Key Teaching Points.
-
•
Cardiac contractility modulation is a device-based therapeutic option for patients with advanced heart failure with reduced ejection fraction and a left ventricular ejection fraction between 25% and 45%, who do not benefit from resynchronization therapy and are still symptomatic despite optimal medical therapy.
-
•
By generating nonexcitatory electrical signals during the myocardial absolute refractory period, cardiac contractility modulation therapy acts directly on cardiac contractility. These high-voltage biphasic electrical impulses do not cause myocardial contraction but appear to have an effect on cellular pathways that regulate calcium cycling, improving myocardial contractility.
-
•
Management of chronic refractory heart failure with reduced ejection fraction in heart transplant patients is challenging. Cardiac contractility modulation may represent a new option for the heart transplant care team involved in the treatment of this specific cohort of patients.
Introduction
Cardiac contractility modulation (CCM) is a device-based therapeutic option for patients with advanced heart failure with reduced ejection fraction (HFrEF) and a left ventricular ejection fraction (LVEF) between 25% and 45%, who do not benefit from cardiac resynchronization therapy (CRT) and are still symptomatic despite optimal medical therapy.1
By generating nonexcitatory electrical signals during the myocardial absolute refractory period, CCM therapy acts directly on cardiac contractility. These high-voltage biphasic electrical impulses do not cause myocardial contraction but appear to have an effect on cellular pathways that regulate calcium cycling, improving myocardial contractility without a significant effect on LVEF.2,3 From a clinical standpoint, this leads to an improvement of the functional exercise capacity and exercise tolerance, reducing the number of hospitalizations for HF.4 Notably, CCM has been proven to be beneficial in terms of symptoms even in patients with shorter QRS duration, which hinders response to CRT.5
Herein, we describe the first case of CCM device implantation in a post-heart transplant patient with refractory heart failure and narrow QRS.
Case report
We present the case of a 74-year-old male patient referred to our electrophysiology department for consideration of device therapy for refractory systolic heart failure and right bundle branch block. This patient underwent orthotopic heart transplant for nonischemic dilated cardiomyopathy 11 years prior. In the immediate postoperative period, he experienced early graft dysfunction, likely secondary to ischemic injury, leading to left ventricular (LV) dysfunction. Subsequently, he suffered late graft dysfunction in the setting of moderate chronic rejection and latent cytomegalovirus infection, contributing to chronic LV dysfunction. Over the years, his LVEF was persistently low (around 30%), despite chronic immunosuppressive therapy (calcineurin inhibitors [560 mg/die]) and heart failure optimal medical therapy (angiotensin receptor blocker [80 mg], beta-blocker [7.5 mg/die], and mineralocorticoid receptor antagonist [100 mg/die]). Of note, neither sodium-glucose cotransporter-2 inhibitors nor angiotensin receptor-neprilysin inhibitors could be used, owing to drug-drug interactions with concomitant immunosuppressive therapy and post-transplant chronic kidney disease.
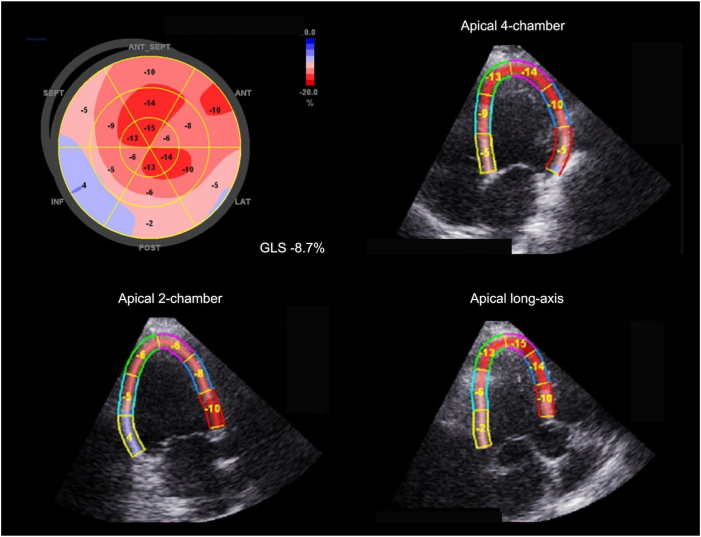
In the year before referral, the patient experienced worsening heart failure, with increasing exertional dyspnea with daily activities (NYHA III); his average blood pressure was 90/60 mm Hg. The most recent transthoracic echocardiogram showed severe LV dilatation (LV end-diastolic volume [LVEDV] 214 mL) and severe LV systolic dysfunction, with both reduced LVEF (25%–30%), stroke volume of 42 mL, and LV global longitudinal strain (LV-GLS -8.7%; Figure 1). To improve his symptoms, we opted for CCM therapy and later consideration of implantable cardioverter-defibrillator implantation for primary prevention in case of persistent LVEF below 30% at follow-up.
