Abstract
We investigated whether Helicobacter pylori cells actively secrete proteins such as the urease subunits UreA and UreB and the GroES and GroEL homologs HspA and HspB or whether these proteins were present in the extracellular compartment as a consequence of autolysis. Using a subcellular fractionation approach associated with quantitative Western blot analyses, we showed that the supernatant protein profiles were very different from those of the cell pellets, even for bacteria harvested in the late growth phase; this suggests that the release process is selective. A typical cytoplasmic protein, a β-galactosidase homolog, was found exclusively associated with the pellet of whole-cell extracts, and no traces were found in the supernatant. In contrast, UreA, UreB, HspA, and HspB were mostly found in the pellet but significant amounts were also present in the supernatant. HspA and UreB were released into the supernatant at the same rate throughout the growth phase (3%), whereas large portions of HspB and UreA were released during the stationary phase (over 30 and 20%, respectively) rather than during the early growth phase (20% and 6, respectively). The profiles of protein obtained after water extraction of the bacteria with those of the proteins naturally released within the liquid culture supernatants demonstrated that water extraction led to the release of a large amount of protein due to artifactual lysis. Our data support the conclusion that a specific and selective mechanism(s) is involved in the secretion of some H. pylori antigens. A programmed autolysis process does not seem to make a major contribution.
Helicobacter pylori is a gram-negative, spiral-shaped pathogenic bacterium. It specifically colonizes the gastric epithelium of primates and is the etiologic agent of chronic gastritis (2). The bacterial properties and both host and various environmental factors can cause gastritis to progress to more severe diseases over a period of years. These diseases include peptic ulcer, gastric lymphoma, gastric atrophy, and gastric carcinoma (5, 26).
H. pylori has properties adapted to life in its unique niche, the viscous and acidic gastric environment (3, 24). In particular, motility and ability to hydrolyze urea are important characteristics of H. pylori (22). Isogenic urease-negative mutants of H. pylori have been constructed and used to demonstrate that urease was not required for the survival of H. pylori in vitro but was essential for colonization of the gastric mucosa in piglets (12) and mice (32). Like the other bacterial ureases, the H. pylori urease is a metalloenzyme that requires nickel ions for activity (6); however, unlike these other ureases, it consists of a heteropolymer of two [(AB)3], not three [(ABC)3], subunits (4, 19, 21). Although mostly found in the cytoplasmic compartment, it is also present in association with the outer membrane and as released subcellular material (14, 17). This pattern of distribution is similar to that of H. pylori HspA and HspB, the GroES and GroEL homologs (13, 20), and superoxide dismutase (28, 30), catalase (18), and ferritin (7, 8, 16). All these proteins lack leader sequences and are exclusively cytoplasmic in other bacteria. Thus, the extracytoplasmic location of these abundant proteins may be artifactual or, alternatively, due to the activity of a specific but uncharacterized secretion pathway.
Recently, Dunn et al. (11) published convincing evidence that H. pylori organisms present on the surface of human gastric biopsies, in their natural environment, produced extracellular urease and HspB. These observations established that urease and HspB were present on the cell surface and as released material. This distribution could result from one of several possible mechanisms. One model, recently proposed by Phadnis et al. (29), suggests that the proteins are released by autolysis and become associated with the surface of intact bacteria by reabsorption. We thus investigated whether autolysis of H. pylori cells during the growth phase accounts for the subcellular localization of urease, HspA, and HspB.
We present data demonstrating that these proteins were found in the supernatants of H. pylori liquid cultures and that their extracellular location could not be explained by autolysis. Using a subcellular fractionation approach associated with quantitative Western blot analyses, we showed (i) that the supernatant protein profiles were different from those cell pellets of bacteria harvested even in the late growth phase, (ii) that the secretion rate for each of the studied antigens was different, and (iii) that a cytoplasmic protein could not be found in the supernatant.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
H. pylori 85P (21) was grown on horse blood agar plates supplemented with vancomycin (12.5 mg/liter), polymyxin B (310 μg/liter), trimethoprim (6.25 mg/liter), and amphotericin B (2.5 mg/liter). Plates were incubated at 37°C under microaerobic conditions in an anaerobic jar with a microaerobic gas-generating kit (Oxoid BR56) in the presence of a catalyst. Liquid cultures were grown in flasks containing brain heart infusion broth (BHI; Difco Laboratories, Detroit, Mich.) supplemented with 0.2% β-cyclodextrin (Sigma) (25) and the selective antibiotic cocktail described above. The flasks were incubated at 37°C with constant agitation at 150 rpm under microaerobic conditions in an anaerobic jar with a microaerobic gas-generating kit (Oxoid BR56) in the presence of a catalyst.
