Abstract
In this case report, a 33-year-old male with a history of smoking presented with recurrent palpitations and chest discomfort. Holter monitoring revealed atrial flutter, and imaging showed a giant left atrial appendage aneurysm. Due to the risk of arrhythmias and thromboembolic events, surgical resection was performed successfully. This case underscores the importance of considering uncommon structural cardiac abnormalities in the evaluation of arrhythmia symptoms in young patients.
Keywords: Left atrium, Left atrial appendage aneurysm, Arrhythmia, Cardiac imaging, Left atrial appendage aneurysm resection
Introduction
Left atrial appendage aneurysms (LAAA) are rare entities characterized by abnormal enlargement of the left atrial appendage [1]. Such cases have been rarely reported in the literature. The first case report that documented LAAA was published in 1962 [2]. Since then, multiple articles have addressed the significance of the left atrial appendage (LAA) and the role it plays in the formation of blood clots under specific conditions, including atrial fibrillation and mitral valve disease [3]. The suggested higher risk of thromboembolism is thought to be associated with the hemodynamic alterations related to the enlarged orifice of the atrial appendage [4]. Here, we discuss the clinical diagnosis and therapeutic interventions employed in the management of a symptomatic patient with a giant aneurysm of the LAA.
Case presentation
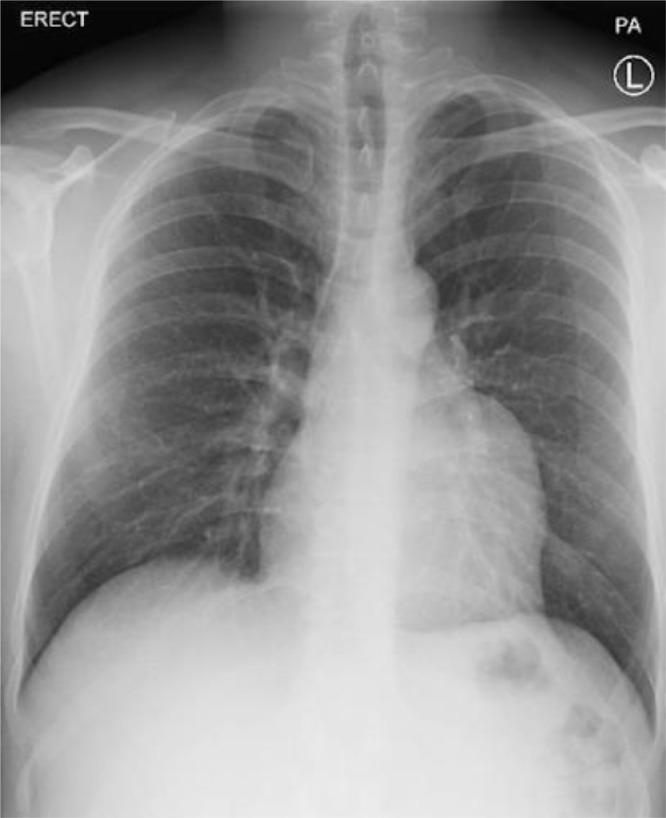
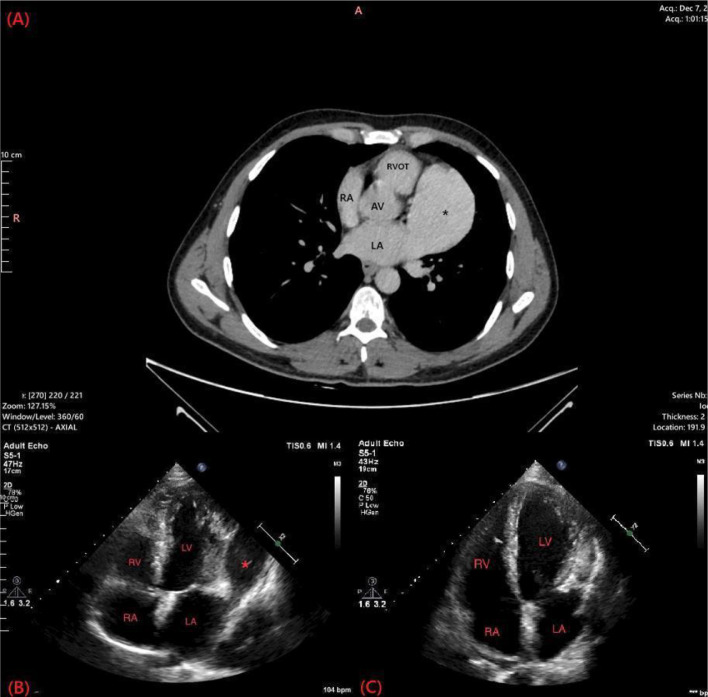
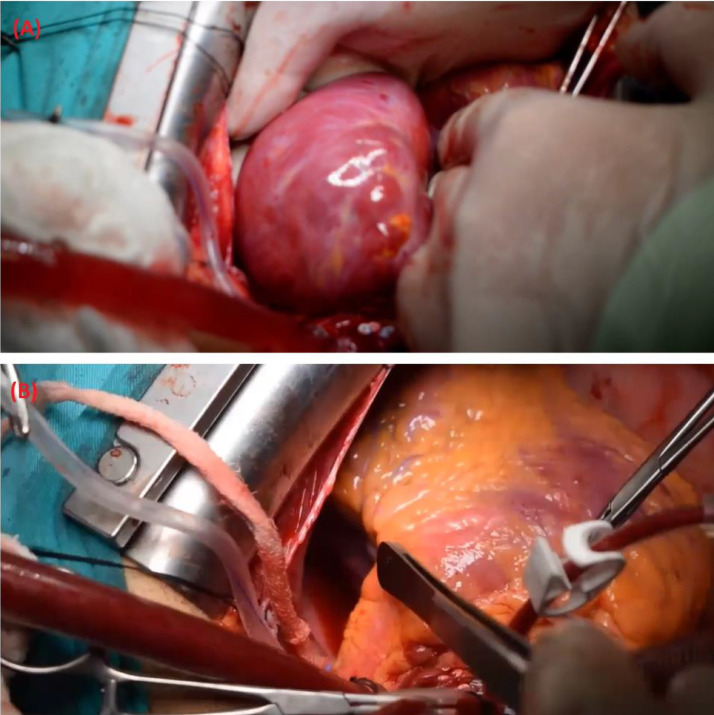
We present a 33-year-old male patient with a 20-pack-year smoking history complaining of recurrent attacks of palpitation and chest discomfort for the past 5 years. The patient symptoms were intermittent which led the patient to ignore these symptoms. Over a period of two months, the frequency of palpitations increased drastically with the most recent