To the Editor:
Inborn errors of metabolism, prominently urea cycle defects and organic acidemias, are common etiologic bases for hyperammonemic disorders. Delays in the clearance of ammonia in young children and newborns increase the risk of adverse outcomes, such as cell swelling, metabolic decompensation, coma, and mortality.1 Initial medical management of hyperammonemia relies on restricting protein intake, maximizing nonprotein caloric intake from intravenous glucose and/or intralipid infusions, and sequestering ammonia through nitrogen scavengers (such as glycerol phenylbutyrate, sodium phenylbutyrate, sodium phenylacetate, and sodium benzoate).2,3 In cases of severe hyperammonemia, kidney replacement therapy (KRT) accelerates the clearance of excess ammonium to alleviate its toxic effect, preserve neurocognitive functions, and improve survival rates.2,4
Before the 1990s, peritoneal dialysis (PD) was primarily used for ammonia clearance due to the technical challenges and risks associated with extracorporeal techniques.3 Currently, PD largely remains an alternative option in the absence of other extracorporeal detoxifications or in newborns requiring timely vascular access.2,4,5 Over the past few decades, hemodialysis (HD) and continuous kidney replacement therapies (CKRTs) have become preferred modalities because of higher flow rates for optimal rapid ammonium clearance. After discontinuation, patients treated by HD often experience complications, including hypotension and transiently increased ammonia levels.2,3 Comparatively, CKRT decreases serum ammonia levels with a reduced risk of hemodynamic instability or ammonia rebound.6 Despite advancements in CKRT technology, overall KRT-associated mortality rates remain high in children with inborn errors of metabolism and even greater in newborns.5, 6, 7 This meta-analysis aimed to identify KRT-related mortality across a global cohort of children with inborn errors of metabolism.
This retrospective study considered eligible children diagnosed with an inborn error of metabolism and receiving any KRT treatment modality. All children aged <18 years were included in this review. Results were reported on the efficacy of KRT measured via survival rate and sensitivity analysis on source heterogeneity. Publication bias for the survival analysis was measured with Egger’s test (P < 0.05). A complete methodology is provided in Item S1.
Our integrative review of KRT-associated mortality in children with inborn errors of metabolism identified 249 articles, of which 14 studies published after 2000 met the inclusion criteria for survival analysis (Table S1). We identified a total of 231 patients with inborn errors of metabolism, and the mean age of this population was 2.3 years (ranging from 3 days to 12.5 years), with the proportion of various inborn errors of metabolism disorders shown in Table S2.
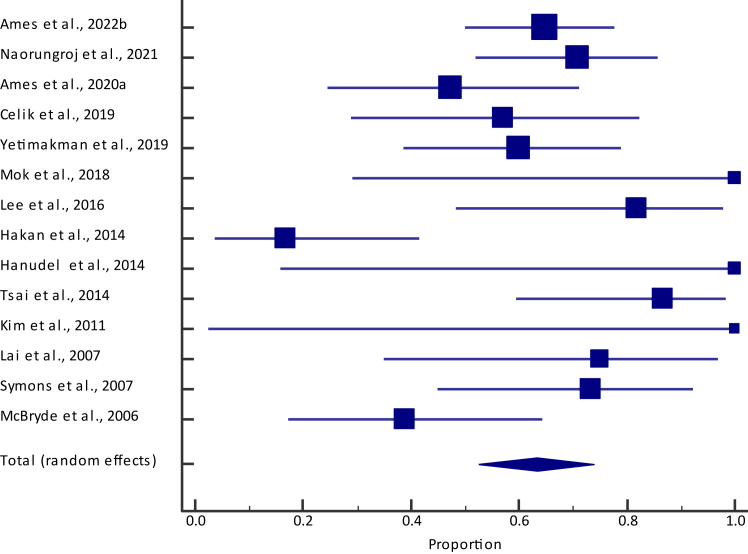
Pooled survival among children who received KRT was 63.4% (95% confidence interval [CI], 52.4%-73.7%) (Fig 1 and Table S3), with no visual indication of publication bias (Fig S1). In 7 studies with a mean/median age >0.7 years (Table 1), our sensitivity analysis revealed a slightly lower survival rate of 59.8% (95% CI, 38.5%-79.3%) than the overall survival rate of 63.4% (95% CI, 52.4%-73.7%). This was also the case in 5 studies limited to only the United States, where the pooled proportion for survival was 59.2% (95% CI, 49.4%-68.5%).
Figure 1.
Forest plot of survival among children with an inborn error of metabolism across collected studies. Total (lower diamond) represents pooled estimate.
Table 1.
Sensitivity Analyses for Survival Among Children With Inborn Errors of Metabolism Treated With Kidney Replacement Therapy
| Criteria | Number of Studies (n) | Number of Children (n) | % Proportion (95% CI) | I2 (95% CI); P value | Egger’s Test P Value |
|---|---|---|---|---|---|
| Overall | 14 | 231 | 63.4 (52.4-73.7) | 63.2% (34.5%-79.3%); P < 0.001 | 0.31 |
| Age (>0.7 ya) | 7 | 96 | 59.8 (38.5-79.3) | 78.4% (55.4%-89.6%); P < 0.001 | 0.19 |
| Sample size (>15a) | 6 | 162 | 50.9 (35.8-65.9) | 74.3% (41.4%-88.7%); P = 0.002 | 0.08 |
| Geography (United States) | 5 | 105 | 59.2 (49.4-68.5) | 57.7% (20.0%-80.8%); P = 0.01 | 0.77 |
| Study Quality (Good) | 12 | 192 | 62.3 (49.4-74.4) | 67.1% (39.7%-82.1%); P < 0.001 | 0.30 |
Note: Values were reported either as mean, median, or range; range was converted into mean by averaging min and max values.
