Abstract
Polycystic ovarian syndrome (PCOS), frequently associated to obesity, is the main reproductive disorder in women in age to procreate. Some evidence suggests that pesticides can result in alterations of the female reproductive system, including polycystic ovary syndrome (PCOS). Here, we detected two fungicides, Tebuconazole (Tb) and Epoxiconazole (Epox) in the soils and waters of French area. Our hypothesis is that these two triazoles could be associated to the etiology of PCOS. We used the human KGN cell line and primary human granulosa cells (hGCs) from different group of patients: normal weight non PCOS (NW), normal weight PCOS (PCOS NW), obese (obese) and obese PCOS (PCOS obese). We exposed in vitro these cells to Tb and Epox from 0 up to 10 mM for 24 and 48 h and analysed cell viability and steroidogenesis. In hGCs NW, cell viability was reduced from 12.5 µM for Tb and 75 µM for Epox. In hGCs NW, Epox decreased progesterone (Pg) and estradiol (E2) secretions and inhibited STAR, HSD3B and CYP19A1 mRNA expressions from 25 µM and increased AHR mRNA expression from 75 µM. Tb exposure also reduced steroid secretion and STAR and CYP19A1 mRNA expressions and increased AHR mRNA expression but at cytotoxic concentrations. Silencing of AHR in KGN cells reduced inhibitory effects of Tb and Epox on steroid secretion. Tb and Epox exposure decreased more steroid secretion in hGCs from obese, PCOS NW and PCOS obese groups than in NW group. Moreover, we found a higher gene expression of AHR within these three groups. Taken together, both Epox and Tb reduced steroidogenesis in hGCs through partly AHR and Tb was more cytotoxic than Epox. These triazoles alter more strongly PCOS and/or obese hGCs suggesting that human with reproductive disorders are more sensitive to triazoles exposure.
Keywords: PCOS, Tebuconazole, Epoxiconazole, Viability, Proliferation, Steroidogenesis, Obesity, AHR
Graphical Abstract
Highlights
-
•
Tebu-(Tb) and Epoxi-conazole (E) reduce steroid secretion by human granulosa cells.
-
•
Tb and E increase Aryl Hydrocarbon Receptor mRNA level in human granulosa cells.
-
•
Tb was more cytotoxic than Epox in human granulosa cells (hGCs).
-
•
The metabolic status modulates Tb and E effects on hGCs viability and steroidogenesis.
1. Introduction
Epoxiconazole (Epox) and tebuconazole (Tb) are azole molecules used as fungicides in the conventional agriculture [70]. They effectively combat fungal diseases by inhibiting sterol demethylation [70]. Around 76 Epox-base fungicides representing about 200 tonnes per year were used in France last decades (French National Agency for Food, Environmental and Occupational Health and Safety, 2019). Furthermore, Tb, applied on more 3 million of hectares, is the one of the most used pesticide in France [56]. In some countries and regions, they have been found in soils (from 0.16 to 0.31 mg/kg for both pesticides) [53], wastewater (from 1 to 30 ng/L for Tb) [32] and surface waters (maximum value of 17 mg/L for Epox) [58] but also in vegetables [16]. With half-life above 198 days or 68 days in water and 91 days or 59 days in soils for Tb and Epox, respectively [22], [24], these triazoles are considered as persistent pollutants [25]. Epoxiconazole and Tb have a similar structure consisting of a five-membered conjugated ring with three nitrogens [13]. Their main activity is to inhibit sterol 14-demethylase (CYP51), a member of the P450 enzymes family important for the metabolism of endogenous and xenobiotics substances [23]. CYP51 leads to the conversion of lanosterol into ergosterol, an essential component of the cell membrane of fungi and yeast and the inhibition of CYP51 by triazoles leads to membrane permeability and cell death [70]. In mammals, CYP51 converts lanosterol into follicular fluid meiosis-activating-sterols (FF-MAS), a precursor of cholesterol, essential for the biosynthesis of sexual steroid like estradiol (E2), testosterone (T) and progesterone (Pg) by the ovarian granulosa cells in females ( [70]). Residues of Epox (minimum value of 10.38 µg/L of urine) [39] and Tb (2.22 µg/kg of hair’s workers in agriculture field) [51] were found in Human. It has been shown that 98% of Tb are absorbed within 48 h in mammals and are distributed into liver and kidneys tissues without potential of accumulation [24], [27]. Regarding Epox, 50% are absorbed within 2 h and they are distributed into blood, liver, kidneys, spleen, lungs and adrenal glands without accumulation [22]. Moreover, Tb and Epox can act on other enzymes from the P450 enzyme family such as cytochrome P450 family 19 subfamily A member 1 (CYP19A1), enabling the conversion of T into E2 [13], [36]. It has been already shown that an exposure to Epox and Tb increased the risk of infertility. Indeed, prenatal exposure to Epox or Tb (50 mg/kg body weight/day (mg/kg bw/d)) in rats resulted in a higher rate of post implantation loss [55], [60] and a decrease in maternal serum cholesterol [55]. Epox, also, decreased E2 secretions and increased Pg and T secretions which can led to precocious puberty onset in rodent [20], [60], [59]. This was also observed after Tb exposure in rats [15], [38]. In previous study of our lab, we have demonstrated that, other triazoles, metconazole (from 100 µM), difenoconazole (from 1 µM), tetraconazole (from 10 µM) and cyproconazole (from 10 µM) reduced Pg and E2 secretions in human granulosa cells after 48 h (Serra, 2023). Thus, Epox and Tb could also contribute to some ovarian disorders. However, the effects of these conazoles on the human ovarian cells and their mechanisms used to alter fertility are still unknown. One potential candidate to explain triazoles effect could be the involvement of the aryl hydrocarbon receptor (AHR). It is originally a sensor of xenobiotic chemicals and it is constitutively expressed in granulosa cells [4]. It is activated by binding to triazoles such as Tb in human [35] or Epox in rat [28] hepatocytes. Furthermore, in a previous study conducted in our lab on triazoles exposure for 48 h, tetraconazole (10 µM), metconazole (100 µM) and prothioconazole (1000 µM) increased AHR mRNA expression in primary human granulosa cells [52]. AHR also regulates the production of E2 in mouse ovarian cells as well as the estrus cycle through the inhibition of CYP19A1 expression [7]. Indeed, AHR has a xenobiotic region element in the promoter of CYP19A1 where it binds and modulates its expression [4] Moreover AHR regulates the formation of primordial and antral follicles in mice [7] and it can be activated by the follicle-stimulating hormone (FSH) and the luteinizing hormone (LH) and be inhibited by phosphokinase A [4], [40]. We hypothesize that Epox and Tb use AHR signalling to impair granulosa cells steroidogenesis.
The polycystic ovarian syndrome (PCOS) and obesity are among the main women sub or infertility causes in worldwide [44], [61], [71]. PCOS affects approximately one in ten women in age to procreate in France [61]. Three criteria have been retained and two of these are sufficient to diagnose PCOS: i) hyperandrogenism, ii) oligo or anovulation, iii) and polycystic ovaries [61]. Dysregulations of hormones from the reproductive axis are observed with an increase of luteinizing hormone (LH) and T secretions, an abnormal secretion of E2 and a low or normal secretion of follicular stimulating hormone (FSH) [21], [62], [71], [76]. Although the knowledge about this syndrome is increasing, the origins remain unknown and can be multiple. In recent years, endocrine disruptors (EDCs) disturbing the reproductive axis have been shown as a potential cause of PCOS [18], [47]. For example, neonatal exposure of female rats to bisphenol A (BPA) led to a PCOS-like phenotype in adults associated with increased T and E2 serum levels and an increase number of ovarian cysts [26]. Obesity is another factor of subfertility [44], around 15% of women in childbearing age had a body mass index (BMI) above 30 in the world in 2016 [63]. Often associated with PCOS with 50% of obese women presenting a PCOS phenotype [45] this physiological status leads to low-grade chronic inflammation [54]. Therefore, it reduces the chances to get a correct number of retrieved oocytes and, at least, decreases the quality of embryos obtained during an in vitro fertilization (IVF) program [66], [76]. In addition, obesity influences androgen levels [21], [54], [76]. Pollutants such as phthalates have been already shown to be positively correlated with BMI, leptin concentration in blood, lipids concentration and visceral adipose index in women [41] Our hypothesis is that ovarian cells from PCOS women with or without obesity could be more sensitive to Epox and Tb than those from normal-weight women and could contribute to explain some ovarian disorders.
In the present study, we evaluated the exposure levels of Epox and Tb in the soil, surface water and groundwater in the French Centre-Val de Loire area, which were found at maximal values of 50 µg/kg and 30 µg/kg; 0.3 and 9 µg/L; 0.45 and 6 µg/L respectively. We then investigated the Epox and Tb effects on cell viability and steroidogenesis (Pg and E2 release) in the human tumor granulosa cell line, KGN and in primary granulosa cells (hGCs) from four different groups of patients: normo-weight and non PCOS (NW), normo-weight and PCOS (PNW), obese non PCOS (O) and obese with PCOS (PO). Finally, we analysed the molecular mechanism of the triazoles effects by developing a CRISP-R/Cas9 mediated deletion of Aryl hydrocarbon Receptor (AHR) in KGN cells.