Figure 1.
Left ventricular longitudinal strain before cardiac contractility modulation therapy. Longitudinal strain analysis was assessed with 2D speckle tracking using GE Vivid E9 (GE, Chicago, IL). The bull’s-eye diagram displays segmental peak systolic strain, with an average global longitudinal strain (GLS) of -8.7%.
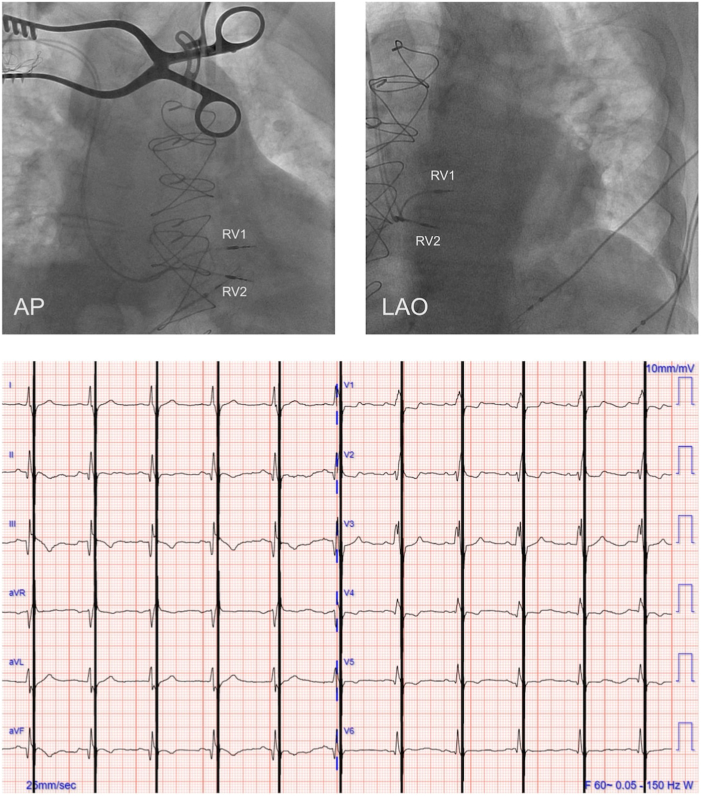
The patient underwent implantation of a 2-lead CCM system, consisting of an Optimizer Smart implantable pulse generator (IPG; Impulse Dynamics, Marlton, NJ) located in the right infraclavicular fossa connected to 2 ventricular pacing leads (4076-52; Medtronic, Minneapolis, MN) placed in the interventricular septum (Figure 2, top). The right-sided venous system was chosen to keep the left-sided venous system free and available for other potential future devices, as customary. At the time of implant, good pacing and sensing parameters were recorded, with an adequate acute increase of dP/dtmax for each lead (ie, ≥5%). There was no diaphragmatic or chest wall capture, and the patient had no complaints of chest discomfort during high-output stimulation. The device was programmed in OVO-LS-CCM mode (Figure 2, bottom) with a CCM train of 7.5 V with 2 pulses (?) over a 89.56 ms duration and a CCM programmed dose of 5 hours periods per day.
Figure 2.
Cardiac contractility modulation implant procedure. Top: Fluoroscopy images of lead placement for cardiac contractility modulation showing 2 leads implanted in the mid right ventricular septum. Bottom: Electrocardiogram during cardiac contractility modulation treatment. RV1, lead 1 in right ventricle; RV2, lead 2 in right ventricle.