Subcellular fractionation.
Proteins released during H. pylori growth were analyzed by using strain 85P because of its good growth in liquid medium. The methods described by Allaoui et al. (1) and Ménard et al. (27) for the study of protein secretion in Shigella flexneri were used. H. pylori cells were grown on blood agar plates for 72 h and used to inoculate 200 ml of BHI to an initial optical density at 600 nm (OD600) of 0.1. Four flasks, each containing 50 ml of the 200 ml of inoculated broth, were incubated in parallel in identical jars under microaerobic conditions in the same incubator for 13, 24, 36, or 46 h. The cultures were then centrifuged for 30 min at 5,000 × g. A sample of 75 μl of the whole-cell fraction (semiliquid pellet) from a total volume of about 900 μl was then mixed with 25 μl of 4× loading buffer (denatured buffer). Each supernatant was filtered through a 0.2-μm-pore-size filter to eliminate intact bacterial cells. Proteins present in the culture supernatant were precipitated by adding 0.1 volume of 100% (wt/vol) trichloracetic acid (TCA) and resuspended in 500 μl of 1× loading buffer–0.5 M (final concentration) Tris (pH 9.0).
Preparation of water extract of H. pylori.
The protocol described by Phadnis et al. (29) was used for the preparation of the water extract of H. pylori. Briefly, bacterial cells grown on one agar plate (for 24 or 48 h) were collected with Q-tips in 30 ml of cold phosphate-buffered saline (20 mM phosphate buffer containing 0.15 mM NaCl [pH 7.0]) and centrifuged (5,000 × g for 20 min at 4°C). The cells were resuspended in 30 ml of distilled water, vortex mixed for 1 min, and then sedimented by centrifugation (5,000 × g for 20 min). The pellet fractions (450 μl) were resuspended in 150 μl of 4× loading buffer. Proteins present in the water were filtered through a 0.2-μm-pore-size filter, TCA precipitated, and resuspended in 200 μl of 1× loading buffer–0.5 M (final concentration) Tris (pH 9.0). Protein profiles of the washed whole-cell fraction and the water extract were analyzed and compared.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting techniques.
Solubilized protein preparations were analyzed on slab gels, consisting of a 4.5% acrylamide stacking gel and a 12.5% resolving gel, in accordance with the procedure of Laemmli (23). Electrophoresis was performed at 200 V on a mini-slab gel apparatus (Bio-Rad Laboratories, Richmond, Calif.). Proteins were either stained with Coomassie brilliant blue or transferred to nitrocellulose membranes in a mini-Trans-Blot transfer cell (Bio-Rad) set at 100 V for 1 h, with cooling. Immunoreactants were detected by chemiluminescence (ECL system; Amersham) as described previously (15). The primary antibodies were rabbit antibodies raised against the maltose binding protein (MBP) fused to UreA, UreB, HspA, or HspB (antibodies were diluted 1:5,000 for use) or raised against recombinant Escherichia coli β-galactosidase (diluted 1:2,000). Secondary antibodies were diluted 1:20,000.
RESULTS AND DISCUSSION
Evidence for protein secretion during growth phase of H. pylori in liquid culture.