2. Materials and Methods
2.1. Epoxiconazole and Tebuconazole analyses in French soils and waters
The characterization of agricultural soils close to the patients’residence in French “Région Centre Val de Loire” area was carried out at the INRAE soil analysis laboratory in Arras (France), accredited by the French committee of accreditation (COFRAC). The analysis of soils sieved at 2 mm was performed by LC QTOF MS (Liquid Chromatography Quadrupole Time-Of-Flight Mass Spectrometry). The quantification limit (LOQ) determined for each triazole in soil were 10 µg/kg. A total of 76 sites was analysed in duplicates. The results were expressed in µg/kg of soil dried at 105 °C. A precise description of the triazole analyses in French soils is explained below:
2.1.1. Chemicals and standards
Organic solvents, acids, bases and salts used were methanol, isopropanol, ammonium formate (Biosolve Chimie, France), acetone, formic acid, acetic acid, sodium hydroxide (Carlo Erba, France) and ultrapure water. Acetone and methanol were used respectively to rinse glass flasks and to prepare standard solutions and for PLE (pressurized liquid extraction or accelerated solvent extraction) extraction. Celite 545 (diatomea earth) and pure Fontainebleau sand (VWR Chemicals, France) were added at each soil in the metallic extraction cell. These reagents were of analytical quality for mass spectrometry analysis. The extractor was a BÜCHI Speedextractor E-916 with 6 ports of ectraction. Evaporators were rotoevaporators BÜCHI R-134and Zymark Turbovap II. Soil extracts were filtered using PTFE filters at 0.20 µm (Pall corporation)and stored in 2 ml amber glass vial (Agilent technonologies). The individual triazoles standard solutions at 10 mg/L in methanol were purchased from Cluzeau Info Labo company (France). The targeted compounds were epoxiconazole, and tebuconazole.
2.1.2. Preparation of solutions
The calibrant solution, injected at the beginning of each analysis for calibration of mass spectrometrer, was prepared with 25 ml of ultrapure water (50%), 25 ml of isopropanol (50%), 0.5 ml of NaOH 1 M (1%), 75 µL of acetic acid (0.15%) and 25 µL of formic acid (0.05%) in mixture. Two mobile phases were used for liquid chromatography in concentration gradient mode. The mobile phase A was prepared with 900 ml of ultrapure water (90%), 100 ml of methanol (10%), 100 µL of formic acid (0.01%) and 315 mg of ammonium formate (5 mM) in mixture. The mobile phase B was prepared with 500 ml of methanol (100%), 50 µL of formic acid (0.01%) and 157.5 mg of ammonium formate (5 mM) in mixture.
Triazoles individual solutions and an internal standard solution of difenoconazole-d6 were used at 10 mg/L in methanol. Triazoles solutions in mixture and individual internal standard solution were prepared at 2 mg/L in methanol in order to prepare standard solutions for internal calibration and spiked soils for the recovery study.
2.1.3. LC QTOF MS analysis
2.1.3.1. Chromatographic and spectrometric conditions
The LC QTOF MS was a HPLC Thermofisher Ultimate 3000 coupled with a high resolution and time-of-flight mass spectrometer BRUKER Impact II with an electrospray ionization source. The QTOF-MS was programmed to work with a mass range from 20 to 40,000 m/z in MS mode and a selection of parent ions with mass less than 3000 m/z in MS/MS mode. A resolution was around 50,000 FWHM (full width at half maximum) for ions of mass greater than 800 m/z obtained in V mode, single return trip in the reflectron, electrospray ionization in positive or negative mode, bbCID and MS fragmentation and optimal primary and secondary vacuum conditions. The chromatographic column was an Acclaim C18 column of dimension 100 mm length, 2.1 mm internal diameter and 2.2 µm of particle size. The HPLC system was equipped with an automatic sampler thermostated at 4 °C for extracts storage and stability. The column furnace was at 30 °C for the repeatability and reproducibility of the retention time of each triazoles. The injection volume of standard solutions and soil extracts was 5 µL. The calibrant solution was delivered at the beginning of each analysis at 0.005 µL/min flow during 2 min for the calibration of mass spectrometer. Triazoles chromatographic separation was achieved in 20 min at 250 µL/min flow in concentration gradient mode of the mobile phase A from 90% at the beginning of analysis to 0% at the end of the run. The softwares were Hystar and Otof for the management of analytical system and Tasq for the treatment of data. -Internal calibration.
Calibration solutions from 0.010 µg/L to 500 µg/L were prepared with an internal standard, difenoconazole-d6, at the concentration of 40 µg/L. The internal calibration curves of triazoles were built from 0.025 µg/L to 500 µg/L with determination coefficient up to 0.99.
2.1.4. Soils analysis
2.1.4.1. preparation
After site sampling, the fresh agricultural soils were freezed at − 20 °C. Before analysis, the soils were stored at ambient temperature. The soils were sieved at 5 mm mesh for homogeneous samples.-Humidity at 105 °C: An aliquot of 30 g of each fresh soil was put in aluminium cup for drying at 105 °C in oven during a night. - Soil characterization: Fresh soils were put in a shelf in a room at 35 °C during 5 days for drying. Dried soils were sieved at 2 mm mesh with titanium sieve. Each dried and sieved soils were stored at 2 mm mesh in glass flasks for the characterization of soils. A sub-sample of 50 g of dried soil sieved at 2 mm mesh was sampled and grinded at 250 µm of particle size using grinder with zirconium bowl and balls. Grinded soils were stored in glass flasks before certain soil characterization analysis. The agronomic characterization of agricultural soils was carried out at the INRAE soil analysis laboratory in Arras (France), accredited by the French committee of accreditation (COFRAC). The analysis of soils sieved at 2 mm was performed according to standard NF ISO 11464 method. The water pH and particle size were determined according to standard NF ISO 10390 and NF X31–107 methods respectively. The total nitrogen, total organic carbon (TOC) and the carbonate content in grinded soils at 250 µm were determined according to standard NF ISO 13878, ISO 10694 and NF EN ISO 10693 methods respectively. The results are presented below:
Characteristics of the 5 agricultural soils for the recovery study:
| Agricultural soils from the North of France | Clay | Fine silt | Coarse silt | Fine sand | Coarse sand | Organic Carbon | Total Nitrogen | CaCO3 | pH water | |
|---|---|---|---|---|---|---|---|---|---|---|
| Origin site | Nature | g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | g/kg | |
| Liévin | Loess silt | 205 | 255 | 457 | 74 | 9 | 22.31 | 1.53 | 1 | 6.6 |
| Airon-Saint-Vaast | Reconstituted sands on glaze |
111 | 109 | 200 | 341 | 239 | 11.71 | 1.17 | 16 | 8 |
| Dompierre-sur-Helpe | Silty alluvium | 380 | 321 | 268 | 22 | 9 | 37.9 | 4.03 | < 1 | 5.5 |
| Steenwerck | Alluvions from Lys plain | 312 | 260 | 293 | 102 | 33 | 12.49 | 1.36 | < 1 | 7.5 |
| Marcq-en-Ostrevent | Loess | 194 | 236 | 446 | 112 | 12 | 12.4 | 1.22 | 3 | 7.9 |
2.1.5. PLE extraction
Fresh soils sieved at 5 mm mesh, an aliquot of 10 g of soil was mixed at 5 g of celite in the bowl. The mixture of solid matrix was put in a 40 ml extraction metallic cell and spiked with 20 µL of internal standard solution of difenoconazole-d6 at 40 µg/L. The cell was completed with pure Fontainebleau sand and overcome with a glass fiber filter. The extraction metallic cell was put in the extractor furnace and extracted by PLE mode using pure methanol, a temperature of 80 °C, a pressure of 150 Bars and 2 static extraction cycles of 5 min. The methanolic extracts received 20 µL of a keeper, n-docécane, before a partial rotative evaporation at 60 °C and 300 mBars of pressure. The residual liquid extract was evaporated to dryness under a gentle nitrogen flow. The dried residual extract was dissolved in 2 ml of pure methanol. After a contact time of 45 min for dissolution, the extract was filtered by a 0.20 µm PTFE filter and stored in a 2 ml amber glass vial.
2.1.6. LC QTOF MS analysis
The LC ESI+ QTOF MS analysis sequence was made of the internal calibration solutions followed by soil extracts with quality control standard solutions each ten samples. Triazoles were analyzed by positive ionization and the data were treated Tasq soft. The identification of triazoles was obtained by the retention time and the exact mass.
2.1.7. Recovery study and method validation
Five agricultural soils were used for recovery study and method validation. The 5 soils were of different sites from the North of France and had different characteristics (see § 2.3). Each soil was analyzed before without spiking in order to measure the eventual residual concentration of triazoles in the 5 agricultural soils. Each soil was spiked at two different levels, 20% and 80% of the application domain, and analyzed two times at 6 different dates. Mean recoveries calculated for each soil were expressed for the two different levels. Mean recoveries were obtained after substraction of triazoles residual concentration in the soils without spiking. Triazoles mean recoveries were from 74.3% to 94.4% at the 80% level of application domain with determination coefficient from 4.4% to 8.8%. Triazoles mean recoveries were from 91.5% to 119.3% at the 20% level of application domain with determination coefficient from 2.5% to 6.5%. The quantification limits determined for each triazole in soil were 10 µg/kg for epoxiconazole and tebuconazole.
Concerning surface and underground waters, triazole concentrations were obtained for the different cities in the Centre Val de Loire region near the patients' residence from the ADES (Accès aux Données sur les Eaux Souterraines, www.ades.eaufrance.fr; [64]) and NAIADES (https://www.brgm.fr/en/website/naiades-data-surface-water-quality) databases. For analysis of surface waters, 721 stations were used related to 448 cities and 26 measurements or each triazole were assessed per cities. For underground waters, 18,006 records for 641 cities for each triazole were made. The results were expressed in µg/L of water.