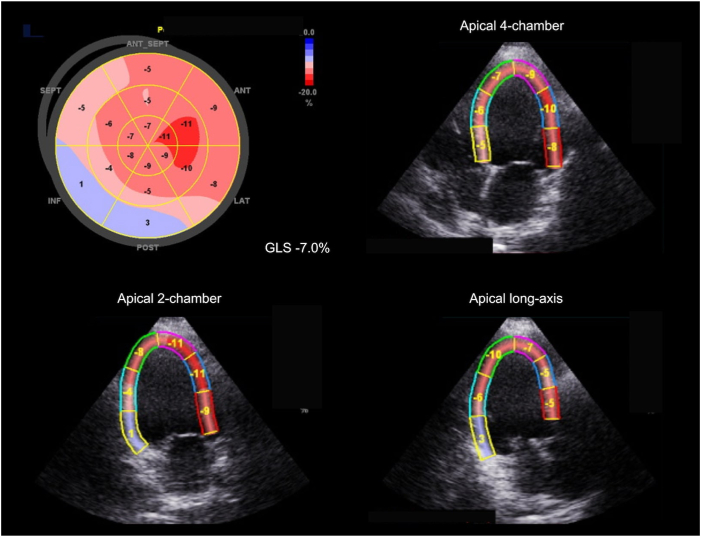
Two weeks later, the patient underwent the first clinical follow-up after IPG implantation; subjectively, exertional dyspnea slightly improved. On IPG interrogation, CCM therapy was deemed not adequate (86%). Therefore, the CCM programmed dose was increased to 7 1-hour periods per day hours a day. At the second follow-up, performed 6 weeks after implantation, CCM therapy was deemed adequate (95%) and the programmed dose was not changed further. Additionally, to quantify the subjective improvement of dyspnea, the patient also underwent a 6-minute walking test (6MWT), reaching a distance 329 meters. At 6-month follow-up, the patient reported further improvement of symptoms, denying any exertional dyspnea; his average blood pressure was 110/75 mm Hg. Furthermore, transthoracic echocardiogram showed worsening LV dilatation (LVEDV 269 mL), with a small increase of the LVEF (30%–35%), stroke volume 68 mL, but slight decline of the LV-GLS (-7.0%), mostly driven by decrease in strain rate of the apical segments (Figure 3). At the 6MWT, the patient was able to walk a distance of 366 meters, correlating with the subjective improvement in dyspnea.
Figure 3.
Left ventricular global longitudinal strain after 6 months of cardiac contractility modulation therapy. Longitudinal strain analysis was assessed with 2D speckle tracking using GE Vivid E9 (GE, Chicago, IL). The bull’s-eye diagram displays segmental peak systolic strain, with an average global longitudinal strain (GLS) of -7.0%.
Discussion
To the best of our knowledge, this is the first worldwide application of CCM in a patient with refractory HFrEF following heart transplant. Despite guidelines-based optimal medical therapy, our patient was experiencing worsening heart failure in the setting of low ejection fraction (around 30%) and a non–left bundle branch block QRS morphology, in which implantable cardioverter-defibrillator / CRT device implantation might not be beneficial.5 CCM may represent a new opportunity for this specific subgroup of patients.
Management of systolic heart failure after heart transplant is challenging. While a better understanding of graft rejection and widespread use of preventive immunosuppressive therapy have improved life expectancy after heart transplant, long-term survival is still affected by various conditions, such as graft failure, cardiac allograft vasculopathy, infections, and malignancy.6 Additionally, we still lack a specific therapy for HFrEF after heart transplant beyond the standard therapy for heart failure (“optimal medical therapy”), which is often limited by concomitant renal insufficiency and drug-drug interactions.