BHI supplemented with β-cyclodextrin (0.2%) and not fetal calf serum was used for liquid cultures to prevent contamination of supernatant protein preparations with heterologous proteins. Under the conditions used, H. pylori 85P cells multiplied and the OD600 increased from 0.1 to 1.9 (stationary phase) after 36 h of incubation; protein profiles of H. pylori whole cells and culture supernatants harvested at 13, 24, 36, and 46 h (corresponding to cultures at OD600s of 0.1, 0.5, 1.3, 1.9, and 1.8, respectively) were determined by SDS-PAGE (Fig. 1A). The protein profiles originating from whole cells, visualized by Coomassie blue staining, remained qualitatively unchanged throughout the time course. However, they clearly differed from the respective profiles of the corresponding supernatants at each time point (13, 24, 36, and 46 h). The supernatant profiles also differed from that of sterile BHI (the culture broth) (Fig. 1A, lane T) treated under the same conditions, indicating that some H. pylori proteins were present in the supernatant. These results were in conflict with the model recently proposed by Phadnis et al. (29), where autolysis of the bacteria explains the presence of some of the major antigens of H. pylori as released material. This model was drawn from results obtained by use of a protein extraction procedure employed by several authors (9, 10, 29) that produces what is called a water extract; the procedure consists of washing the bacteria in phosphate-buffered saline, vortexing them in water, and then concentrating and analyzing the proteins in suspension in the water extract. By using this procedure, Phadnis et al. (29) reported that the protein content of this water extract was qualitatively similar to that of the whole-cell extract; they concluded that the proteins found in the water extract were there as the result of lysis and demonstrated that the lysis was more prevalent during late growth phases than early phases. They extrapolated these findings to propose that the presence of major antigens previously identified as material associated with the subcellular fraction and visualized by electron microscopy was due to autolysis. To confirm whether this extrapolation was valid, we thus prepared water extracts from H. pylori and concentrated these water extracts by TCA precipitation. The protein profiles of our water extracts (Fig. 1B) were indeed very similar to those of the water-washed whole cells from the corresponding cultures (solid-medium culture); however, they were very different from those of the supernatants of the liquid cultures (Fig. 1A). Our results suggest that water extraction of the proteins associated with the cells led to a cell lysis that does not seem to occur spontaneously and therefore are not compatible with autolysis being the major mechanism of the release of these proteins.
FIG. 1.
(A) Proteins from whole-cell extracts (2 μl of a 100-μl sample) and culture supernatants (3 μl of a 500-μl sample) from independent broth cultures (13, 24, 36, and 46 h) of H. pylori 85P were separated by SDS–12.5% PAGE and stained with Coomassie brilliant blue. Lane T, sterile BHI. (B) Protein analysis of water-washed cells (5 μl of a 600-μl sample) and water extracts (5 μl of a 200-μl sample) prepared after 24 and 48 h of culture as described in Materials and Methods is shown. The positions and sizes (in kilodaltons) of the protein standards and the positions of UreA, UreB, and HspB proteins and the multimeric forms of the HspA protein are indicated.
Secretion of specific antigens of H. pylori that accumulate in the cytoplasm.
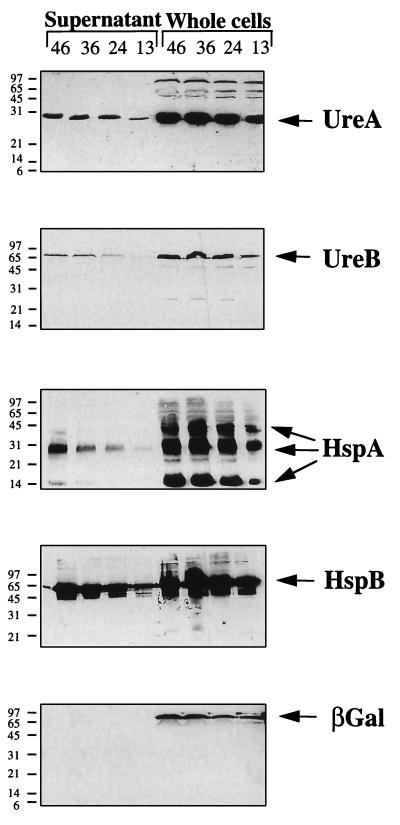
We then attempted to confirm that some H. pylori antigens are present in the supernatants and others are not. The supernatants and the whole-cell fractions were analyzed by immunoblotting with rabbit polyclonal antibodies raised against recombinant UreA and UreB, the two urease subunits, as well as HspA and HspB, the GroES and GroEL homologs. UreA, UreB, and HspB have been shown to be present in the extracellular compartment of H. pylori. The subcellular location of a protein cross-reacting with an anti-rabbit recombinant E. coli β-galactosidase antibody and exhibiting an apparent molecular mass of 96 kDa was also tested. H. pylori liquid culture was sonicated, centrifuged, filtered, and TCA precipitated. This material was then analyzed by immunoblotting with the E. coli