2.2. Ethical issues
The study was carried out in accordance with the Declaration of Helsinki principles and free informed consent was obtained from all participants. The biological samples and identity of patients remained anonymous. Study protocol was previously approved by the Ethics Committee of the University Hospital of Tours as part of the “HAPOFERTI” and PESTIFERTI projects (authorisation number 2016_075). No conflict of interest is declared and researchers bought fungicides independently of user’s companies.
2.3. Study population
The study was carried out in accordance with the Declaration of Helsinki [65]. One hundred and sixty women undergoing an IVF (In vitro Fertilization) procedure between 2019 and 2022 at the reproductive medicine center of Tours Hospital were included in this study. For each triazole studied, four groups of patients were constituted as follow. Forty women normal weight (BMI 18–25 kg/m2) without PCOS consulting for men infertility and considered as a control group (NW). Forty women suffering from PCOS with normal weight (BMI 18–25 kg/m2; PNW group). Forty obese women ((BMI > 30 kg/m2; O group) and 40 obese women suffering from PCOS (PO group). Diagnosis of PCOS followed 2003 Rotterdam Consensus Conference Criteria [1]. All women with PCOS presented an oligo/anovulation and the number of follicles per ovary ≥ 12.
2.4. Collection and isolation of human granulosa cells and culture of KGN cells
Follicular fluid was recovered after isolation of luteinized cumulus–oocyte complexes, and centrifuged at 1800 rpm for 15 min to pellet cell remnants. Human primary granulosa cells (hGCs) from this pellet were then isolated, percoll purified, and cultured as previously described [17] for 24 h before in vitro treatments. For each biological replicates, a pool of cells from about 3 patients was made. Granulosa cells present FSH and LH receptors and secrete mediators such as growth factors and steroids to exchange with oocyte for maturation [10]. KGN derived from luteinized granulosa tumoral cells collected from a 63 years-old woman in 1994 was provided by Nishi and collaborators [67]. They express FSH and E2 receptors and have steroidogenic capacity [67]. Cells were cultivated with Dulbecco’s Modified Eagle’s Medium (DMEM; PAA, Thermo Fisher, Waltham, USA) with 10% fetal bovine serum (FBS) (Sigma-Aldrich; Saint-Louis, USA) and 1% of penicillin 10.000 UI/ml / streptomycin 10.000 µg/ml (Eurobio, Les Ulis, France).
2.5. CRISPR/Cas9 methodology
KGN AHR-/- were obtained by developing a CRISP-R/Cas9 mediated deletion of Aryl hydrocarbon Receptor gene. A KGN-Cas9 line was established after infection with Cas9-RFP lentiviral particles (Sigma Aldrich) at a multiplicity of infection of 2. Then, single-cell (RFP positive cell) cloned this cell line by cytometry (MoFlo AstriosEQ, Beckman Coulter, USA). After, KGN-Cas9 RFP cells were transfected with a small guide RNA targeting human AHR gene (NM_001621) (AhR sqRNA Crisp-GFP vector, target 1, ABM, Euromedex, France) or with a non –targeted guide (GTAGGCGCGCCGCTCTCTAC) creating the control cells, called Scr-KGN cells. After 3 days, GFP cells were sorted by Fluorescent Activated Cell Sorter and cell line cloned from a single-cell was performed. After two weeks, clones (Scr KGN and KGN AHR-/-) were tested by qPCR and western-blotting for AHR expression.
2.6. Cells treatments
Tebuconazole and Epoxiconazole were purchased from Sigma-Aldrich and diluted with methanol 100%. Different concentrations of Tb and Epox were prepared from 0 µM to 10 mM, so 1400 times higher than the reproductive and offspring non-observable adverse effect level (NOAEL) in rats at 2.3 mg/kg body weight/day (mg/kg bw/d; around 7 µMa) for Epox [22] and 143 times higher than the parental and offspring NOAEL for Tb at 21.6 mg/kg bw/d (around 70 µM [24]). First, we used a large range of triazoles concentrations on KGN cells (until 10 mM) and according to results, we focused on concentrations below 100 µM for hGCs. This last concentration corresponding to 3333 times the Epox urinary concentration [39] and 14 285 times the Tb hair concentration [51] found in farm workers using these fungicides. The dilutions were prepared with Mc Coy medium containing FBS (10%) for hGCs or DMEM containing FBS (10%) for KGN.
2.7. Cell viability
Cells were treated in 96 wells plate with Tb or Epox from 0 to 10 mM (KGN) or from 0 to 100 µM (hGCs) for 24 h and at 100 µM for 48 h. Cell counting-kit 8 (CCK-8; Sigma-Aldrich, Saint-Louis, USA) was used for the cell viability assay.
2.8. RNA isolation and real-time quantitative PCR (qPCR)
Cells were treated for 48 h with Tb or Epox (from 0 to 100 µM) and then stored at − 20 °C until RNA isolation. Total RNA from granulosa cells was extracted with QIAzol® Lysis Reagent (Qiagen Sciences, Maryland, USA) following the manufacturing recommendations. The cDNA was obtained by reverse transcription (RT) from total RNA (2 µg). Briefly, a mix of RT buffer (2 M), deoxyribonucleotide triphosphate (dATP, dCTP, dGTP and dTTP; 0.5 mM each), 15 µg/µL of oligodT, 0.125 U of ribonuclease inhibitor and 0.05 U of Moloney murine leukemia virus reverse transcriptase (MMLV) was prepared and mixed with samples before an incubation for 1 h at 37 °C. The efficiency of primers was tested before the qPCR as explained in Bongrani et al. (Bongrani et al., 2019). The couple primers used are for reference genes: human β-actin (F: 5’-AAGAGAGGCATCCTCACCCT-3’; R: 5’-TACATGGCTGGGGTCTTGAA-3’) which encodes actin, an highly conserved protein playing a role in networks in the cytoplasm of cells (https://www.proteinatlas.org/ENSG00000075624-ACTB/tissue#rna_expression), human Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (F: 5’-ATGGAAATCCCATCACCATCTT-3’; R: 5’-CGCCCCACTTGATTTTGG-3’) which encodes a protein playing a role in glycolysis (https://www.proteinatlas.org/ENSG00000111640-GAPDH/tissue#rna_expression), human peptidylprolyl isomerase A (PPIA) (F: 5’-CGCGTCTCCTTTGAGCTGTT-3’; R: 5’-ATTTTCTGCTGTCTTTGGGACC-3’) which encodes protein catalyzing the cis-trans isomerization of proline imidic peptide bonds in oligopeptides and improving the folding of proteins (https://www.proteinatlas.org/ENSG00000196262-PPIA/tissue#rna_expression). These three reference genes are constantly expressed in human ovarian cells. For tested genes we used: human Steroidogenic acute regulatory (STAR) (F: 5’-AAACTTACGTGGCTACTCAGCATC-3’; R: 5’-GACCTGGTTGATGCTCTTG-3’), CYP11A1 (F: 5’-CAGGAGGGGTGGACACGAC-3’; R: 5’-AGGTTGCGTGCCATCTCATAC-3’), human 3 beta-hydroxysteroid dehydrogenase (HSD3B) (F: 5’-GCCTTCCAGACCAGAATTGAGAGA-3’; R: 5’-TCCTTCAAGTACAGTCAGCTTGGT-3’), human Cytochrome P450 Family 19 subfamily A member 1 (CYP19A1) (F: 5’-CCATAAAGACCCGATTCCACCA-3’; R: 5’-GCTGAGGCATAAATCGACAGAC-3’), human aryl hydrocarbon receptor (AHR) (F: 5’AGAGTTGGACCGTTTGGCTA-3’; R: 5’-AGTTATCCTGGCCTCCGTT-3’) and human Anti-Müllerian hormone (AMH) (F: 5’-GCATGTTGACACATCAGGC-3’; R: 5’-GAGTGGCCTTCTCAAAGAGC −3’). A new mixture of the cDNA of samples (3 µL) diluted 10 times with SYBR Green Supermix 1X reagent (Bio-rad, Marnes la Coquette, France), 250 nM of specific human primers and distilled water was prepared before performed the PCR procedure. After incubation for 2 min at 50 °C and a denaturation step of 10 min at 95 °C, the samples were subjected to 40 cycles (30 s at 95 °C, 30 s at 60 °C, 30 s at 72 °C) followed by the acquisition of the melting curve. The efficiency (E) of the primers was determined from a serial dilution of cDNA and was calculated as E = 10 − 1/slope value, and was between 1.9 and 2. For each gene, relative abundance was calculated according to primer efficiency (E) and quantification cycle (Cq), where expression = E − Cq. Melting curve analysis were performed to verify the presence of a single amplicon. The expression of the target gene was expressed relative to the geometric mean of three specific reference genes (Beta Actin, PPIA and GAPDH). The geometric mean of housekeeping genes has been reported as an accurate normalization factor [31].
2.9. Progesterone, estradiol and testosterone assays
Progesterone and estradiol concentrations in GC culture medium were measured employing a previously described ELISA [9] and ELISA kits provided by Cayman Chemicals, respectively, and normalized to the protein concentration of each well. For all tests, intra- and inter-assay coefficients of variation averaged < 10%.
