40520-016-0668-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu Z, Zhang P, Chen Y, Jiang J, Zhou Z, Zhu H. Comparing SARC-CalF with SARC-F for screening sarcopenia in adults with type 2 diabetes mellitus. Front Nutr (2022) 9:803924. doi: 10.3389/fnut.2022.803924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. da Luz MCL, Pinho CPS, Bezerra GKA, da Conceição Chaves de Lemos M, da Silva Diniz A, Cabral PC. SARC-F and SARC-CalF in screening for sarcopenia in older adults with Parkinson's disease. Exp Gerontol (2021) 144:111183. doi: 10.1016/j.exger.2020.111183 [DOI] [PubMed] [Google Scholar]
- 42. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle (2019) 10(1):207–17. doi: 10.1002/jpen.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papadopoulos E, Jin R, Monginot S, Alibhai SMH. Performance of the SARC-F in identifying low grip strength and physical performance in older adults with cancer. J Geriatr Oncol (2023) 14(2):101424. doi: 10.1016/j.jgo.2022.10142 [DOI] [PubMed] [Google Scholar]
- 44. Tosato M, Marzetti E, Cesari M, Savera G, Miller RR, Bernabei R, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res (2017) 29(1):19–27. doi: 10.1007/s40520-016-0717-0 [DOI] [PubMed] [Google Scholar]
- 45. Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, van Kan GA, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle (2012) 3(3):181–90. doi: 10.1007/s13539-012-0078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ackermans LLGC, Rabou J, Basrai M, Schweinlin A, Bischoff SC, Cussenot O, et al. Screening, diagnosis and monitoring of sarcopenia: When to use which tool? Clin Nutr ESPEN (2022) 48:36–44. doi: 10.1016/j.clnesp.2022.01.027 [DOI] [PubMed] [Google Scholar]
- 47. Lee SY, Choo PL, Pang BWJ, Lau LK, Jabbar KA, Seah WT, et al. SPPB reference values and performance in assessing sarcopenia in community-dwelling Singaporeans - Yishun study. BMC Geriatr (2021) 21(1):213. doi: 10.1186/s12877-021-02147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bergland A, Strand BH. Norwegian reference values for the Short Physical Performance Battery (SPPB): the Tromsø Study. BMC Geriatr (2019) 19(1):216. doi: 10.1186/s12877-019-1234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. He P, Du G, Qin X, Li Z. Reduced energy metabolism contributing to aging of skeletal muscle by serum metabolomics and gut microbiota analysis. Life Sci (2023) 323:121619. doi: 10.1016/j.lfs.2023.121619 [DOI] [PubMed] [Google Scholar]
- 50. Lim J-Y, Frontera WR. Skeletal muscle aging and sarcopenia: Perspectives from mechanical studies of single permeabilized muscle fibers. J Biomechanics (2023) 152:111559. doi: 10.1016/j.jbiomech.2023.111559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dos Santos MR, Storer TW. Testosterone treatment as a function-promoting therapy in sarcopenia associated with aging and chronic disease. Endocrinol Metab Clinics North America (2022) 51(1):187–204. doi: 10.1016/j.ecl.2021.11.012 [DOI] [PubMed] [Google Scholar]
- 52. Scicchitano BM, Pelosi L, Sica G, Musarò A. The physiopathologic role of oxidative stress in skeletal muscle. Mech Ageing Dev (2018) 170:37–44. doi: 10.1016/j.mad.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 53. Sandiford SD, Kennedy KA, Xie X, Pickering JG, Li SS. Dual oxidase maturation factor 1 (DUOXA1) overexpression increases reactive oxygen species production and inhibits murine muscle satellite cell differentiation. Cell Commun Signal (2014) 12:5. doi: 10.1186/1478-811x-12-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sestili P, Barbieri E, Martinelli C, Battistelli M, Guescini M, Vallorani L, et al. Creatine supplementation prevents the inhibition of myogenic differentiation in oxidatively injured C2C12 murine myoblasts. Mol Nutr Food Res (2009) 53(9):1187–204. doi: 10.1002/mnfr.200800504 [DOI] [PubMed] [Google Scholar]
- 55. Vasilaki A, Jackson MJ. Role of reactive oxygen species in the defective regeneration seen in aging muscle. Free Radic Biol Med (2013) 65:317–23. doi: 10.1016/j.freeradbiomed.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fealy CE, Grevendonk L, Hoeks J, Hesselink MKC. Skeletal muscle mitochondrial network dynamics in metabolic disorders and aging. Trends Mol Med (2021) 27(11):1033–44. doi: 10.1016/j.molmed.2021.07.013 [DOI] [PubMed] [Google Scholar]