β-galactosidase antibody; the β-galactosidase immunoreactivity was found in the soluble fraction (data not shown). Immunoblotting analysis was performed to detect UreA, UreB, HspA, and HspB. UreA, UreB, and HspB were found predominantly associated with the whole cells, each as a single form with a mobility in SDS-PAGE corresponding to its calculated molecular mass (Fig. 2). HspA was detected as multimeric forms, with the dimeric form being more abundant than the monomeric and the trimeric forms. The intensity of these antigenic bands in Western blots of the whole-cell fractions was proportional to the cell culture density and thus to the number of bacterial cells. The four antigens UreA, UreB, HspA, and HspB were also unambiguously detected in the supernatant fraction (Fig. 2). In contrast, the β-galactosidase homolog was found exclusively in the whole-cell fraction; no trace of this protein was detected in the supernatant (Fig. 2) even following 150-fold concentration of the sample or prolonged exposure of the X-ray film (24 h). However, the intensity of the β-galactosidase homolog signal in the whole-cell fraction was similar to that of UreB, which was detected in the supernatant fraction. We thus concluded that this cytoplasmic protein was not found in the supernatant. We tested its sensitivity to proteolytic degradation: extracts from the liquid culture were sonicated directly and incubated at 37°C for 1 h. The β-galactosidase homolog present in this incubated extract remained detectable and in apparently similar amounts before and after incubation, indicating that its absence from the supernatant of growing cultures was not the result of its proteolysis. Moreover, after centrifugation of these sonicated extracts, the β-galactosidase homolog was found in the supernatants, indicating that its location was cytoplasmic rather than membrane associated (data not shown). The β-galactosidase immuno-cross-reactive protein, which was found by fractionation study to be a typical cytosolic protein, could not be detected in the supernatant of culture even during the stationary phase. This suggests that not all proteins present in the cytoplasmic fraction are released by a nonspecific mechanism such as autolysis but rather that some proteins are selectively released into the supernatant.
FIG. 2.
Western blot analysis of protein from whole-cell extracts and culture supernatants after various times of growth by using, from top to bottom, antiserum raised against MBP-UreA, MBP-UreB, MBP-HspA, MBP-HspB, and β-galactosidase. Apparent molecular masses (in kilodaltons) of the protein standards are indicated on the left.
Antigens are not released at the same rate.
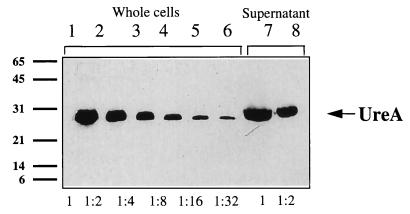
The relative rates of release of the four extracytoplasmic antigens were determined by testing dilutions of samples for different time points of the culture (see Fig. 3 for quantification of the UreA antigen). The four antigens were detected by immunoblotting. The relative accumulation rates for the four antigens were then calculated (Table 1). They were not secreted at the same rate: 3% of HspA and 3% of UreB were present in the supernatant during the exponential growth phase, compared to 11% of UreA and 20% of HspB. More UreA and HspB were secreted during the late stationary phase (around 20% and over 30%, respectively), whereas the rate of accumulation for the two other antigens (UreB and HspA) did not vary with time. In this study, strain 85P was used because of its good growth in liquid medium; however, the possibility that there might be a difference in secretion rate when another isolate is tested cannot be ruled out. For strain 85P, the rates of release of the different proteins were different. If autolysis was responsible for the release of antigens, the same proportion of the total amount of each protein would be found in the supernatant. This was clearly not the case since 20 and 11% of total HspB and UreA, respectively, and only 3% of total UreB and HspA were extracellular. Possibly, UreA, UreB, and HspA were not found at higher levels in the medium because they were more sensitive to a proteolytic activity. However, using a sensitive technique (Western immunoblotting), we were unable to detect any proteolytic degradation of the protein in the supernatant. Furthermore, such putative sensitivity of UreA is not compatible with its accumulation in the supernatant during growth.