The postulated mechanism of CCM is different than that of traditional optimal medical therapy. CCM influences the intrinsic contraction properties of the heart by directly acting on calcium pathways and bypassing autonomic regulation, which is impaired in patients following heart transplant.7,8 On a cellular level, CCM appears to normalize the phosphorylation levels of phospholamban and to enhance calcium handling through upregulation of sarco/endoplasmic reticulum calcium ATPase (SERCA2a) and restoration of the sodium/calcium exchanger.9 Taken together, these mechanisms can improve myocardial contractility without increasing myocardial oxygen consumption, which can be detrimental in patients with failing hearts. The potential beneficial effect of CCM on the transplanted heart can be compared to that of levosimendan, which improves cardiac contractility enhancing myofilament calcium sensitivity without increasing myocardial ATP consumption.10 Indeed, levosimendan has been shown to benefit cardiac function in patients with primary graft dysfunction after heart transplant.11
In our patient, CCM led to improvement of symptoms, as shown by an increase in walked distance at the 6MWT without a corresponding change in LV function. A discordance between improvement of functional capacity and no significant change in LVEF has also been observed in large CCM clinical trials.12 Similarly, while our patient’s LVEF has slightly improved, LVEDV increased and LV-GLS was essentially unchanged, if not slightly worsened. Of note, it is difficult to reconcile this latter finding with the postulated mechanism for CCM. Indeed, GLS expresses the longitudinal shortening of myocardial fibers and, as such, is a more direct measurement of contractility and should be a better indicator of CCM response.13,14 Nevertheless, our patient’s functional capacity improved significantly, an important observation in this challenging cohort of patients.15
Conclusion
This is the first described case of CCM in a patient with refractory systolic heart failure following orthotopic heart transplant. CCM led to improvement of symptoms, without a corresponding change in LV function. Further reports and dedicated studies are needed to confirm the beneficial effect of CCM in this peculiar cohort of patients.
Disclosures
No conflicts of interest relevant to this manuscript.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:E895–E1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 2.Brunckhorst C.B., Shemer I., Mika Y., Ben-Haim S.A., Burkhoff D. Cardiac contractility modulation by non-excitatory currents: studies in isolated cardiac muscle. Eur J Heart Fail. 2006;8:7–15. doi: 10.1016/j.ejheart.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Kahwash R., Burkhoff D., Abraham W.T. Cardiac contractility modulation in patients with advanced heart failure. Expert Rev Cardiovasc Ther. 2013;11:635–645. doi: 10.1586/erc.13.48. [DOI] [PubMed] [Google Scholar]
- 4.Abraham W.T., Kuck K.H., Goldsmith R.L., et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail. 2018;6:874–883. doi: 10.1016/j.jchf.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Glikson M., Nielsen J.C., Kronborg M.B., et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 6.McCartney S.L., Patel C., Del Rio J.M. Long-term outcomes and management of the heart transplant recipient. Best Pract Res Clin Anaesthesiol. 2017;31:237–248. doi: 10.1016/j.bpa.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Grupper A., Gewirtz H., Kushwaha S. Reinnervation post-heart transplantation. Eur Heart J. 2018;39:1799–1806. doi: 10.1093/eurheartj/ehw604. [DOI] [PubMed] [Google Scholar]
- 8.Masarone D., Vastarella R., Melillo E., Petraio A., Pacileo G. Beta-blocker therapy in heart transplant recipients: a review. Clin Transplant. 2020;34(11) doi: 10.1111/ctr.14081. [DOI] [PubMed] [Google Scholar]
- 9.Marks A.R. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figgitt D.P., Gillies P.S., Goa K.L. Levosimendan. Drugs. 2001;61:613–627. doi: 10.2165/00003495-200161050-00006. [DOI] [PubMed] [Google Scholar]
- 11.Immohr M.B., Akhyari P., Boettger C., et al. Levosimendan for treatment of primary graft dysfunction after heart transplantation: optimal timing of application. Exp Clin Transplant. 2021;19:473–480. doi: 10.6002/ect.2020.0342. [DOI] [PubMed] [Google Scholar]
- 12.Giallauria F., Vigorito C., Piepoli M.F., Stewart Coats A.J. Effects of cardiac contractility modulation by non-excitatory electrical stimulation on exercise capacity and quality of life: an individual patient’s data meta-analysis of randomized controlled trials. Int J Cardiol. 2014;175:352–357. doi: 10.1016/j.ijcard.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Potter E., Marwick T.H. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–274. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Karlsen S., Dahlslett T., Grenne B., et al. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc Ultrasound. 2019;17:18. doi: 10.1186/s12947-019-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkaryoni A., Altibi A.M., Khan M.S., et al. Global longitudinal strain assessment of the left ventricle by speckle tracking echocardiography detects acute cellular rejection in orthotopic heart transplant recipients: a systematic review and meta-analysis. Echocardiography. 2020;37:302–309. doi: 10.1111/echo.14586. [DOI] [PubMed] [Google Scholar]