- 57. Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Biran M, Deschodt-Arsac V, et al. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell (2014) 13(1):39–48. doi: 10.1111/acel.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gonzalez-Freire M, Scalzo P, D'Agostino J, Moore ZA, Diaz-Ruiz A, Fabbri E, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell (2018) 17(2):e12725. doi: 10.1111/acel.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sousa-Victor P, García-Prat L, Muñoz-Cánoves P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol (2022) 23(3):204–26. doi: 10.1038/s41580-021-00421-2 [DOI] [PubMed] [Google Scholar]
- 60. Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol (2010) 340(2):330–43. doi: 10.1016/j.ydbio.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Verdijk LB, Dirks ML, Snijders T, Prompers JJ, Beelen M, Jonkers RA, et al. Reduced satellite cell numbers with spinal cord injury and aging in humans. Med Sci Sports Exerc (2012) 44(12):2322–30. doi: 10.1249/MSS.0b013e3182667c2e [DOI] [PubMed] [Google Scholar]
- 62. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol (2017) 8:1045. doi: 10.3389/fphys.2017.01045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc (2002) 50(12):1947–54. doi: 10.1046/j.1532-5415.2002.50605.x [DOI] [PubMed] [Google Scholar]
- 64. Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci (2004) 59(3):242–8. doi: 10.1093/gerona/59.3.m242 [DOI] [PubMed] [Google Scholar]
- 65. Haren MT, Malmstrom TK, Miller DK, Patrick P, Perry HM, 3rd, Herning MM, et al. Higher C-reactive protein and soluble tumor necrosis factor receptor levels are associated with poor physical function and disability: a cross-sectional analysis of a cohort of late middle-aged African Americans. J Gerontol A Biol Sci Med Sci (2010) 65(3):274–81. doi: 10.1093/gerona/glp148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bautmans I, Onyema O, Van Puyvelde K, Pleck S, Mets T. Grip work estimation during sustained maximal contraction: validity and relationship with dependency and inflammation in elderly persons. J Nutr Health Aging (2011) 15(8):731–6. doi: 10.1007/s12603-010-0317-1 [DOI] [PubMed] [Google Scholar]
- 67. Alemán H, Esparza J, Ramirez FA, Astiazaran H, Payette H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing (2011) 40(4):469–75. doi: 10.1093/ageing/afr040 [DOI] [PubMed] [Google Scholar]
- 68. Giallauria F, Cittadini A, Smart NA, Vigorito C. Resistance training and sarcopenia. Monaldi Arch Chest Dis (2016) 84(1-2):738. doi: 10.4081/monaldi.2015.738 [DOI] [PubMed] [Google Scholar]
- 69. Ibata N, Terentjev EM. Why exercise builds muscles: titin mechanosensing controls skeletal muscle growth under load. Biophys J (2021) 120(17):3649–63. doi: 10.1016/j.bpj.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 71. de Souza LF, Danielewicz AL, Rech CR, d'Orsi E, Mendonça VA, Lacerda ACR, et al. How much time in sedentary behavior is associated with probable sarcopenia in older adults? Geriatric Nurs (2022) 48:127–31. doi: 10.1016/j.gerinurse.2022.09.007 [DOI] [PubMed] [Google Scholar]
- 72. Nagai K, Matsuzawa R, Wada Y, Tsuji S, Itoh M, Sano K, et al. Impact of isotemporal substitution of sedentary time with physical activity on sarcopenia in older Japanese adults. J Am Med Directors Assoc (2021) 22(4):876–8. doi: 10.1016/j.jamda.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 73. Wahlen BM, Mekkodathil A, Al-Thani H, El-Menyar A. Impact of sarcopenia in trauma and surgical patient population: A literature review. Asian J Surg (2020) 43(6):647–53. doi: 10.1016/j.asjsur.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 74. Stefanaki C, Pervanidou P, Boschiero D, Chrousos GP. Chronic stress and body composition disorders: implications for health and disease. Hormones (Athens) (2018) 17(1):33–43. doi: 10.1007/s42000-018-0023-7 [DOI] [PubMed] [Google Scholar]
- 75. Li X, He J, Sun Q. Sleep duration and sarcopenia: an updated systematic review and meta-analysis. J Am Med Directors Assoc (2023) 24(8):1193–206.e5. doi: 10.1016/j.jamda.2023.04.032 [DOI] [PubMed] [Google Scholar]