Abbreviations: CI, confidence interval.
Median across the studies.
We present a retrospective meta-analysis of KRT-associated survival in a large cohort of children with inborn errors of metabolism. Our meta-analysis revealed a mortality rate between 40% and 50%, consistent with reports in the current literature (20%-50%).5, 6, 7 Greater peak ammonia levels and longer coma durations are associated with poorer outcomes and survival.8 A multisite study from 2000-2015 also identifies several other risk factors for mortality, including HD, number of repeat KRTs, and KRT complications, thus highlighting the importance of considering the patient's complete clinical status before determining KRT initiation and modality. CKRT is often better tolerated than intermittent HD and PD, resulting in improved long-term outcomes following prompt initiation in children.7 A retrospective trial by Arbeiter et al5 (2010) evaluated the effectiveness of continuous venovenous hemodialysis and continuous PD in 21 children with inborn errors of metabolism and showed a greater rate of survival in patients (82%) with continuous venovenous hemodialysis than in the continuous PD cohort (50%) in the first 6 months after treatment, and long-term survival was associated with improved neurologic outcomes. Newer generations of CKRT machines, with ultrafiltration and high-dose capabilities, have improved CKRT clearance rates to levels comparable with intermittent hemodialysis. Presently, high-dose CKRT or biphasic therapies involving CKRT following intermittent hemodialysis are best suited for initial clearance of blood ammonia levels while maintaining hemodynamics in children diagnosed with inborn errors of metabolism, ultimately reducing mortality risk.9,10
Inborn errors of metabolism represent a heterogeneous group of disorders, and the cause of mortality in these children is often infrequently reported across studies. There is a noticeable scarcity of clinical trials and cross-sectional studies that investigate and validate the effectiveness of newer CKRT modalities in comparison with existing treatments among patients with inborn errors of metabolism. Future developments should consider focusing on models to assess prognostic indicators for nonsurvival risk in children with inborn errors of metabolism and optimize their intervention course for long-term positive outcomes.
Article Information
Authors’ Contributions
Research idea, study design, data acquisition, data analysis/interpretation, statistical analysis: all authors. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received June 25, 2023. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form September 4, 2023.
Footnotes
Figure S1: Funnel plot evaluating publication bias in studies reporting survival.
Item S1: Detailed methods.
Table S1: General characteristics of included studies.
Table S2: Inborn errors of metabolism disorders among 231 children reported across gathered studies.
Table S3: Survival among children with an inborn error of metabolism.
Supplementary Material
Figure S1, Item S1, Tables S1-S3.
References
- 1.Ames E.G., Luckritz K.E., Ahmad A. A retrospective review of outcomes in the treatment of hyperammonemia with renal replacement therapy due to inborn errors of metabolism. Pediatr Nephrol. 2020;35(9):1761–1769. doi: 10.1007/s00467-020-04533-3. [DOI] [PubMed] [Google Scholar]
- 2.Raina R., Bedoyan J.K., Lichter-Konecki U., et al. Consensus guidelines for management of hyperammonaemia in paediatric patients receiving continuous kidney replacement therapy. Nat Rev Nephrol. 2020;16(8):471–482. doi: 10.1038/s41581-020-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gortner L., Leupold D., Pohlandt F., et al. Peritoneal dialysis in the treatment of metabolic crises caused by inherited disorders of organic and amino acid metabolism. Acta Paediatr Scand. 1989;78(5):706–711. doi: 10.1111/j.1651-2227.1989.tb11130.x. [DOI] [PubMed] [Google Scholar]
- 4.Picca S., Dionisi-Vici C., Bartuli A., et al. Short-term survival of hyperammonemic neonates treated with dialysis. Pediatr Nephrol. 2015;30(5):839–847. doi: 10.1007/s00467-014-2945-x. [DOI] [PubMed] [Google Scholar]
- 5.Arbeiter A.K., Kranz B., Wingen A.M., et al. Continuous venovenous haemodialysis (CVVHD) and continuous peritoneal dialysis (CPD) in the acute management of 21 children with inborn errors of metabolism. Nephrol Dial Transplant. 2010;25(4):1257–1265. doi: 10.1093/ndt/gfp595. [DOI] [PubMed] [Google Scholar]
- 6.Lai Y.C., Huang H.P., Tsai I.J., et al. High-volume continuous venovenous hemofiltration as an effective therapy for acute management of inborn errors of metabolism in young children. Blood Purif. 2007;25(4):303–308. doi: 10.1159/000106102. [DOI] [PubMed] [Google Scholar]
- 7.Ames E.G., Powell C., Engen R.M., et al. Multisite retrospective review of outcomes in renal replacement therapy for neonates with inborn errors of metabolism. J Pediatr. 2022;246:116–122.e1. doi: 10.1016/j.jpeds.2022.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S., Fenves A.Z., Hootkins R. The role of RRT in hyperammonemic patients. Clin J Am Soc Nephrol. 2016;11(10):1872–1878. doi: 10.2215/CJN.01320216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinale J.M., Laskin B.L., Sondheimer N., et al. High-dose continuous renal replacement therapy for neonatal hyperammonemia. Pediatr Nephrol. 2013;28(6):983–986. doi: 10.1007/s00467-013-2441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanudel M., Avasare S., Tsai E., Yadin O., Zaritsky J. A biphasic dialytic strategy for the treatment of neonatal hyperammonemia. Pediatr Nephrol. 2014;29(2):315–320. doi: 10.1007/s00467-013-2638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Item S1, Tables S1-S3.