attack being treated with urgent cardioversion at the emergency department. Physical examination findings were unremarkable. The patient had a free past medical history and a surgical history of traumatic splenectomy 3 years ago. The patient sought our outpatient cardiology clinic for further evaluation. Electrocardiography (ECG) showed a normal sinus rhythm with no abnormal findings. A Holter monitor recorded multiple episodes of atrial flutter over a period of 7 days. Chest radiography showed a prominent left heart border (Fig. 1). Transthoracic echocardiogram demonstrated the presence of a large cyst-like structure adjacent to the left atrium. The cardiac valves demonstrated normal structure with minimal regurgitations observed in the mitral and tricuspid valves (Fig. 2B). Computed tomography showed the presence of a giant LAAA measuring 8.0 × 6.0 cm arising from the left atrial wall. No other focal cardiac or structural abnormalities were observed (Fig. 2A). Due to the significant risk of life-threatening arrhythmias and thromboembolic events associated with the patient's condition, a left atrial aneurysmectomy via median sternotomy was performed. The surgical procedure involved opening the chest by median sternotomy and instituting a total cardiopulmonary bypass. Perioperative findings confirmed the presence of a giant left atrial appendage aneurysm dilation as visualized in (Fig. 3A) (Video S1, Video S2). The aneurysmal dilatation of the left atrium was opened, and the blood was evacuated. No clots were found, and the aneurysmal wall was resected, leaving an adequate margin for closure. Following the resection of the aneurysms, a large indentation on the lateral myocardial wall was visualized indicating the chronic compression caused by the LAAA (Fig. 3B). The left atrium opening was closed using polypropylene suture 4/0 double sutures. The surgery went well with no postoperative arrhythmias or complications.