- 76. Gallagher EJ, Leroith D, Karnieli E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt Sinai J Med (2010) 77(5):511–23. doi: 10.1002/msj.20212 [DOI] [PubMed] [Google Scholar]
- 77. Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell (2013) 152(4):673–84. doi: 10.1016/j.cell.2013.01.041 [DOI] [PubMed] [Google Scholar]
- 78. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care (2009) 32 Suppl 2(Suppl 2):S157–63. doi: 10.2337/dc09-S302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia (2016) 59(1):44–55. doi: 10.1007/s00125-015-3751-0 [DOI] [PubMed] [Google Scholar]
- 80. Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab (2005) 31:5S20–5S6. doi: 10.1016/S1262-3636(05)73648-X [DOI] [PubMed] [Google Scholar]
- 81. Gareev I, Beylerli O, Aliev G, Pavlov V, Izmailov A, Zhang Y, et al. The role of long non-coding RNAs in intracranial aneurysms and subarachnoid hemorrhage. Life (Basel) (2020) 10(9):155. doi: 10.3390/life10090155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc (2011) 59(7):1217–24. doi: 10.1111/j.1532-5415.2011.03472.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr (2005) 25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155 [DOI] [PubMed] [Google Scholar]
- 84. Beilerli A, Kudriashov V, Sufianov A, Kostin A, Begliarzade S, Ilyasova T, et al. Regulation and mechanism of action of miRNAs on insulin resistance in skeletal muscles. Non-coding RNA Res (2023) 8(2):218–23. doi: 10.1016/j.ncrna.2023.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Umegaki H. Sarcopenia and diabetes: Hyperglycemia is a risk factor for age-associated muscle mass and functional reduction. J Diabetes Investig (2015) 6(6):623–4. doi: 10.1111/jdi.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee H, Kim SY, Lim Y. Lespedeza bicolor extract supplementation reduced hyperglycemia-induced skeletal muscle damage by regulation of AMPK/SIRT/PGC1α–related energy metabolism in type 2 diabetic mice. Nutr Res (2023) 110:1–13. doi: 10.1016/j.nutres.2022.12.007 [DOI] [PubMed] [Google Scholar]
- 87. Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci (2010) 11(4):1509–26. doi: 10.3390/ijms11041509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mori H, Kuroda A, Ishizu M, Ohishi M, Takashi Y, Otsuka Y, et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J Diabetes Investig (2019) 10(5):1332–40. doi: 10.1111/jdi.13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rato L, Duarte AI, Tomás GD, Santos MS, Moreira PI, Socorro S, et al. Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating mitochondrial bioenergetics and oxidative stress. Biochim Biophys Acta (2014) 1837(3):335–44. doi: 10.1016/j.bbabio.2013.12.00 [DOI] [PubMed] [Google Scholar]
- 90. Li W, Liang X, Zeng Z, Yu K, Zhan S, Su Q, et al. Simvastatin inhibits glucose uptake activity and GLUT4 translocation through suppression of the IR/IRS-1/Akt signaling in C2C12 myotubes. BioMed Pharmacother (2016) 83:194–200. doi: 10.1016/j.biopha.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 91. Yoo JI, Ha YC, Choi H, Kim KH, Lee YK, Koo KH, et al. Malnutrition and chronic inflammation as risk factors for sarcopenia in elderly patients with hip fracture. Asia Pac J Clin Nutr (2018) 27(3):527–32. doi: 10.6133/apjcn.082017.02 [DOI] [PubMed] [Google Scholar]
- 92. Brioche T, Lemoine-Morel S. Oxidative stress, sarcopenia, antioxidant strategies and exercise: molecular aspects. Curr Pharm Des (2016) 22(18):2664–78. doi: 10.2174/1381612822666160219120531 [DOI] [PubMed] [Google Scholar]
- 93. Kozakowska M, Pietraszek-Gremplewicz K, Jozkowicz A, Dulak J. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil (2015) 36(6):377–93. doi: 10.1007/s10974-015-9438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med (1999) 340(6):448–54. doi: 10.1056/nejm199902113400607 [DOI] [PubMed] [Google Scholar]
- 95. Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules (2019) 9(6):223. doi: 10.3390/biom9060223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts (2022) 15(3):321–35. doi: 10.1159/000521241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gao Q, Mei F, Shang Y, Hu K, Chen F, Zhao L, et al. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin Nutr (2021) 40(7):4633–41. doi: 10.1016/j.clnu.2021.06.009 [DOI] [PubMed] [Google Scholar]