2.10. Statistical analysis
All statistical analyses were performed using GraphPad Prism 6® (San Diego, California USA). Data are presented as mean ± standard error of the mean (SEM). The means of independent and random replicates were used. Bartlett’s test was run to test the homogeneity of variance, and distribution was verified by the Shapiro-Wilk test. For parametric values, test of Student (comparison between Scr-KGN and KGN AHR -/-), one-way ANOVA test followed by a Tukey multiple comparison test (comparison between all concentrations for cell viability, Pg and E2 secretions, genes expressions in primary or KGN cells without O, PO and PN), or a two-way ANOVA test followed by a Tukey multiple comparison test (comparison between all concentrations and all status (PCOS, obese…) for cell viability, Pg and E2 secretions, genes expressions in primary), were applied as appropriate. Means were considered different at p < 0.05, 95% confidence interval. For each analysis, N means the number of experimental replicates and n means the number of samples for each condition used for each experiment.
3. Results
3.1. Detected fungicides substances in soils and surface and underground water in the French Centre Val de Loire area
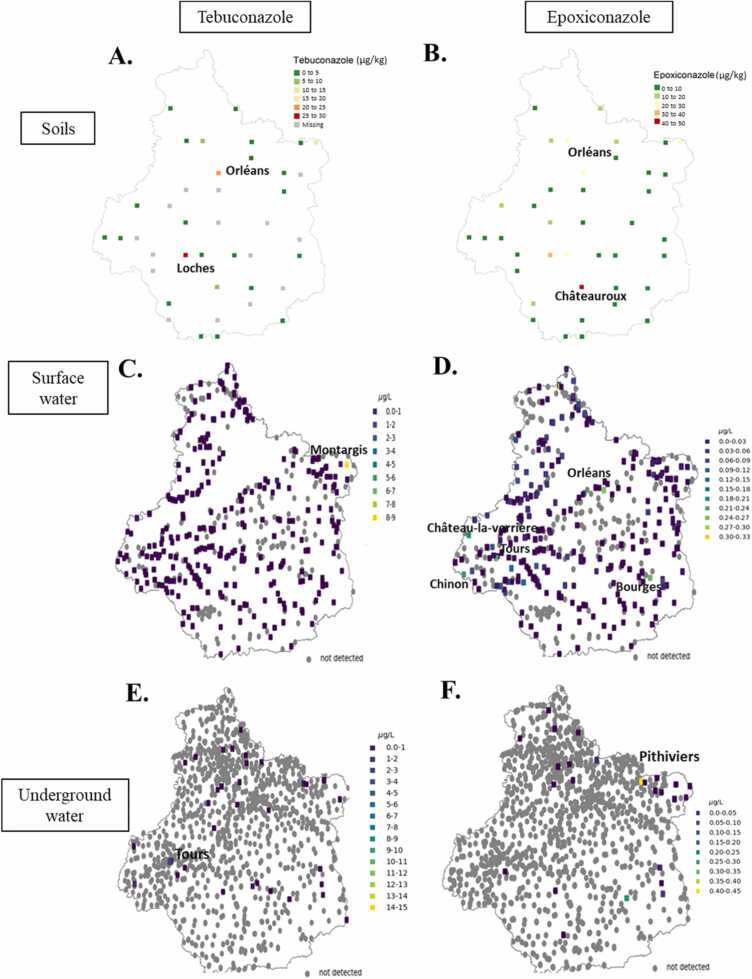
The analysis performed on the samples from the soil of the Centre Val de Loire area showed that Tb residues were accumulated in the soils of two areas: Orléans (from 20 to 25 µg/kg, around 65 nMb) and Loches area (from 25 to 30 µg/kg, around 81 nMc)(Fig. 1A). Epoxiconazole residues were also found in Orléans area (from 20 to 30 µg/kg, around 61 nMd) and at higher level in Châteauroux area (from 40 to 50 µg/kg, around 121 nMe) (Fig. 1B). For the surface and the underground water, the highest concentrations of Tb residues were found in Montargis area between 8 and 9 µg/L (around 30 nMf) and in Tours area (4–5 µg/L, around 16 nMg), respectively (Figs. 1C and 1E). Epoxiconazole residues were found at many places (Orléans, Bourges, Tours, Chinon and Château-La-Verrière) at concentrations between 0.18 and 0.27 µg/L (around 0.8 nMh) in the surface water and in Pithiviers area (0.4–0.45 µg/L, around 1.4 nMi) in the underground water (Fig. 1D and F).
Fig. 1.
Map of the French Region Centre-Val de Loire and location of exposed sites to Tb and Epox in soils and waters. A-C-E) Exposed sites to Tb in soils, surface water and underground water. The concentrations were in range of µg/kg and µg/L respectively. B-D-F) Exposed sites to Epox in soils, surface water and underground water. Each point represents the mean value of the triazoles concentration measured on 26 samples for surface waters, 28 samples for underground waters and 2 samples for soils.
3.2. Effect of tebuconazole (Tb) and epoxiconazole (Epox) exposure on cell viability, and steroidogenesis in KGN cells
Tebuconazole, after 24 h or 48 h of exposure, decreased the viability of KGN cells from 500 µM (p < 0.001, 99.9% confidence interval) (Supp 1A-B). A tendency for decrease and a significative decrease in cell viability were observed after 10 mM of Epox exposure for 24 h and 48 h, respectively (p = 0.0645; p < 0.05, 95% confidence interval) (Supp 1 C-D). The secretions of Pg and E2 in the culture medium of KGN were significantly reduced from 75 µM of Tb (p < 0.0001, 99.99% confidence interval; Supp 1E and 1 F) and 25 µM of Epox (p < 0.0001, 99.99% confidence interval) (Supp 1 G and 1 H) after 48 h. Secretion of Pg is possible thanks to the transport of cholesterol from the outer to the inner mitochondrial membrane by STAR, then CYP11A1 converts cholesterol into pregnenolone. After passing into the smooth endoplasmic reticulum, pregnenolone is converted by HSD3B to Pg [68]. Moreover, the binding of FSH to its receptor, FSHR activates FSHR/cAMP signaling which is necessary for increased expression of gene encoding steroidogenic enzymes (STAR, CYP11A1, HSD3B, CYP19A1) [69]. FSH signaling also enables aromatase activity (CYP19A1) converting testosterone into estradiol [69].
As shown in Supplemental data 2A to D, Tb decreased the mRNA expression of STAR, HSD3B, CYP19A1 of KGN from 75 µM (p < 0.0001, 99.99% confidence interval) whereas CYP11A1 mRNA expression remained unchanged. Epoxiconazole inhibited the mRNA expression of STAR, HSD3B and CYP19A1 from 75 µM (p < 0.01, 99% confidence interval) and the expression of CYP11A1 from 100 µM (p < 0.05, 95% confidence interval) (Supp 2E to H).
3.3. Clinical and hormonal parameters of different group of patients recruited in the study
For each cohort of patients (Tb and Epox), women included into NW groups consulted for male infertility, had normal BMI (18–25 kg/m2) and no sign of PCOS (number of follicles per ovary retrieved ≤12, no oligo or anovulation and normal concentration of testosterone ≤ 0.5 ng/ml). The PNW women had two of three criteria of Rotterdam [1]: number of follicles per ovary retrieved ≥ 12, no oligo or anovulation and concentration of testosterone ≥ 0.5 ng/ml, but presented BMI between 18 and 25 kg/m2. Obese women included in O group presented BMI above 30 but no PCOS phenotype. Finally, PO women presented both PCOS phenotype and BMI above 30.
Granulosa cells from eighty patients were selected for the study of each triazole (Tb and E, Table 1). In the cohort for Tb, women from PNW group were younger than women from O group (p = 0.0188, Table 1). As expected, the BMI of O and PO women were significantly higher than the other groups (p < 0.0001, 99.99% confidence interval). The number of follicles was similar in NW and O group but it was increased in PO group and higher in PNW group (p < 0.0001, 99.99% confidence interval). Additionally, the number of days per cycle were higher in PCOS groups (p < 0.0001, 99.99% confidence interval). The plasma T was higher for both PCOS groups when compared to the other groups (p < 0.0001, 99.99% confidence interval). The highest plasma concentration of AMH within the blood was found in both PCOS groups (p = 0.0007) as well as the highest secretion of LH (p = 0.0011) and ratio LH/FSH (p < 0.0001, 99.99% confidence interval). No difference was found for the plasma E2 secretion and the number of mature oocytes between the four groups. However higher number of embryos was found in PNW group compared to the obese groups (p = 0.0165). The results are reported in the Table 1.A.
Table 1.