Fig. 1.
Posterior-anterior chest radiography showing a prominent left heart border.
Fig. 2.
Computed tomography and transthoracic echocardiography demonstrating the giant left atrial appendage aneurysm. The following section of the CT shows a significantly enlarged fifth heart chamber representing the giant left atrial appendage aneurysm (A). (B and Fig. 3B) represent the echocardiogram findings in these patients. (B) demonstrated the presence of the left atrial appendage aneurysm while (C) shows the resolution of the aneurysm following resection. LA; Left atrium, RA; Right atrium; RVOT; Right ventricular outflow tract, AV; Aortic valve, *; Left atrial appendage aneurysm, RV; Right ventricle, RA; Right atrium, LA; Left atrium, LV; Left ventricle, *; left atrial appendage aneurysm.
Fig. 3.
(A) the giant left atrial appendage aneurysm is visualized. Following the resection of the aneurysm, a large indentation was observed on the myocardial wall due to the chronic compression caused by the left atrial appendage aneurysm. This finding is illustrated in (B).
Discussion
The left wall of the major atrium, which develops during the fourth week of embryonic development, gives rise to the LAA. It differs from the left atrium itself in terms of development, ultrastructure, and physiological properties [5]. The LAAA is an extremely rare entity characterized by the expansion of the LAA either in a localized or diffuse manner [5].
LAAA can be congenital or acquired. While acquired LAAA results from left atrial enlargement brought on by mitral valve disease or myocardial pathologic conditions like mitral stenosis, mitral regurgitation, syphilitic myocarditis, and tuberculosis, congenital LAAA is caused by dysplasia of pectinate muscles and other related atrial muscle bands [6]. Distinguishing between the acquired and congenital forms is frequently challenging, as moderate alterations in the mitral valve function can be a cause or a result of LAAA. LAAA can also be divided into intrapericardial (with intact pericardium) and extraepicardial (with pericardium defect) [7].
While some patients are asymptomatic, others may have palpitations, chest discomfort, exertional dyspnea, and embolic impairments of cerebral circulation brought on by the production of thrombi, arrhythmias, or compression of coronary arteries [8]. Occasionally, it can irritate the left phrenic nerve, leading to hiccups, and exerting pressure on the left recurrent laryngeal nerve, creating a persistent cough [9,10].
Because of the ambiguous clinical picture, the illness is either discovered by coincidence or during a patient examination when complications emerge. TTE is frequently used since it is noninvasive, simple to administer, and helps to detect LAAA, but because of its low sensitivity [11], further tests like CT scans or transesophageal echocardiography are required when TTE reveals evidence that LAAA may be present [12]. When necessary, MRI can give the best detailed assessment of the heart. Nevertheless, MRI does possess certain limitations, including the need for a consistent cardiac rhythm and the introduction of nephrotoxic contrast agents to the patient's body.
Foale et al. [13] outlined the following diagnostic criteria for the congenital forms of LAAA: (1) originating from a normally functioning atrial chamber; (2) displaying a clearly defined connection with the atrial cavity; (3) being situated within the pericardium; and (4) causing distortion of the left ventricle due to an aneurysm.
The majority of patients should have a surgical intervention for the treatment of LAAA [11,12]. However, anticoagulant medication under strict observation should be taken into consideration if the patient is asymptomatic and does not have a thrombus. There have been several effective techniques for performing aneurysmectomy, including median sternotomy, left thoracotomy, mini-thoracotomy, and endoscopy. A unique noninvasive method of treating LAAA was published, resulting in the catheter closure of a massive LAAA [14]. Depending on the surgical technique, different postoperative imaging evaluation points must be made. Most importantly, it is vital to evaluate whether there is a left atrial deformity or persistent aneurysm. With a thin-walled aneurysm, fibrosis of the endocardium or myocardium is a frequent histological finding. In rare circumstances, myocardial hypertrophy and fatty infiltration of myocardial fibers may be seen. After LAAA resection, no medical interventions were given. The patient was followed up at 2 weeks, 1 month, and 6 months after surgery and reported complete resolution of symptoms.