- 98. Gandham A, Mesinovic J, Jansons P, Zengin A, Bonham MP, Ebeling PR, et al. Falls, fractures, and areal bone mineral density in older adults with sarcopenic obesity: A systematic review and meta-analysis. Obes Rev (2021) 22(5):e13187. doi: 10.1111/obr.13187 [DOI] [PubMed] [Google Scholar]
- 99. Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina (Kaunas) (2019) 55(9):546. doi: 10.3390/medicina55090546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhou Y-y, Wang J-f, Yao Q, Jian Q-f, Luo Z-p. Prevalence of sarcopenic obesity in patients with diabetes and adverse outcomes: A systematic review and meta-analysis. Clin Nutr ESPEN (2023) 58:128–35. doi: 10.1016/j.clnesp.2023.09.920 [DOI] [PubMed] [Google Scholar]
- 101. Xu J, Li X, Chen W, Zhang Z, Zhou Y, Gou Y, et al. Myofiber Baf60c controls muscle regeneration by modulating Dkk3-mediated paracrine signaling. J Exp Med (2023) 220(9):e20221123. doi: 10.1084/jem.20221123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Basiri R, Spicer MT, Ledermann T, Arjmandi BH. Effects of nutrition intervention on blood glucose, body composition, and phase angle in obese and overweight patients with diabetic foot ulcers. Nutrients (2022) 14(17):3564. doi: 10.3390/nu14173564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sadiya A, Ahmed SM, Carlsson M, Tesfa Y, George M, Ali SH, et al. Vitamin D3 supplementation and body composition in persons with obesity and type 2 diabetes in the UAE: A randomized controlled double-blinded clinical trial. Clin Nutr (2016) 35(1):77–82. doi: 10.1016/j.clnu.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 104. Yoshimoto Y, Oishi Y. Mechanisms of skeletal muscle-tendon development and regeneration/healing as potential therapeutic targets. Pharmacol Ther (2023) 243:108357. doi: 10.1016/j.pharmthera.2023.108357 [DOI] [PubMed] [Google Scholar]
- 105. Wu J, Egusa A, Nishimura T. Carnosine synthase deficiency in mice affects protein metabolism in skeletal muscle. Biochem Biophys Res Commun (2022) 612:22–9. doi: 10.1016/j.bbrc.2022.04.075 [DOI] [PubMed] [Google Scholar]
- 106. Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res (2017) 29(1):43–8. doi: 10.1007/s40520-016-0709-0 [DOI] [PubMed] [Google Scholar]
- 107. Matsunaga T, Furuya T, Deto T, Masuda H, Nakanishi K. Two older hematological Malignancy patients for whom nutrition rehabilitation was effective against global leadership initiative on malnutrition-defined malnutrition and sarcopenia. Cureus (2023) 15(8):e43069. doi: 10.7759/cureus.43069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chan L-C, Yang Y-C, Lin H-C, Wahlqvist ML, Hung Y-J, Lee M-S. Nutrition counseling is associated with less sarcopenia in diabetes: A cross-sectional and retrospective cohort study. Nutrition (2021) 91-92:111269. doi: 10.1016/j.nut.2021.111269 [DOI] [PubMed] [Google Scholar]
- 109. Hwang J, Zmuda JM, Kuipers AL, Bunker CH, Santanasto AJ, Wheeler VW, et al. Serum vitamin D and age-related muscle loss in Afro-Caribbean men: the importance of age and diabetic status. J Frailty Aging (2019) 8(3):131–7. doi: 10.14283/jfa.2018.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Henson J, Arsenyadis F, Redman E, Brady EM, Coull NA, Edwardson CL, et al. Relative protein intake and associations with markers of physical function in those with type 2 diabetes. Diabetes Med (2022) 39(8):e14851. doi: 10.1111/dme.14851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fanelli SM, Kelly OJ, Krok-Schoen JL, Taylor CA. Low protein intakes and poor diet quality associate with functional limitations in US adults with diabetes: A 2005-2016 NHANES analysis. Nutrients (2021) 13(8):2582. doi: 10.3390/nu13082582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Buchanan A, Villani A. Association of adherence to a Mediterranean diet with excess body mass, muscle strength and physical performance in overweight or obese adults with or without type 2 diabetes: two cross-sectional studies. Healthcare (Basel) (2021) 9(10):1255. doi: 10.3390/healthcare9101255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Okamura T, Miki A, Hashimoto Y, Kaji A, Sakai R, Osaka T, et al. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort. J Diabetes (2019) 11(6):477–83. doi: 10.1111/1753-0407.12874 [DOI] [PubMed] [Google Scholar]