Clinical and hormonal parameters of different groups of patients.
| Tebuconazole | NW (n = 10) | Obese (n = 10) | PCOS NW (n = 10) | PCOS obese (n = 10) | P-value |
|---|---|---|---|---|---|
| Age | 31.1 ± 1.1ab | 34 ± 1.1a | 29.5 ± 0.9b | 30.4 ± 0.9ab | 0.0188 * |
| BMI (kg/m²) | 21.8 ± 0.6a | 33 ± 0.4b | 20.7 ± 0.5a | 33.3 ± 0.5b | < 0.0001 * ** * |
| Number of follicles | 17.4 ± 1.4a | 9.7 ± 1.2a | 44 ± 4.1b | 31.2 ± 1.3c | < 0.0001 * ** * |
| Cycles | 28.5 ± 0.6a | 29.2 ± 0.7a | 98.5 ± 19.5b | 93.9 ± 17.8b | 0.0001 * ** |
| Testosterone (µg/l) | 0.2 ± 0.1a | 0.2 ± 0.1a | 0.8 ± 0.1b | 0.8 ± 0.1b | < 0.0001 * ** * |
| AMH (ng/ml) | 3 ± 0.5a | 4.6 ± 0.8ac | 17.1 ± 4.9bc | 10.8 ± 1.6c | 0.0007 * ** |
| FSH (UI/L) | 6.8 ± 0.9 | 7 ± 1.1 | 5.3 ± 0.3 | 4.6 ± 0.4 | 0.0333 * |
| LH (UI/L) | 4.1 ± 0.3a | 3.7 ± 0.4a | 8.1 ± 1.2b | 6.1 ± 0.9ab | 0.0011 * * |
| Ratio LH/FSH | 0.7 ± 0.1a | 0.6 ± 0.1a | 1.6 ± 0.2b | 1.4 ± 0.2b | < 0.0001 * ** * |
| Estradiol (ng/l) | 43.3 ± 4.3 | 41.5 ± 5.3 | 41.5 ± 4.2 | 36.8 ± 2.2 | 0.7028 |
| Number of mature ovocytes | 5.8 ± 0.7 | 3.9 ± 0.8 | 7.6 ± 1.2 | 7.3 ± 1.1 | 0.0467 * |
| Number of embryos | 3.4 ± 0.6ab | 2 ± 0.6a | 5.3 ± 0.8b | 4.2 ± 0.7ab | 0.0165 * |
| A. Epoxiconazole | |||||
| NW (n = 10) | Obese (n = 10) |
PCOS NW (n = 10) |
PCOS obese (n = 10) | P-value | |
| Age | 30.92 ± 0.9ab | 33.67 ± 0.6b | 29.62 ± 0.8a | 30.42 ± 0.9a | 0.0054 * * |
| BMI (kg/m²) | 21.58 ± 0.4a | 32.08 ± 0.4b | 20.85 ± 0.3a | 33.08 ± 0.4b | < 0.0001 * ** * |
| Number of follicles | 17.42 ± 1.2a | 9.5 ± 0.9a | 43.23 ± 3.8b | 30.92 ± 1.0c | < 0.0001 * ** * |
| Cycles | 27.17 ± 1.0a | 29.21 ± 0.7a | 103.08 ± 18.5b | 94.92 ± 16.8b | < 0.0001 * ** * |
| Testosterone (µg/l) | 0.13 ± 0.02a | 0.19 ± 0.02a | 0.8 ± 0.1b | 0.77 ± 0.1b | < 0.0001 * ** * |
| AMH (ng/ml) | 3.03 ± 0.4a | 4.59 ± 0.6a | 17.53 ± 4.3b | 10.06 ± 1.4b | < 0.0001 * ** * |
| FSH (UI/L) | 6.63 ± 0.8 | 6.96 ± 0.7 | 5.71 ± 0.3 | 4.81 ± 0.4 | 0.0636 |
| LH (UI/L) | 3.96 ± 0.2a | 3.63 ± 0.3a | 8.7 ± 1.1b | 6.78 ± 0.5b | < 0.0001 * ** * |
| Ratio LH/FSH | 0.66 ± 0.1a | 0.57 ± 0.1a | 1.57 ± 0.1b | 1.48 ± 0.1b | < 0.0001 * ** * |
| Estradiol (ng/l) | 44.75 ± 4.3 | 41.18 ± 4.0 | 42.58 ± 4.3 | 40.24 ± 1.7 | 0.8364 |
| Number of mature ovocytes | 5.83 ± 0.6ab | 3.92 ± 0.5a | 7.62 ± 1.0b | 7.5 ± 0.8b | 0.0034 * * |
| Number of embryos | 3.67 ± 0.4ab | 2.08 ± 0.6a | 4.54 ± 0.6b | 4.17 ± 0.6ab | 0.0232 * |
A) Characteristics of patients for the tebuconazole exposure. B) Characteristics of patients for the epoxiconazole exposure. BMI: body mass index; AMH: anti-Mullerian hormone; FSH: follicular stimulating hormone; LH: luteinizing hormone. Data are mean ± SEM.* p < 0.05, 95% confidence interval; * * p < 0.01, 99% confidence interval; * ** p < 0.001, 99.9% confidence interval; * ** *p < 0.0001, 99.99% confidence interval
Focusing on the cohort for E, the O group was significantly older than the other groups (p < 0.05, 95% confidence interval). BMI was, as expected, higher in O group and PO group when compared to the other groups (p < 0.0001, 99.99% confidence interval). The number of follicles was higher in PNW group compared to the three other groups (p < 0.01, 99% confidence interval), and higher in PO group compared to the NW and the O group (p < 0.01, 99% confidence interval). Higher number of days per cycle was found in both PCOS groups (p < 0.0001, 99.99% confidence interval). The plasma concentrations for T (p < 0.0001, 99.99% confidence interval), AMH (p < 0.05, 95% confidence interval), LH (p < 0.05, 95% confidence interval) and the ratio of LH/FSH (p < 0.001, 99.9% confidence interval) were increased by obesity and PCOS statues. No variation of the concentration of FSH and E2 were noticed. The number of mature oocytes were higher in the PNW and PO group compared to the O group (p < 0.01, 99% confidence interval) and the number of embryos conceived by IVF was higher in PNW compared to PO (p < 0.05, 95% confidence interval). The results are reported in the Table 1.B.
3.4. Effect of Tb and Epox exposure on the viability of hGCs from Normal weight or Obese women with or without PCOS
The viability of hGCs from each group of patients (NW, O, PNW and PO) was firstly measured without comparison between groups (Supp 3). The cell viability began to decrease from 12.5 of Tb for NW group (Supp 3 A, p < 0.0001, 99.99% confidence interval) whereas, this decrease was observed from 75 µM of Tb for O, PNW and PO groups (Supp 3 C, E and G, p < 0.0001, 99.99% confidence interval). At 75 µM, Epox began to decrease cell viability of hGCs for NW group (Supp 3B, p < 0.0001, 99.99% confidence interval). However, 25 µM of Epox reduced the cell viability of hGCs from O and PNW groups (Supp 3D and F, p < 0.0001, 99.99% confidence interval) whereas the cells from PO group showed the same pattern as NW group (Supp 3 H, p < 0.0001, 99.99% confidence interval).
Then, the viability of hGCs from each group of patients (NW, O, PNW and PO) was measured without exposure to any triazole for comparison between groups (Supp 4 A). At the basal condition, the viability of the hGCs from Obese, PNW and PO groups was lower than those from the NW group (p < 0.0001, 99.99% confidence interval), with a decrease of 19 ± 1.58%; 22.25 ± 1.89% and 22.5 ± 2.63% respectively when compared to the NW group (p < 0.01, 99% confidence interval) (Supp 4 A). Then, hGCs from each group were exposed to Tb and Epox and cell viability compared between groups. For Tb, we found a significant interaction between the patient status and the effect of Tb (p < 0.0001, 99.99% confidence interval) (Supp 4B). The lowest number of alive hGCs was found at 100 µM of Tb and Epox with a decrease of 49.25 ± 1.11% and 47 ± 3.63% for NW, 53 ± 1.29% and 66 ± 2.92% for O, 52.25 ± 1.25% and 71.25 ± 0.48% for PNW and 57.25 ± 1.11% and 74 ± 2.35% for PO, respectively (Supp 4B and C). At 25 µM of Tb, the percentage of alive hGCs was significantly higher in O, PNW and PO group as compared to the NW group (p < 0.0001, 99.99% confidence interval) (Supp 4B). p < 0.0001, 99.99% confidence intervalp< 0.0001, 99.99% confidence intervalIn addition, at 100 µM of Tb, PNW group had higher viability than the control NW group (p < 0.0001, 99.99% confidence interval) (Supp 4B). No significant difference between patient groups was observed concerning hGCs viability after Epox exposure whatever the concentration used (Supp 4 C). p < 0.0001, 99.99% confidence intervalp< 0.0001, 99.99% confidence interval.
