Key Teaching Points.
-
•
The incidence of atrial arrhythmia is 5%–16% in patients taking Bruton’s tyrosine kinase inhibitors, a class of chemotherapeutic agents that is used first line for many hematological malignancies. The main risk factors for developing arrhythmia on treatment are age >65 years, hypertension, prior cardiovascular disease, and a dilated left atrium.
-
•
Animal studies have shown ibrutinib leads to off-target kinase inhibition in the myocardium. This leads to increased proinflammatory signaling and impaired cardioprotective mechanisms in the atria, culminating in pathologic remodeling that promotes triggered activity and substrate for reentrant arrhythmias.
-
•
A rate control strategy is presently recommended as the first-line treatment option for atrial arrhythmias, with a rhythm control strategy reserved for those who remain symptomatic or develop a rate-related cardiomyopathy. Our case highlights atrial flutter on ibrutinib is amenable to an ablation strategy and should be considered in appropriately selected patients.
Introduction
Bruton’s tyrosine kinase (BTK) inhibitors are first-line therapy for many hematological malignancies, including chronic lymphocytic leukemia (CLL).1 Ibrutinib was the first-in-class agent developed and its use has significantly improved progression-free survival2; however, it is associated with an increased risk of atrial arrhythmia, with an incidence between 5% and 16%.3, 4, 5 The underlying mechanism responsible for this increase in arrhythmogenicity is not fully understood but may relate to off-target effects on tyrosine kinases expressed in the atrial myocardium.1 The commonest reported atrial arrhythmia is paroxysmal atrial fibrillation (AF),4 and although atrial flutter has been recognized, the anatomical and mechanistic basis of the common flutter circuits has not been described.
We present a patient with CLL managed with ibrutinib who developed recurrent cavotricuspid isthmus (CTI)-dependent flutter and, after successful block of the CTI, developed an atypical right atrial flutter using a macroreentrant circuit with a slowly conducting isthmus through a region of lateral scar. We explore whether BTK inhibitors have a direct myopathic effect on the atrium by summarizing the potential proarrhythmic mechanisms that may produce the substrate necessary for complex reentrant arrhythmias.
Case report
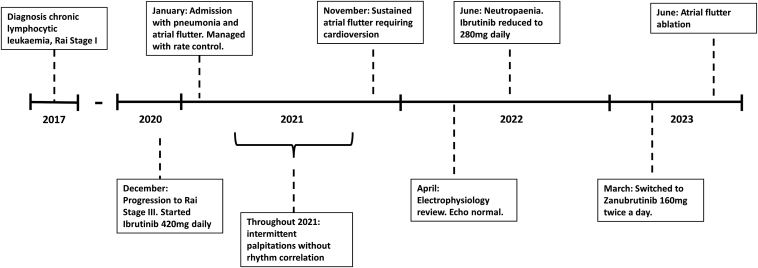
We describe the case of a 57-year-old man who was originally found to have an asymptomatic lymphocytosis 6 years prior. A summary of the timeline can be seen in Figure 1. Immunophenotyping confirmed a diagnosis of CLL and a staging computed tomography scan showed diffuse small-volume lymphadenopathy (Rai stage I). He developed symptomatic anemia (Rai stage III) in 2020 and was initiated on 420 mg daily of ibrutinib.
Figure 1.
A summary of the timeline relevant to the patient’s presentation, from initial diagnosis to the time of atrial flutter ablation.
Within a month of starting treatment, he presented to his local hospital with breathlessness and was found to be in atrial flutter with ventricular rates of 130–150 beats per minute, which was managed by a rate control strategy including oral metoprolol and digoxin, as well as anticoagulation with apixaban. The atrial flutter spontaneously cardioverted within 72 hours, whereupon the digoxin was stopped and he was asked to stop apixaban after a 4-week course, given a CHA2DS2-VASC score of 0.
During the rest of 2021, he reported intermittent palpitations associated with elevated heart rates (130–140 beats/min) on his smartwatch; however, these were less than 1 hour in duration and no correlation to ambulatory rhythm monitoring could be established. In November 2021, he had a sustained episode of palpitations requiring presentation to his local hospital. The presenting electrocardiogram (ECG) showed atrial flutter. He underwent DC cardioversion upon the advice of his cardiologist given a duration of <48 hours and his metoprolol was up-titrated. He was restarted on apixaban and referred to our electrophysiology service for consideration of a flutter ablation.