- 114. Takahashi F, Hashimoto Y, Kaji A, Sakai R, Kawate Y, Okamura T, et al. Vitamin intake and loss of muscle mass in older people with type 2 diabetes: A prospective study of the KAMOGAWA-DM cohort. Nutrients (2021) 13(7):2335. doi: 10.3390/nu13072335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Okamura T, Hashimoto Y, Miki A, Kaji A, Sakai R, Iwai K, et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: a cross-sectional study of KAMOGAWA-DM cohort study. J Clin Biochem Nutr (2020) 66(3):233–7. doi: 10.3164/jcbn.19-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Casals-Vázquez C, Suárez-Cadenas E, Estébanez Carvajal FM, Aguilar Trujillo MP, Jiménez Arcos MM, Vázquez Sáchez M. [Relationship between quality of life, physical activity, nutrition, glycemic control and sarcopenia in older adults with type 2 diabetes mellitus]. Nutr Hosp (2017) 34(5):1198–204. doi: 10.20960/nh.1070 [DOI] [PubMed] [Google Scholar]
- 117. Izquierdo M, Laosa O, Cadore EL, Abizanda P, Garcia-Garcia FJ, Hornillos M, et al. Two-year follow-up of a multimodal intervention on functional capacity and muscle power in frail patients with type 2 diabetes. J Am Med Dir Assoc (2021) 22(9):1906–11. doi: 10.1016/j.jamda.2021.06.022 [DOI] [PubMed] [Google Scholar]
- 118. Low S, Wang J, Moh A, Ang SF, Ang K, Shao YM, et al. Amino acid profile of skeletal muscle loss in type 2 diabetes: Results from a 7-year longitudinal study in asians. Diabetes Res Clin Pract (2022) 186:109803. doi: 10.1016/j.diabres.2022.109803 [DOI] [PubMed] [Google Scholar]
- 119. da Silva Soares DB, Shinjo SK, Santos AS, de Cassia Rosa de Jesus J, Schenk S, de Castro GS, et al. Skeletal muscle gene expression in older adults with type 2 diabetes mellitus undergoing calorie-restricted diet and recreational sports training - a randomized clinical trial. Exp Gerontology (2022) 164:111831. doi: 10.1016/j.exger.2022.111831 [DOI] [PubMed] [Google Scholar]
- 120. Martínez-Arnau FM, Fonfría-Vivas R, Buigues C, Castillo Y, Molina P, Hoogland AJ, et al. Effects of leucine administration in sarcopenia: A randomized and placebo-controlled clinical trial. Nutrients (2020) 12(4):932. doi: 10.3390/nu12040932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee HX, Yeo A, Tan CN, Yew S, Tay L, Ding YY, et al. Combined impact of positive screen for sarcopenia and frailty on physical function, cognition and nutrition in the community dwelling older adult. Ann Geriatr Med Res (2021) 25(3):210–6. doi: 10.4235/agmr.21.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Millward DJ, Layman DK, Tomé D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr (2008) 87(5):1576s–81s. doi: 10.1093/ajcn/87.5.1576S [DOI] [PubMed] [Google Scholar]
- 123. Wu Y, de Crom TO, Schaft NVD, Chen Z, Rivadeneira F, Boer CG, et al. Dietary protein intake with body composition, hand grip strength, and sarcopenia in older adults: A population-based study. Clin Nutr ESPEN (2023) 54:476. doi: 10.1016/j.clnesp.2022.09.062 [DOI] [Google Scholar]
- 124. Fuchs CJ, Hermans WJH, Holwerda AM, Smeets JSJ, Senden JM, van Kranenburg J, et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial. Am J Clin Nutr (2019) 110(4):862–72. doi: 10.1093/ajcn/nqz120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol (2006) 41(2):215–9. doi: 10.1016/j.exger.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 126. Hou Y, Yoon Y, Oh E, Sung B, Kim Y. Effects of soy protein hydrolysates on antioxidant activity and inhibition of muscle loss. Int Food Res J (2022) 29(6):1458–67. doi: 10.47836/ifrj.29.6.22 [DOI] [Google Scholar]
- 127. Lewgood J, Oliveira B, Korzepa M, Forbes SC, Little JP, Breen L, et al. Efficacy of dietary and supplementation interventions for individuals with type 2 diabetes. Nutrients (2021) 13(7):2378. doi: 10.3390/nu13072378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr (2014) 33(6):929–36. doi: 10.1016/j.clnu.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc (2013) 14(8):542–59. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 130. Coletta G, Phillips SM. An elusive consensus definition of sarcopenia impedes research and clinical treatment: A narrative review. Ageing Res Rev (2023) 86:101883. doi: 10.1016/j.arr.2023.101883 [DOI] [PubMed] [Google Scholar]