3.5. Effect of Tb and Epox exposure on steroid production in human primary granulosa cells (hGCs) from Normal weight or Obese women with or without PCOS
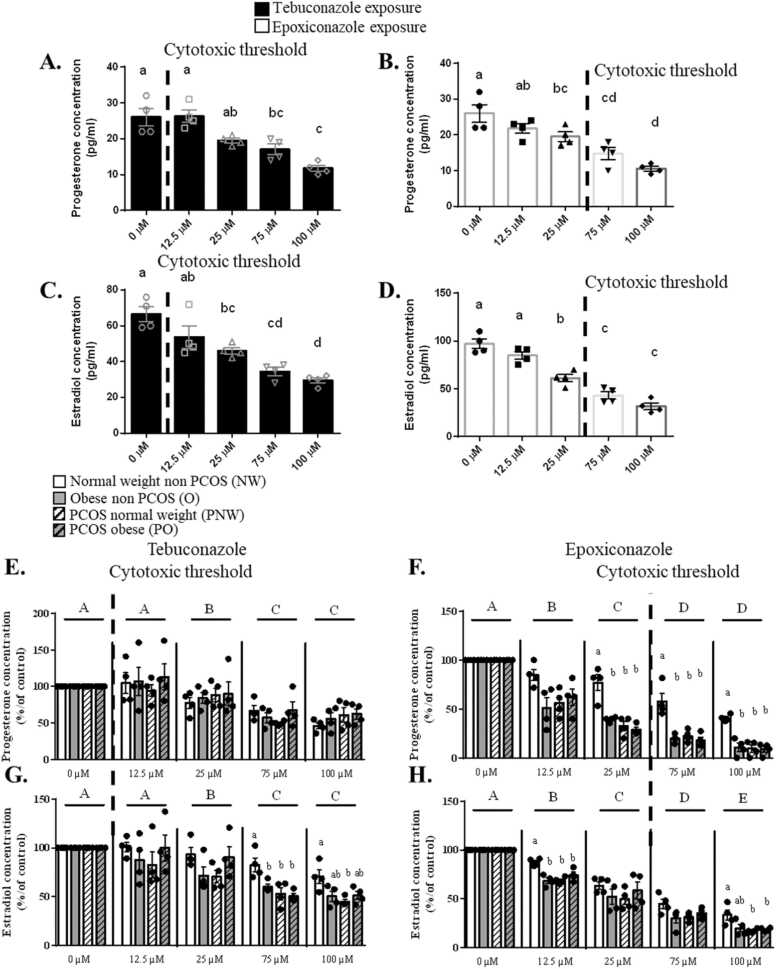
Then, the impact of Tb and Epox on steroidogenesis was evaluated on hGCs after 48 h of exposition. As shown in Fig. 2A and B, we observed a reduced level of Pg secretion in conditioned media from 75 µM of Tb (cytotoxic dose) and 25 µM of Epox (p < 0.0001, 99.99% confidence interval). In addition, E2 levels were decreased from 25 µM of Tb (cytotoxic dose) and Epox (p < 0.0001, 99.99% confidence interval) (Fig. 2C and D). These decreases of Pg and E2 secretions can affect ovarian cycle, oocyte maturation, ovulation and the retro-control on hypothalamo-pituitary axis leading to the alteration of fertility [12], [33], [73]. Moreover, Pg concentration is also correlated with top quality embryos during in vitro fertilization [30].
Fig. 2.
Effects of Tb and Epox on steroids production of human primary granulosa cells (hGCs) from normal weight (NW), obese (O), PCOS normal weight (PNW) and PCOS obese (PO) groups. A-D. Progesterone (A-B) and Estradiol (C-D) secreted in the culture medium by hGCs (pg/ml) after the exposure to Tb (A-C) or Epox (B-D) for 48 h. Lowercase letters (i.e a, b) correspond to the ordinary one-way ANOVA significance followed by the multiple comparison test of Tukey between all concentrations (p < 0.05, 95% confidence interval). Data are presented as mean ± SEM of N = 4 and n = 3. E-F. Percentage of Pg (E-F) and E2 (G-H) secreted in the culture medium by hGCs compared to the basal condition in NW, O, PNW and PO groups after the exposure to Tb (E-G) or Epox (F-H) for 48 h. For each group and concentration, the values were normalized with the control condition* 100. Lowercase letters (i.e a, b) correspond to the 2-way ANOVA significance followed by the multiple comparison test of Tukey between all groups under one concentration (p < 0.05, 95% confidence interval). Uppercase letters (i.e A, B) correspond to the 2-way ANOVA significance followed by the multiple comparison test of Tukey (p < 0.05, 95% confidence interval) between all concentrations regardless of the group. Data are presented as mean ± SEM of N = 4 and n = 3. For each analysis, N means the number of experimental replicates and n means the number of samples used for each experiment.
The supplemental data 5A and B illustrates the secretion of testosterone in conditioned media after Tb or Epox exposure, respectively, on hGCs from NW group. The secretion of testosterone was decreased by 25 µM of Tb (cytotoxic dose) (p < 0.0001, 99.99% confidence interval) and 12.5 µM of Epox (p < 0.0001, 99.99% confidence interval). The reduction of testosterone concentration could lead to the decrease of E2 secretion observed earlier.
In the absence of Tb and Epox exposure, hGCs from O, PNW and PO groups had a reduced secretion of Pg, E2 and testosteronewhen compared to the NW group (p < 0.0001, 99.99% confidence interval) (Supp 5 C, D and E). As shown in Fig. 2E and F, the Pg secretion was significantly decreased in a dose dependent from 25 µM of Tb (cytotoxic dose) and 12.5 µM of Epox (p < 0.0001, 99.99% confidence interval). Similar effect was found for E2 secretion for both triazoles (p < 0.0001, 99.99% confidence interval, Fig. 2G and H). We observed a significant interaction between the patient status and the effect of Tb for E2 secretion (p = 0.0019, Fig. 2G). We showed that at 25 µM of Epox, a non-cytotoxic dose for PO cells, the secretion of Pg was decreased in this group as compared to NW patients (p < 0.0001, 99.99% confidence interval) (Fig. 2F). Estradiol secretion was reduced in O, PNW and PO groups at 12.5 µM (non-cytotoxic dose) of Epox as compared to NW group (p < 0.0001, 99.99% confidence interval) (Fig. 2H). A fall of 50% of Pg and E2 secretion was observed at 100 µM of Epox whereas for Tb, the decrease was less important. The secretion of testosterone was decreased in O, PNW and PO from the cytotoxic dose 75 µM of Tb compared to NW group (p < 0.0001, 99.99% confidence interval) (Supplemental data 5F). Regarding Epox exposure, the testosterone level was reduced in O group compared to NW and PO at the non-cytotoxic dose 12.5 µM (p < 0.0001, 99.99% confidence interval) (Supplemental data 5E).
3.6. Effect of Tb or epox exposure on STAR, CYP11A1, HSD3B, CYP19A1, AMH and AHR mRNA expression in hGCs from obese or normal weight women with or without PCOS
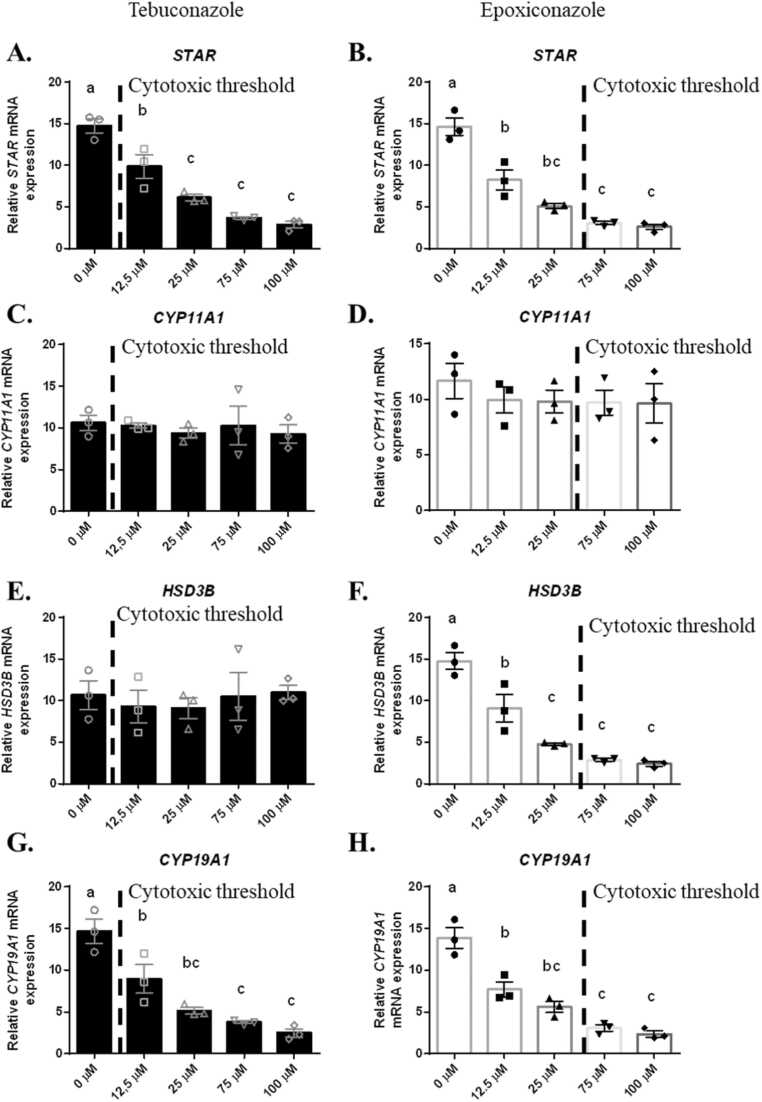
At the molecular level, we have also shown an inhibition of some components involved in the steroidogenesis process: STAR and CYP19A1 mRNA expression from 12.5 µM of Tb (cytotoxic dose) (p < 0.001, 99.9% confidence interval) (Fig. 3A, C, E and G) and an inhibition of STAR, HSD3B and CYP19A1 from 12.5 µM of Epox (p < 0.001, 99.9% confidence interval) (Fig. 3B, D, F and H) whereas no effect of Tb and Epox was observed on the CYP11A1 mRNA expression in hGCs (Figs. 3C and 3D). The expression of STAR and HSD3B mRNA expression could lead to the reduction of proteins involved at the beginning of the steroidogenesis process and explain the reduced level of Pg content. Aromatase protein is coded by CYP19A1, and its inhibition after triazole exposure could explain the decreased level of E2 secretion observed. However, these observations need to be confirmed with protein concentration analysis (STAR, 3 bHSD, Aromatase).
Fig. 3.