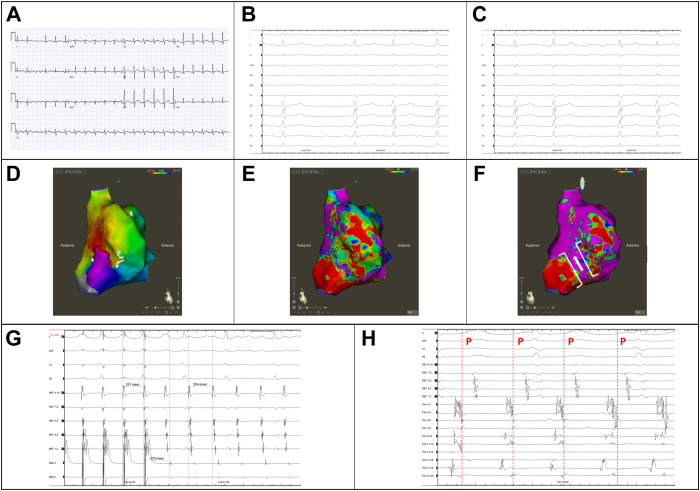
Evaluation in our clinic in April 2022 noted a man who was overweight (body mass index 27.1 kg/m2) and normotensive and had no other conventional risk factors for atrial arrhythmias, including sleep disordered breathing, diabetes mellitus, prior thromboembolism, and alcohol excess. His 12-lead ECGs were consistent with counterclockwise CTI-dependent flutter (Figure 2A). His echocardiogram demonstrated normal biventricular size and function, normal left ventricular wall thickness, no mitral valve disease, trivial tricuspid regurgitation, no interatrial shunt, and normal atrial dimensions (left atrium diameter: 35 mm). An atrial flutter ablation was offered; however, the patient had initial concerns about potential complications and therefore was switched from metoprolol to bisoprolol.
Figure 2.
A: A 12-lead electrocardiogram (ECG) of the clinical atrial flutter (AFL) prior to ablation. The negative F waves in the inferior leads and positive F wave in V1 is consistent with typical flutter. B, C: Intraprocedure 12-lead ECG of (B) the typical flutter (AFL1) and (C) the atypical atrial flutter (AFL2). The F waves are positive inferiorly and in V1. D: An activation map of AFL2 showing the lateral wall of the right atrium. E: The corresponding voltage map showing patchy scar on the lateral wall. F: The same voltage map using a lower cut-off of 0.2 mV for healthy tissue shows a potential channel that corresponds to the critical isthmus, which was the site of successful ablation. G: Entrainment from within this channel shows can in-circuit response with a postpacing interval minus tachycardia cycle length of 16 ms. H: Electrograms from the proposed exit site of the isthmus mapped with the PentaRay catheter (Biosense Webster, Irvine, CA). The onset of surface P wave is marked in AFL2 and late diastolic fractionated electrograms can be observed.
In June 2022, he developed neutropenia and had a dose reduction in his ibrutinib to 280 mg daily. Despite this, he continued to have intermittent palpitations; therefore, after discussion between electrophysiology and hematology, he was switched to zanubrutinib 160 mg twice daily in March 2023. Upon further discussion, he agreed to be listed for a flutter ablation.
Electrophysiology study and ablation
The study was performed under local anesthesia with sedation using the CARTO 3 electroanatomical mapping system (Biosense Webster, Irvine, CA). A decapolar catheter with 5 mm spacing (Inquiry; Abbott, Chicago, IL) was placed into the coronary sinus and a duodecapolar catheter with 2/10/2 mm spacing (Livewire; Abbott) was placed around the tricuspid annulus. The presenting rhythm was atrial flutter (AFL1) with a tachycardia cycle length (TCL) of 240 ms (Figure 2B). Entrainment was performed from the CTI at 6 o’clock on the tricuspid annulus with a 3.5 mm bidirectional ThermoCool SmartTouch SF catheter (Biosense Webster) with a postpacing interval (PPI) minus TCL of 25 ms. Ablations of 35–40 watts for 60 seconds duration were performed across the CTI. During ablation, there was a subtle change in TCL (250 ms) and change in the F-wave morphology on surface ECG consistent with a new atrial flutter (AFL2, Figure 2C). Once a linear lesion set through the CTI was completed, repeat entrainment from the lateral aspect of the line was concealed with a PPI − TCL of 20 ms; however, entrainment from the medial aspect of the line was manifest with a PPI − TCL of 148 ms, suggesting the CTI was blocked. Activation and voltage mapping of the right atrium was performed with a PentaRay catheter (Biosense Webster). The left atrium was not mapped. The activation window was set at 95% of TCL and the voltage cut-offs were 0.05 mV for scar and 0.5 mV for healthy tissue. The activation map demonstrated a potential reentrant circuit using a slow-conducting isthmus on the inferior aspect of the lateral right atrial wall (Figure 2D). The voltage map showed patchy areas of low-voltage tissue, particularly on the lateral wall (Figure 2E). Using a lower healthy voltage cut-off of 0.2 mV suggested there was a channel through the scar acting as the isthmus (Figure 2F). Entrainment from this isthmus was concealed with a PPI − TCL of 16 ms (Figure 2G and 2H). Further ablation was performed to transect this isthmus, resulting in termination of AFL2. Repeat induction was negative for atrial arrhythmias and confirmed bidirectional block across the CTI with a trans-isthmus time of 170 ms.