- 131. Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr (2011) 93(5):997–1005. doi: 10.3945/ajcn.110.008102 [DOI] [PubMed] [Google Scholar]
- 132. Rennie MJ, Bohé J, Wolfe RR. Latency, duration and dose response relationships of amino acid effects on human muscle protein synthesis. J Nutr (2002) 132(10):3225s–7s. doi: 10.1093/jn/131.10.3225S [DOI] [PubMed] [Google Scholar]
- 133. Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, et al. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab (2005) 288(4):E645–53. doi: 10.1152/ajpendo.00413.2004 [DOI] [PubMed] [Google Scholar]
- 134. Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr (2002) 132(1):95–100. doi: 10.1093/jn/132.1.95 [DOI] [PubMed] [Google Scholar]
- 135. Frexes-Steed M, Lacy DB, Collins J, Abumrad NN. Role of leucine and other amino acids in regulating protein metabolism in vivo . Am J Physiol (1992) 262(6 Pt 1):E925–35. doi: 10.1152/ajpendo.1992.262.6.E925 [DOI] [PubMed] [Google Scholar]
- 136. Kobayashi H. [Amino acid nutrition in the prevention and treatment of sarcopenia]. Yakugaku Zasshi (2018) 138(10):1277–83. doi: 10.1248/yakushi.18-00091-4 [DOI] [PubMed] [Google Scholar]
- 137. Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol (2006) 575(Pt 1):305–15. doi: 10.1113/jphysiol.2006.110742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. van Loon LJ. Leucine as a pharmaconutrient in health and disease. Curr Opin Clin Nutr Metab Care (2012) 15(1):71–7. doi: 10.1097/MCO.0b013e32834d617a [DOI] [PubMed] [Google Scholar]
- 139. Yang C, Dai Y, Li Z, Peng Y, Zhang L, Jia H. Relationship of serum 25-hydroxyvitamin D levels with sarcopenia and body composition in community-dwelling older adults: A paired case-control study. J Am Med Dir Assoc (2023) 24(8):1213–9. doi: 10.1016/j.jamda.2023.06.004 [DOI] [PubMed] [Google Scholar]
- 140. Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab (2010) 95(4):1595–601. doi: 10.1210/jc.2009-2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab (2003) 88(12):5766–72. doi: 10.1210/jc.2003-030604 [DOI] [PubMed] [Google Scholar]
- 142. Bhattacharya S, Bhadra R, Schols AMWJ, van Helvoort A, Sambashivaiah S. Nutrition in the prevention and management of sarcopenia - A special focus on Asian Indians. Osteoporosis Sarcopenia (2022) 8(4):135–44. doi: 10.1016/j.afos.2022.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Seo MH, Kim MK, Park SE, Rhee EJ, Park CY, Lee WY, et al. The association between daily calcium intake and sarcopenia in older, non-obese Korean adults: the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) 2009. Endocr J (2013) 60(5):679–86. doi: 10.1507/endocrj.ej12-0395 [DOI] [PubMed] [Google Scholar]
- 144. Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res (2003) 18(2):343–51. doi: 10.1359/jbmr.2003.18.2.343 [DOI] [PubMed] [Google Scholar]
- 145. Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc (2008) 56(1):84–90. doi: 10.1111/j.1532-5415.2007.01478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Dos Reis AS, Limirio LS, Santos HO, de Oliveira EP. Intake of polyunsaturated fatty acids and ω-3 are protective factors for sarcopenia in kidney transplant patients. Nutrition (2021) 81:110929. doi: 10.1016/j.nut.2020.110929 [DOI] [PubMed] [Google Scholar]
- 147. Bird JK, Troesch B, Warnke I, Calder PC. The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: A scoping systematic review and meta-analysis. Clin Nutr ESPEN (2021) 46:73–86. doi: 10.1016/j.clnesp.2021.10.011 [DOI] [PubMed] [Google Scholar]
- 148. Haghiac M, Yang XH, Presley L, Smith S, Dettelback S, Minium J, et al. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: A randomized double-blind controlled clinical trial. PLoS One (2015) 10(9):e0137309. doi: 10.1371/journal.pone.0137309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pan D, Yang L, Yang X, Xu D, Wang S, Gao H, et al. Potential nutritional strategies to prevent and reverse sarcopenia in aging process: Role of fish oil-derived ω-3 polyunsaturated fatty acids, wheat oligopeptide and their combined intervention. J Advanced Res (2023) S2090-1232(23)00111-X. doi: 10.1016/j.jare.2023.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhao D, Shi W, Bi L, Qi Y, Hu S, Li C, et al. Effect of short-term acute moderate-intensity resistance exercise on blood glucose in older patients with type 2 diabetes mellitus and sarcopenia. Geriatr Gerontol Int (2022) 22(8):653–9. doi: 10.1111/ggi.14437 [DOI] [PubMed] [Google Scholar]