Expression levels of STAR, CYP11A1, HSD3B, CYP19A1 in human primary granulosa cells (hGCs) after Tb and Epox exposure. A-B) Expression of the gene STAR in hGCs after exposure to Tb (A) or Epox (B) for 48 h. C-D) Expression of the gene CYP11A1 in hGCs after exposure to Tb (C) or Epox (D) for 48 h. E-F) Expression of the gene HSD3B in hGCs after exposure to Tb (E) or Epox (F) for 48 h. G-H) Expression of the gene CYP19A1 in hGCs after exposure to Tb (G) or Epox (H) for 48 h. Data are presented as mean ± SEM of N = 3 and n = 3. For each analysis, N means the number of experimental replicates and n means the number of samples used for each experiment. Lowercase letters (i.e a, b) correspond to the ordinary one-way ANOVA significance followed by the multiple comparison test of Tukey between all concentrations (p < 0.05, 95% confidence interval).
We next determined the effect of the triazoles exposure and the metabolic and PCOS status of the patients on the mRNA expression of STAR, CYP11A1, HSD3B, CYP19A1, a diagnostic marker for PCOS (AMH) and AHR. As shown in Supplemental data 6, without any triazole exposure, STAR (Supp 6 A), HSD3B (Supp 6 C) and CYP19A1 (Supp 6D) mRNA expressions were lower in hGCs from O, PNW and PO groups as compared to those from NW patients (p < 0.0001, 99.99% confidence interval). No variation of CYP11A1 mRNA expression was observed between the four groups (Supp 6B). Concerning AMH mRNA, as expected, higher mRNA expressions were found in hGCs from PCOS groups as compared to the other groups in the absence of Tb or Epox exposure (Supp 6E). AHR mRNA expression was similar in hGCs from all studied groups in absence of Tb or Epox exposure (Supp 6 F).
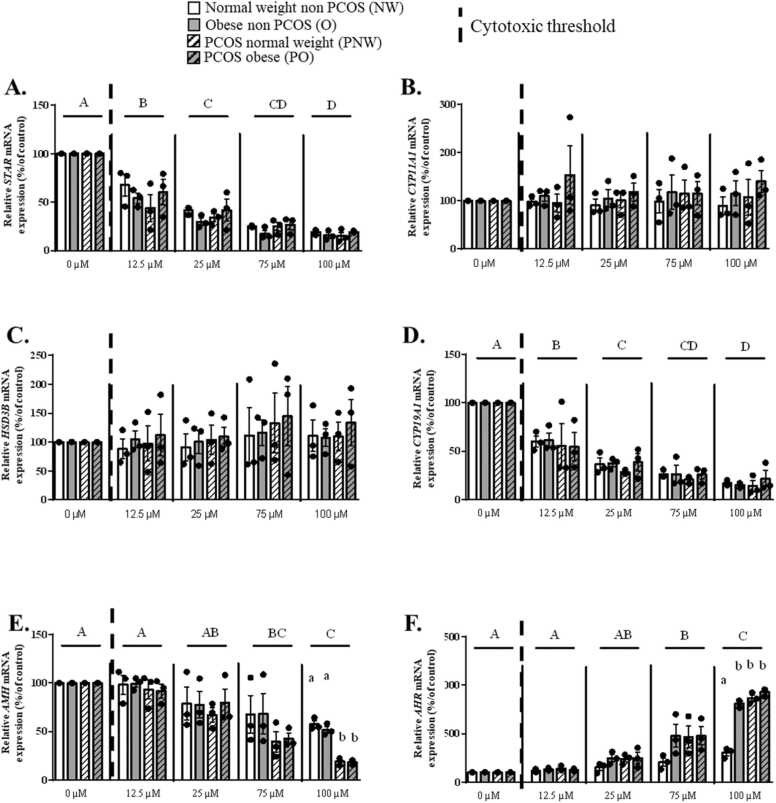
A significant interaction of the patient status and the Tb effect was observed for STAR and CYP19A1 genes (p = 0.0004, Fig. 4A and p = 0.0148, Fig. 4D, respectively). A dose dependent decrease in AMH mRNA expression was observed from 75 µM of Tb (cytotoxic dose) with the largest effect in hGCs from PCOS groups (p < 0.0001, 99.99% confidence interval) (Fig. 4E). In the presence of Tb, a dose dependent increase in AHR mRNA expression was observed in hGCs from 75 µM whatever the patient group. At 100 µM of Tb (cytotoxic dose), O and both PCOS groups had an higher expression of AHR mRNA in hGCs compared to the control NW group (p < 0.0001, 99.99% confidence interval) (Fig. 4F).
Fig. 4.
Expression levels of STAR, CYP11A1, HSD3B, CYP19A1, AMH and AHR mRNA in granulosa cells from normal weight (NW), obese (O), PCOS normal weight (PNW) and PCOS obese (PO) groups in response to Tb exposure. Expression of the STAR (A), CYP11A1 (B), HSD3B (C), CYP19A1 (D), AMH (E) and AHR (F) gene expressions in hGCs from NW, O, PNW and PO groups after exposure to Tb for 48 h as compared to control (without Tb exposure). Data are presented as mean ± SEM of N = 3 and n = 3. For each analysis, N means the number of experimental replicates and n means the number of samples used for each experiment. For each group and concentration, the values were normalized with the control condition* 100. Lowercase letters (i.e a, b) correspond to the 2-way ANOVA significance followed by the multiple comparison test of Tukey between all groups under one concentration (p < 0.05, 95% confidence interval). Uppercase letters (i.e A, B) correspond to the 2-way ANOVA significance followed by the multiple comparison test of Tukey (p < 0.05, 95% confidence interval) between all concentrations regardless of the group.
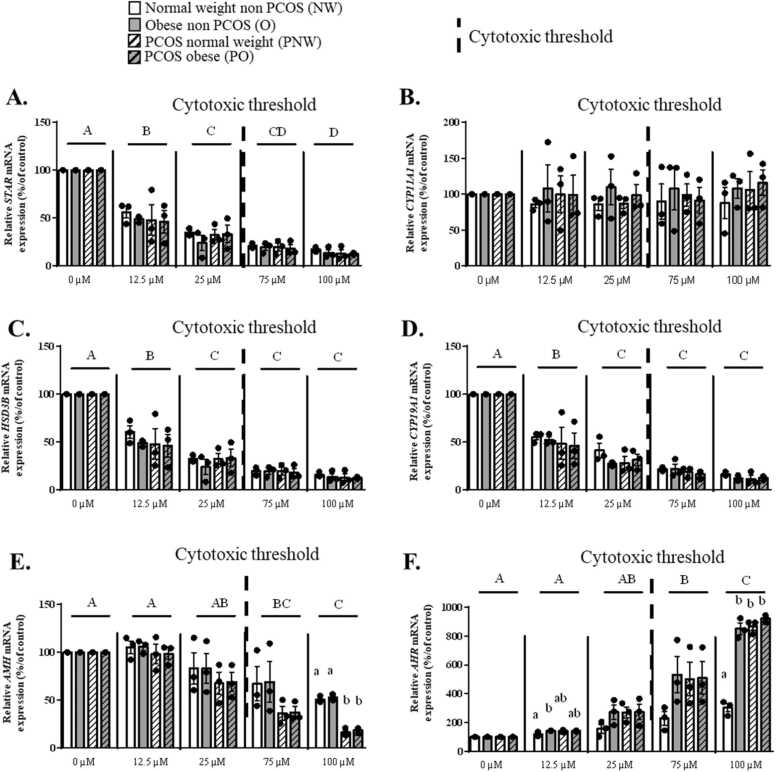
Epoxiconazole exposure did not affect CYP11A1 mRNA expression in hGCs whereas it inhibited STAR, HSD3B and CYP19A1 mRNA expression from 12.5 µM concentration without differences between groups (p < 0.0001, 99.99% confidence interval, Fig. 5A to D). A dose dependent decrease in AMH mRNA expression was observed from 75 µM of Epox (cytotoxic dose) with the largest effect in hGCs from PCOS groups (p < 0.0001, 99.99% confidence interval) (Fig. 5E). Regarding AHR mRNA expression, the same pattern as Tb exposure was observed (Fig. 5F).
Fig. 5.
Expression levels of STAR, CYP11A1, HSD3B, CYP19A1, AMH and AHR mRNA in granulosa cells from normal weight (NW), obese (O), PCOS normal weight (PNW) and PCOS obese (PO) groups in response to Epox exposure. Expression of the STAR (A), CYP11A1 (B), HSD3B (C), CYP19A1 (D), AMH (E) and AHR (F) gene expressions in hGCs from NW, O, PNW and PO groups after exposure to Epox for 48 h as compared to control (without Epox exposure). Data are presented as mean ± SEM of N = 3 and n = 3. For each analysis, N means the number of experimental replicates and n means the number of samples used for each experiment. For each group and concentration, the values were normalized with the control condition* 100. Lowercase letters (i.e a, b) correspond to the 2-way ANOVA significance followed by the multiple comparison test of Tukey between all groups under one concentration (p < 0.05, 95% confidence interval). Uppercase letters (i.e A, B) correspond to the 2-way ANOVA significance followed by the multiple comparison test of Tukey (p < 0.05, 95% confidence interval) between all concentrations regardless of the group.