Discussion
For the first time, we describe the presence of both counterclockwise (typical) CTI-dependent flutter and an atypical atrial flutter based on a reentrant circuit involving patchy scar on the lateral wall of the right atrium in a patient on chronic ibrutinib therapy.
Atrial arrhythmias are increasingly being recognized as a common adverse event associated with BTK inhibitors, particularly ibrutinib, given the longer duration of its use in clinical practice. Ibrutinib increases the risk of atrial arrhythmia between 4- and 16-fold, dependent on the size of the population in the study and whether patients with a history of AF were excluded.4, 5, 6 Most studies do not distinguish between AF and atrial flutter5,7 and, where present, the anatomical basis of the atrial flutter is not reported; therefore, it is unclear what the true incidence of typical and atypical flutters are. It is recognized, however, that most atrial arrhythmias manifest as paroxysmal AF, with just over half having a single symptomatic episode only during follow-up.4 The commonest risk factors for AF in patients on ibrutinib include age >65 years, hypertension, prior cardiovascular disease, and a dilated left atrium, none of which our patient had.4, 5, 6, 7
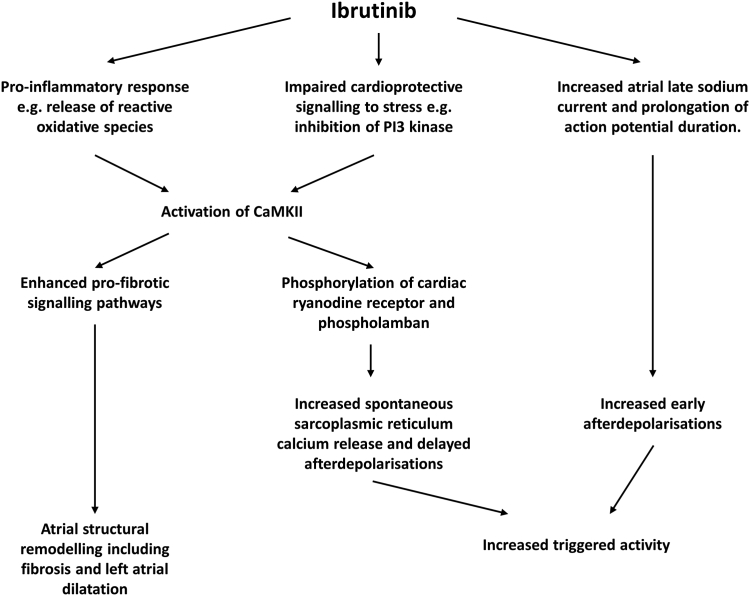
Given the association between BTK inhibitors and atrial arrhythmia, studies have attempted to clarify the electrophysiological effects of ibrutinib in the murine atrium. Mice treated with 2–4 weeks of ibrutinib show an increased susceptibility to induced AF.8, 9, 10 The left atrium undergoes significant pathologic remodeling, including chamber dilatation and fibrosis, as seen on histological examination.8,9 At a cellular level, ibrutinib is believed to have a number of off-target effects on kinases, including C-terminal Src kinase8 and phosphoinositide 3-kinase, the latter of which is important in protection of the myocardium against stress insults.11,12 The combination of impaired cardioprotective signaling and an upregulation in proinflammatory reactive oxygen species culminates in activation of calcium calmodulin kinase type II (CaMKII),8, 9, 10 which is well recognized as a key mediator of electrical and structural remodeling in the pathogenesis of AF.13 Increased CaMKII activity in the murine atrium as a result of chronic ibrutinib therapy is associated with increased spontaneous release of calcium from the sarcoplasmic reticulum, resulting in delayed afterdepolarizations and triggered activity.9,10 In addition, CaMKII activation is known to upregulate a number of profibrotic signaling pathways in the atrium, resulting in fibrosis and chamber dilatation.13 Direct application of ibrutinib to myocytes in vitro has been reported to prolong the action potential from an increase in late sodium current favoring early afterdepolarizations.12 A potential mechanism for the initiation of AF on ibrutinib therapy is shown in Figure 3.
Figure 3.
An overview of potential proarrhythmic mechanism of ibrutinib in atrial arrhythmia as summarized from the literature.8, 9, 10,12
Although these studies have focused primarily on the left atrium, we believe the same process occurs in the right atrium and hypothesize ibrutinib is the primary cause of the atrial myopathy, which provided the substrate for the atypical atrial flutter in our patient. In support of this, there was a temporal association between starting ibrutinib and the occurrence of atrial flutter; and the patient had no conventional risk factors for either atrial arrhythmias or atrial scarring. It can be challenging to prove a direct causation relationship between a drug and an arrhythmia, and it is possible the patient had atrial remodeling from subclinical AF between first presentation and ablation. However, in the 3 months following ablation the patient had no further palpitations, suggesting the right atrial flutter was the primary arrhythmia. Future studies characterizing the nature of atrial remodeling, the timeframe over which this may occur, and whether it is reversible are therefore warranted.