Conclusion(s)
LAAA is a rare condition with serious complications that may be misdiagnosed due to the vague nature of its presentation. Getting familiar with the presentation and imaging findings associated with LAAA is needed to promptly diagnose, treat, and prevent the life-threatening complications that may arise.
Patient consent
In accordance with ethical and legal requirements, written informed consent for the publication of this case has been obtained from the patient.
Footnotes
Acknowledgments: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2023.11.049.
Appendix. Supplementary materials
References
- 1.Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The Left atrial appendage: anatomy, function, and noninvasive evaluation. JACC: Cardiovasc Imaging. 2014;7(12):1251–1265. doi: 10.1016/j.jcmg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Parmley LF. Congenital atriomegaly. Circulation. 1962;25(3):553–558. doi: 10.1161/01.cir.25.3.553. [DOI] [PubMed] [Google Scholar]
- 3.Odell JA, Blackshear JL, Davies E, Byrne WJ, Kollmorgen CF, Edwards WD, et al. Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann Thorac Surg. 1996;61(2):565–569. doi: 10.1016/0003-4975(95)00885-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee JM, Shim J, Uhm JS, Kim YJ, Lee HJ, Pak HN, et al. Impact of increased orifice size and decreased flow velocity of left atrial appendage on stroke in nonvalvular atrial fibrillation. Am J Cardiol. 2014;113(6):963–969. doi: 10.1016/j.amjcard.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 5.Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82(5):547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann U, Hamed N, Herold C, Globits S. Radiological signs of a left atrial aneurysm. Eur Radiol. 2000;10(8):1332–1334. doi: 10.1007/s003309900307. [DOI] [PubMed] [Google Scholar]
- 7.Williams WG. Dilatation of the left atrial appendage. Heart. 1963;25(5):637–643. doi: 10.1136/hrt.25.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseini S, Hashemi A, Saedi S, Jalili F, Maleki M, Jalalian R, et al. Left atrial appendage aneurysm. Ann Thorac Surg. 2016;102(3):e207–e209. doi: 10.1016/j.athoracsur.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Toufan M, Pourafkari L, Afrasiabi A, Sohrabi M, Nader ND. Left atrial appendage aneurysm presenting with chronic cough. Neth Heart J. 2017;25(9):526–527. doi: 10.1007/s12471-017-1021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asfalou I, Boumaaz M, Raissouni M, Sabry M, Benyass A, Zbir EM. Huge left atrial appendage aneurysm revealed by chronic hiccups. J Saudi Heart Assoc. 2017;29(4):293–296. doi: 10.1016/j.jsha.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Li H, Zhang L, He L, Zhang J, Liu C, et al. Congenital left atrial appendage aneurysm: a rare case report and literature review. Medicine (Baltimore) 2018;97(2):e9344. doi: 10.1097/MD.0000000000009344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aryal MR, Hakim FA, Ghimire S, Ghimire S, Giri S, Pandit A, et al. Left atrial appendage aneurysm: a systematic review of 82 cases. Echocardiography. 2014;31(10):1312–1318. doi: 10.1111/echo.12667. [DOI] [PubMed] [Google Scholar]
- 13.Foale RA, Gibson TC, Guyer DE, Gillam L, King M, Weyman A. Congenital aneurysms of the left atrium: recognition by cross-sectional echocardiography. Circulation. 1982;66(5):1065–1069. doi: 10.1161/01.cir.66.5.1065. [DOI] [PubMed] [Google Scholar]
- 14.Kothandam S, Ramasamy R. Planning and execution of catheter closure of a giant left atrial appendage aneurysm causing recurrent cardioembolism. Ann Pediatr Cardiol. 2020;13(4):353. doi: 10.4103/apc.APC_76_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.