- 151. Chien YH, Tsai CJ, Wang DC, Chuang PH, Lin HT. Effects of 12-week progressive sandbag exercise training on glycemic control and muscle strength in patients with type 2 diabetes mellitus combined with possible sarcopenia. Int J Environ Res Public Health (2022) 19(22):228. doi: 10.3390/ijerph192215009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Buch A, Eldor R, Kis O, Keinan-Boker L, Dunsky A, Rubin A, et al. The effect of circuit resistance training, empagliflozin or "vegeterranean diet" on physical and metabolic function in older subjects with type 2 diabetes: a study protocol for a randomized control trial (CEV-65 trial). BMC Geriatr (2019) 19(1):228. doi: 10.1186/s12877-019-1219-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pfeifer LO, Botton CE, Diefenthaeler F, Umpierre D, Pinto RS. Effects of a power training program in the functional capacity, on body balance and lower limb muscle strength of elderly with type 2 diabetes mellitus. J Sports Med Phys Fitness (2021) 61(11):1529–37. doi: 10.23736/s0022-4707.21.11880-8 [DOI] [PubMed] [Google Scholar]
- 154. Botton CE, Umpierre D, Rech A, Pfeifer LO, MaChado CLF, Teodoro JL, et al. Effects of resistance training on neuromuscular parameters in elderly with type 2 diabetes mellitus: A randomized clinical trial. Exp Gerontol (2018) 113:141–9. doi: 10.1016/j.exger.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 155. Hsieh PL, Tseng CH, Tseng YJ, Yang WS. Resistance training improves muscle function and cardiometabolic risks but not quality of life in older people with type 2 diabetes mellitus: A randomized controlled trial. J Geriatr Phys Ther (2018) 41(2):65–76. doi: 10.1519/jpt.0000000000000107 [DOI] [PubMed] [Google Scholar]
- 156. Marcotte-Chénard A, Tremblay D, Mony MM, Brochu M, Dionne IJ, Langlois MF, et al. Low-volume walking HIIT: Efficient strategy to improve physical capacity and reduce the risk of cardiovascular disease in older women with type 2 diabetes. Diabetes Metab Syndr (2021) 15(5):102233. doi: 10.1016/j.dsx.2021.102233 [DOI] [PubMed] [Google Scholar]
- 157. Ferreira LF, Scariot EL, da Rosa LHT. The effect of different exercise programs on sarcopenia criteria in older people: A systematic review of systematic reviews with meta-analysis. Arch Gerontol Geriatr (2023) 105:104868. doi: 10.1016/j.archger.2022.104868 [DOI] [PubMed] [Google Scholar]
- 158. Park S. Association between polygenetic risk scores related to sarcopenia risk and their interactions with regular exercise in a large cohort of Korean adults. Clin Nutr (2021) 40(10):5355–64. doi: 10.1016/j.clnu.2021.09.003 [DOI] [PubMed] [Google Scholar]
- 159. Li L, He Y, Jin N, Li H, Liu X. Effects of protein supplementation and exercise on delaying sarcopenia in healthy older individuals in Asian and non-Asian countries: A systematic review and meta-analysis. Food Chemistry: X (2022) 13:100210. doi: 10.1016/j.fochx.2022.100210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kim H, Suzuki T, Saito K, Yoshida H, Kojima N, Kim M, et al. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. Geriatr Gerontol Int (2013) 13(2):458–65. doi: 10.1111/j.1447-0594.2012.00923.x [DOI] [PubMed] [Google Scholar]
- 161. Scott D, Blizzard L, Fell J, Giles G, Jones G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian Older Adult Cohort Study. J Am Geriatr Soc (2010) 58(11):2129–34. doi: 10.1111/j.1532-5415.2010.03147.x [DOI] [PubMed] [Google Scholar]
- 162. Hashemi R, Motlagh AD, Heshmat R, Esmaillzadeh A, Payab M, Yousefinia M, et al. Diet and its relationship to sarcopenia in community dwelling Iranian elderly: a cross sectional study. Nutrition (2015) 31(1):97–104. doi: 10.1016/j.nut.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 163. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med (2016) 2(1):e000143. doi: 10.1136/bmjsem-2016-000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Codella R, Ialacqua M, Terruzzi I, Luzi L. May the force be with you: why resistance training is essential for subjects with type 2 diabetes mellitus without complications. Endocrine (2018) 62(1):14–25. doi: 10.1007/s12020-018-1603-7 [DOI] [PubMed] [Google Scholar]