Recommendations have been published for the management of AF in patients on BTK inhibitors.3 The cornerstone is rate control therapy, primarily with beta-blockers; however, both calcium channel blockers and digoxin can be used with caution. This is recommended owing to the inhibition of cytochrome P450 3A4 by calcium channel blockers increasing the plasma levels of ibrutinib, and ibrutinib inhibition of P-glycoprotein increasing plasma levels of digoxin.3 Alongside rate control, consideration should be given to either a dose reduction of ibrutinib (which can be immediate or after a break)7 or considering switching to a newer BTK inhibitor such as zanubrutinib, which has been reported to have a much lower incidence of atrial arrhythmias.14 A rhythm control strategy has only been recommended when patients remain symptomatic despite rate control or develop an arrhythmia-induced cardiomyopathy, and even then the focus is on cardioversion or antiarrhythmic drugs rather than ablation. This is in part because of an increased risk of bleeding associated with anticoagulation owing to drug-interactions and thrombocytopenia associated with CLL.3 Successful pulmonary vein isolation has been reported as part of a management plan for a patient with highly symptomatic AF on ibrutinib15 and, as demonstrated from the acute success in our case, we argue should be considered in appropriately selected patients with atrial flutter.
Conclusion
BTK inhibitors not only are associated with AF but may lead to significant atrial remodeling manifesting as an atypical atrial flutter. Off-target inhibition of atrially expressed kinases with ibrutinib activates CaMKII and may lead to enhanced triggered activity and myocardial fibrosis that may predispose to scar-based reentrant circuits. Newer BTK inhibitors such as zanubrutinib are reported to have a lower incidence of atrial arrhythmias; however, longer follow-up studies are required. Cardiologists should be aware of the possibility that patients on chronic ibrutinib presenting with atrial flutter may have complex atrial substrates and the challenges associated with a rate control strategy.
Disclosures
None.
Acknowledgments
Funding Sources
None.
References
- 1.Estupiñán H.Y., Berglöf A., Zain R., Smith C.I.E. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.630942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molica S., Giannarelli D., Baumann T., Montserrat E. Ibrutinib as initial therapy in chronic lymphocytic leukemia: a systematic review and meta-analysis. Eur J Haematol. 2020;104:512–515. doi: 10.1111/ejh.13387. [DOI] [PubMed] [Google Scholar]
- 3.Ganatra S., Sharma A., Shah S., et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4:1491–1500. doi: 10.1016/j.jacep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Brown J.R., Moslehi J., O'Brien S., et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796–1805. doi: 10.3324/haematol.2017.171041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fradley M.G., Gliksman M., Emole J., et al. Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am J Cardiol. 2019;124:539–544. doi: 10.1016/j.amjcard.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Baptiste F., Cautela J., Ancedy Y., et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6 doi: 10.1136/openhrt-2019-001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson P.A., Lévy V., Tam C.S., et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175:462–466. doi: 10.1111/bjh.14324. [DOI] [PubMed] [Google Scholar]
- 8.Xiao L., Salem J.E., Clauss S., et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation. 2020;142:2443–2455. doi: 10.1161/CIRCULATIONAHA.120.049210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang L., Li L., Ruan Y., et al. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16:1374–1382. doi: 10.1016/j.hrthm.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Yang X., An N., Zhong C., et al. Enhanced cardiomyocyte reactive oxygen species signaling promotes ibrutinib-induced atrial fibrillation. Redox Biol. 2020;30 doi: 10.1016/j.redox.2020.101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMullen J.R., Boey E.J., Ooi J.Y., et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829–3830. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 12.Yang T., Moslehi J.J., Roden D.M. Abstract 14587: proarrhythmic effects of ibrutinib, a clinically approved inhibitor of Bruton’s tyrosine kinase (BTK) used in cancer therapy. Circulation. 2015;132 [Google Scholar]
- 13.Denham N.C., Pearman C.M., Caldwell J.L., et al. Calcium in the pathophysiology of atrial fibrillation and heart failure. Front Physiol. 2018;9:1380. doi: 10.3389/fphys.2018.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam C.S., Opat S., D’Sa S., et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136:2038–2050. doi: 10.1182/blood.2020006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor R., Fazal M., Cheng P., et al. Ibrutinib-associated atrial fibrillation treatment with catheter ablation. HeartRhythm Case Rep. 2021;7:713–716. doi: 10.1016/j.hrcr.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]