FIG. 3.
Quantification of the released UreA antigen. Serial dilutions from 1 (i.e., 1:1) to 1:32 of a whole-cell extract (3 μl of a 1,200-μl sample) and of the corresponding supernatant extract (10 μl of a 500-μl sample) were resolved by SDS-PAGE and revealed with anti-MBP-UreA antiserum. The dilution of the supernatant extract that was estimated to be equivalent to the dilution of the whole-cell extract was determined, and the amount of UreA in the supernatant as a proportion of the total present in both the supernatant and the whole-cell extract was calculated. In this UreA example, at the 24-h time point, the 1:2 dilution of supernatant was equivalent to the 1:2 dilution of the corresponding whole-cell extract, indicating that there was the same amount of protein in 0.125% (3/1,200 × 0.5) whole-cell extract as in 1% (10/500 × 0.5) supernatant. It can therefore be estimated that after 24 h of growth, 11% of total UreA was in the supernatant. Apparent molecular masses (in kilodaltons) of the protein standards are indicated on the left.
TABLE 1.
Secretion of UreA, UreB, HspA, and HspB into the culture supernatant during H. pylori growth and stationary phasesa
| Time point (h) | Secreted antigen (%)
|
|||
|---|---|---|---|---|
| UreA | UreB | HspA | HspB | |
| 12 | 6 | 3 | 3 | 20 |
| 24 | 11 | 3 | 3 | 20 |
| 36 | 11 | 3 | 3 | 20 |
| 48 | 20 | 1.5 | 2.5 | >30 |
The results are expressed as the percentage of secreted antigen: the amount of secreted antigen was divided by the total amount of the same antigen in the culture (see legend to Fig. 3).
To confirm the existence of specific mechanisms for the secretion of some of the proteins that accumulate in the cytosolic fraction, genetic evidence is required. Nevertheless, our results argue against a massive autolysis process. Indeed, such autolysis is inconsistent with the general observations made since the discovery of this bacterium, which suggest that it differentiates to a resistant coccoid form rather than spontaneously lysing. It therefore seems likely that specific mechanisms might be involved in the secretion process, as in a large number of other pathogenic and nonpathogenic bacteria. There is genetic evidence that the major H. pylori antigens found in the subcellular fraction are produced as polypeptides with no signal sequence. In view of our general understanding of the secretion pathways in bacteria, the pathway may involve either (i) type III secretion machinery, a highly sophisticated membrane complex of at least 20 specific membrane-associated proteins, or (ii) a simpler system such as the ABC transporter. The recent publication of the whole genomic sequence of H. pylori (31) (Internet address: http://www.tigr.org/) and the analysis of this database for evidence of typical type III secretion machinery were not conclusive. However, sequences possibly encoding ABC transporters were found. ABC transporters are systems formed of three proteins that transport proteins in one step from the cytosolic compartment to the subcellular fraction. Construction of knockout mutants for each putative transporter gene should reveal their involvement in the proteins that we investigated.
ACKNOWLEDGMENTS
We gratefully acknowledge Claude Parsot for constructive discussions and interest throughout our study and thank Agnès Ullmann for providing us with anti-β-galactosidase serum.
Anne Vanet was supported by OraVax Inc., Boston, Mass., and Pasteur-Mérieux Connaught (PMC), Lyon, France.