- 165. Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci (2013) 68(7):769–79. doi: 10.1093/gerona/gls241 [DOI] [PubMed] [Google Scholar]
- 166. Fragala MS, Fukuda DH, Stout JR, Townsend JR, Emerson NS, Boone CH, et al. Muscle quality index improves with resistance exercise training in older adults. Exp Gerontol (2014) 53:1–6. doi: 10.1016/j.exger.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 167. McLeod JC, Stokes T, Phillips SM. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front Physiol (2019) 10:645. doi: 10.3389/fphys.2019.00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Liu Y, Ye W, Chen Q, Zhang Y, Kuo CH, Korivi M. Resistance exercise intensity is correlated with attenuation of hbA1c and insulin in patients with type 2 diabetes: A systematic review and meta-analysis. Int J Environ Res Public Health (2019) 16(1):140. doi: 10.3390/ijerph16010140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yoshimura Y, Bise T, Shimazu S, Tanoue M, Tomioka Y, Araki M, et al. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition (2019) 58:1–6. doi: 10.1016/j.nut.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 170. Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc (2017) 18(6):553.e1–.e16. doi: 10.1016/j.jamda.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 171. Hordern MD, Dunstan DW, Prins JB, Baker MK, Singh MA, Coombes JS. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. J Sci Med Sport (2012) 15(1):25–31. doi: 10.1016/j.jsams.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 172. Zhang X, Zhao Y, Chen S, Shao H. Anti-diabetic drugs and sarcopenia: emerging links, mechanistic insights, and clinical implications. J Cachexia Sarcopenia Muscle (2021) 12(6):1368–79. doi: 10.1002/jcsm.12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Tricarico D, Mele A, Lundquist AL, Desai RR, George AL, Jr., Conte Camerino D. Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc Natl Acad Sci U.S.A. (2006) 103(4):1118–23. doi: 10.1073/pnas.0505974103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tricarico D, Mele A, Camerino GM, Bottinelli R, Brocca L, Frigeri A, et al. The KATP channel is a molecular sensor of atrophy in skeletal muscle. J Physiol (2010) 588(Pt 5):773–84. doi: 10.1113/jphysiol.2009.185835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mele A, Buttiglione M, Cannone G, Vitiello F, Camerino DC, Tricarico D. Opening/blocking actions of pyruvate kinase antibodies on neuronal and muscular KATP channels. Pharmacol Res (2012) 66(5):401–8. doi: 10.1016/j.phrs.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 176. Lee CG, Boyko EJ, Barrett-Connor E, Miljkovic I, Hoffman AR, Everson-Rose SA, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care (2011) 34(11):2381–6. doi: 10.2337/dc11-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sugimoto K, Ikegami H, Takata Y, Katsuya T, Fukuda M, Akasaka H, et al. Glycemic control and insulin improve muscle mass and gait speed in type 2 diabetes: the MUSCLES-DM study. J Am Med Directors Assoc (2021) 22(4):834–8.e1. doi: 10.1016/j.jamda.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 178. Bouchi R, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, et al. Insulin treatment attenuates decline of muscle mass in Japanese patients with type 2 diabetes. Calcif Tissue Int (2017) 101(1):1–8. doi: 10.1007/s00223-017-0251-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ferrari U, Then C, Rottenkolber M, Selte C, Seissler J, Conzade R, et al. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA-Age study. Acta Diabetol (2020) 57(9):1057–63. doi: 10.1007/s00592-020-01523-7 [DOI] [PubMed] [Google Scholar]
- 180. Sallé A, Guilloteau G, Ryan M, Bouhanick B, Ritz P. Effect of insulin treatment on the body composition of Type 2 diabetic patients. Diabetes Med (2004) 21(12):1298–303. doi: 10.1111/j.1464-5491.2004.01335.x [DOI] [PubMed] [Google Scholar]