REFERENCES
- 1.Allaoui A, Sansonetti P J, Parsot C. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri lpa invasins. Mol Microbiol. 1993;7:59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 2.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 3.Calam J. Helicobacter pylori, acid and gastrin. Eur J Gastroenterol Hepatol. 1995;7:310–317. [PubMed] [Google Scholar]
- 4.Clayton C L, Pallen M J, Kleanthous H, Wren B W, Tabaqchali S. Nucleotide sequence of two genes from Helicobacter pylori encoding for urease subunits. Nucleic Acids Res. 1990;18:362. doi: 10.1093/nar/18.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19:S37–S43. [PubMed] [Google Scholar]
- 6.Cussac V, Ferrero R L, Labigne A. Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J Bacteriol. 1992;174:2466–2473. doi: 10.1128/jb.174.8.2466-2473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doig P, Austin J W, Kostrzynska M, Trust T J. Production of a conserved adhesin by the human gastroduodenal pathogen Helicobacter pylori. J Bacteriol. 1992;174:2539–2547. doi: 10.1128/jb.174.8.2539-2547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doig P, Austin J W, Trust T J. The Helicobacter pylori 19.6-kilodalton protein is an iron-containing protein resembling ferritin. J Bacteriol. 1993;175:966–972. doi: 10.1128/jb.175.2.557-560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn B E, Campbell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 10.Dunn B E, Roop II R M, Sung C-C, Sharma S A, Perez-Perez G I, Blaser M J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992;60:1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn B E, Vakil N B, Schneider B G, Miller M M, Zitzer J B, Peutz T, Phadnis S H. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect Immun. 1997;65:1181–1188. doi: 10.1128/iai.65.4.1181-1188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton K A, Brooks C L, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschweiler B, Bohrmann B, Gerstenecker B, Schiltz E, Kist M. In situ localization of the 60 k protein of Helicobacter pylori, which belongs to the family of heat shock proteins, by immuno-electron microscopy. Zentralbl Bakteriol. 1993;280:73–85. doi: 10.1016/s0934-8840(11)80942-4. [DOI] [PubMed] [Google Scholar]
- 14.Evans D J, Jr, Evans D G, Kirkpatrick S S, Graham D Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991;10:15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- 15.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazier B A, Pfeifer J D, Russell D G, Falk P, Olsen A N, Hammar M, Westblom T U, Normark S J. Paracrystalline inclusions of a novel ferritin containing nonheme iron, produced by the human gastric pathogen Helicobacter pylori: evidence for a third class of ferritins. Ann Paediatr. 1993;40:364–367. doi: 10.1128/jb.175.4.966-972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawtin P R, Stacey A R, Newell D G. Investigation of the structure and localization of the urease of Helicobacter pylori using monoclonal antibodies. J Gen Microbiol. 1990;136:1995–2000. doi: 10.1099/00221287-136-10-1995. [DOI] [PubMed] [Google Scholar]
- 18.Hazell S L, Evans D J, Jr, Graham D Y. Helicobacter pylori catalase. J Gen Microbiol. 1991;137:57–61. doi: 10.1099/00221287-137-1-57. [DOI] [PubMed] [Google Scholar]
- 19.Hu L T, Foxall P A, Russell R, Mobley H L. Purification of recombinant Helicobacter pylori urease apoenzyme encoded by ureA and ureB. Infect Immun. 1992;60:2657–2666. doi: 10.1128/iai.60.7.2657-2666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kansau I, Labigne A. Heat shock proteins of Helicobacter pylori. Aliment Pharmacol Ther. 1996;10:51–56. doi: 10.1046/j.1365-2036.1996.22164005.x. [DOI] [PubMed] [Google Scholar]
- 21.Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991;173:1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labigne A, de Reuse H. Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis. 1996;5:191–202. [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lee A. The microbiology and epidemiology of Helicobacter pylori infection. Scand J Gastroenterol Suppl. 1994;201:2–6. [PubMed] [Google Scholar]
- 25.Marchini A, Massari P, Manetti R, Olivieri R. Optimized conditions for the fermentation of Helicobacter pylori and production of vacuolating cytotoxin. FEMS Microbiol Lett. 1994;124:55–59. doi: 10.1111/j.1574-6968.1994.tb07261.x. [DOI] [PubMed] [Google Scholar]
- 26.McColl K E. Helicobacter pylori infection and its role in human disease—an overview. Pharm World Sci. 1996;18:49–55. doi: 10.1007/BF00579705. [DOI] [PubMed] [Google Scholar]
- 27.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori M, Suzuki H, Suzuki M, Kai A, Miura S, Ishii H. Catalase and superoxide dismutase secreted from Helicobacter pylori. Helicobacter. 1997;2:100–105. doi: 10.1111/j.1523-5378.1997.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 29.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiegelhalder C, Gerstenecker B, Kersten A, Schiltz E, Kist M. Purification of Helicobacter pylori superoxide dismutase and cloning and sequencing of the gene. Infect Immun. 1993;61:5315–5325. doi: 10.1128/iai.61.12.5315-5325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L X, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 32.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]