3.7. Aryl hydrocarbon receptor (AHR) gene expression in KGN and hGCs in response to Tb and Epox exposures, deletion of AHR gene in KGN cells and consequences on steroidogenesis
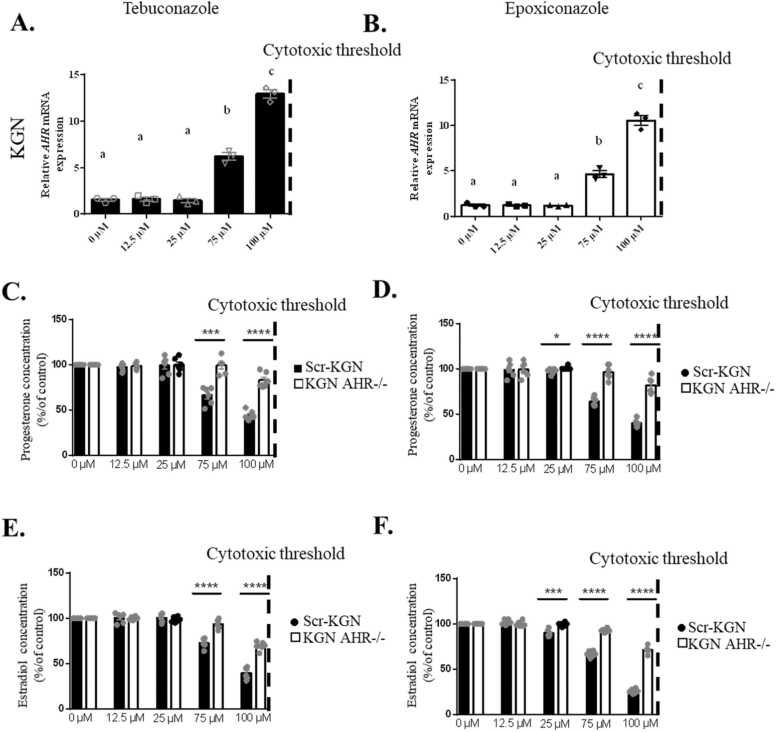
The effect of Tb and Epox exposures for 48 h on AHR mRNA expression was assessed in KGN (Fig. 6A and B) and in NW hGCs (Supp 7 A and B). For both triazoles and cell types, an increase of the expression of AHR was observed from 75 µM to 100 µM (p < 0.0001, 99.99% confidence interval). To assess the role of AHR in the Tb and Epox effects on the hGCs, we silenced AHR gene in KGN cells by using the sgRNA system. As shown in Supplemental data 7C, and D, AHR mRNA and protein expression were significantly reduced in KGN AHR-/- cell line as compared to parental KGN cells as determined by qPCR,Western-blot (p < 0.001, 99.9% confidence interval), respectively. These two types of cells were exposed for 48 h with Tb or Epox (0–100 µM, non-cytotoxic doses). From 75 µM of Tb (p < 0.001, 99.9% confidence interval) and 25 µM of Epox (p < 0.05, 95% confidence interval), Pg and E2 concentrations were significantly more decreased in parental KGN cells than in KGN AHR-/- in response to triazoles exposure suggesting that AHR could partly contribute to explain the negative effect of two triazoles in the hGCs steroidogenesis (Fig. 6C-F).
Fig. 6.
Evolution of AHR and steroids production after exposure to Tb or Epox in Scr-KGN and KGN AHR-/-. A-B) Relative mRNA expression of AHR in KGN cells exposed to Tb (A) or Epox (B) for 48 h of culture. Data are presented as mean ± SEM of N = 3 and n = 1. For each analysis, N means the number of experimental replicates and n means the number of samples used for each experiment. C-D) Progesterone secreted in the culture medium by KGN cells or KGN AHR-/- (pg/ml) after 48 h of culture with Tb (C) or Epox (D). Data are presented as mean ± SEM of N = 6 and n = 1. E-F) Estradiol secreted in the culture medium by KGN cells or KGN AHR-/- (pg/ml) after 48 h of culture with Tb (E) or Epox (F) Data are presented as mean ± SEM of N = 6 and n = 1. For each analysis, N means the number of experimental replicates and n means the number of samples used for each experiment. Lowercase letters (i.e a, b) correspond to the ordinary one-way ANOVA significance followed by the multiple comparison test of Tukey between all concentrations (p < 0.05, 95% confidence interval). Stars correspond to the comparison test of Student between Scr-KGN and KGN AHR-/- (* p < 0.05, 95% confidence interval, ** p < 0.01, 99% confidence interval, *** p < 0.001, 99.9% confidence interval, **** p < 0.0001, 99.99% confidence interval).
4. Discussion
In the present study we showed for the first time that Epox and Tb are able to alter hGCs viability and steroidogenesis. These effects were observed at largely higher concentration than those detected in the soils or in the surface or underground water however for Tb, the cytotoxic concentration (12.5 µM, 24 h for hGCs) was 5.6 times lower than the reproductive NOAEL [24]. Therefore, the reproductive NOAEL for Tb should be reevaluated. Concerning the steroidogenesis process, we demonstrated that the decrease in Pg and E2 production induced by Epox and Tb in hGCs was associated to a reduction in the mRNA expression of several steroidogenesis components and an increase in AHR mRNA expression. However, data for Tb exposure in hGCs cells were obtained after the cytotoxic threshold (12.5 µM). In addition, we showed that the silencing of AHR in KGNs led to a decrease in the negative effects of Epox and Tb in the steroid production by hGCs suggesting that AHR could be partly involved in the negative effect of both triazoles in granulosa cell steroidogenesis. Interestingly, we showed that cells from O, PNW and PO were less sensitive than NW groups for viability (12.5 µM versus 75 µM, 24 h) after Tb exposure whereas after Epox exposure, cells from O and PNW (25 µM, 24 h) were more affected than NW group (75 µM, 24 h). Moreover, we demonstrated that for the Epox (12.5 μM, 48 h) exposure,estradiol secretion by hGCs was higher decreased in Obese, and PCOS NW or Obese patient than in NW patients and the higher decrease of testosterone secretion was found in obese group. At 25 µM of Epox for 48 h, the progesterone secretion by the cells from PO group was the most altered. Surprisingly, in these conditions, AHR mRNA expression was increased. Taken together, all these data suggest that in term of steroid production by hGCs, the alterations of Tb and Epox exposure could be more important in patients with metabolic disorders or PCOS.
Our investigations showed that KGN and hGCs viability determined after 24 h of exposure was reduced from 500 µM and 12.5 µM of Tb, respectively. This last concentration corresponds to 125 times the maximal concentration found within the soil for Tb, and it is 780 times and 431 times higher than those found within the under and surface waters, respectively. Compared to the literature, this concentration is 37 times lower than the concentration found in hair workers in farmland [48] and 7.4 higher than the concentration found in bird’s blood living in vineyard in France [2]. Epox decreased the cell viability of hGCs at 75 µM after 24 h of exposure, however, no effect was found in KGN. Thus, at the concentrations found in soil and water in the French area, no impact was demonstrated on this parameter. Our data are in good agreement with the results found in Cao et al.’s study where Tb did not impact human choriocarcinoma JEG3 cells line (JEG3 cells) viability below 100 µM [11], but also in Rieke et al.’s study where 10 µM of Epox did not alter JEG3 cell viability too [49]. However, 20 µM of Tb reduced human colon HCT116 cells line’ viability [43]. Epoxiconazole exposure (from 0.1 to 100 µg/L for 48 h) in the germ cells of C. elegans led to the arrest of cell proliferation [37] whereas higher doses (6.25 and 75 µM of E) stimulated the proliferation of MCF-7 BUS, a human breast cancer cells line [34]. Thus, the effects of these triazoles are dependent on the type of cells. If we compare Tb and Epox effects on KGN cells’ viability, Tb induced higher cytotoxic effects than Epox. Moreover, we found higher toxicity of triazoles on hGCs compared to KGN which could be related with the fact that KGN are derived from tumoral granulosa cells and they can survive in culture for extended cell generation. However, despite their nature, it has been shown that KGN cells keep the same physiological regulation of apoptosis as hGCs [67].
Interestingly, the opposite effect between Tb and Epox was observed in terms of steroidogenesis after 48 h of exposure. In this study, the mRNA expression of 4 genes coding for the steroidogenesis were assessed regarding their important role in the transport of cholesterol (STAR), the conversion of cholesterol into pregnenolone (CYP11A1), the conversion of pregnenolone into Pg (HSD3B) and the conversion of testosterone into E2 (CYP19A1). Here, Tb reduced Pg and E2 secretions of KGN from 75 μM, a non-cytotoxic dose, as well as the expression of mRNAs of STAR, HSD3B and CYP19A1. Regarding hGCs, Tb from 75 µM reduced Pg level, STAR and CYP19A1 mRNA expression whereas E2 level was decreased from 25 µM. These concentrations are at cytotoxic level. While Pg and E2 secretions decreased from 25 µM of Epox and the expression of STAR, HSD3B and CYP19A1 were inhibited from 12.5 µM of Epox, a non-cytotoxic dose for both cell types. This concentration is 83 times higher than the maximal concentration found within the soil, 8929 times and 15625 times higher than those found in the under and surface water, respectively. Compared to the literature, it is 208 times higher than the concentration found in worker’s hair [51] and 31.25 times higher than the maximal concentration found in human urine in the literature [39]. However, this concentration is only 1.79 times higher than the reproductive NOAEL in Europe [22] and 20.83 times higher than maximal residue limits (MRL) for wheat in Europe. Thus, the most deleterious impact of both triazoles was observed on steroidogenesis at doses higher than the environmental concentrations. This result was also observed within the literature where Pg secretion of JEG3 cells was reduced at 10 µM of Epox and 15 µM of Tb [49]. The decrease of E2 secretion was also shown after exposure to Epox (up to 90 mg/kg/bw/d) during the gestational period of guinea pig [50]. An interesting study [36] focused on a computational approach to predict which human proteins could be impacted by Epox exposure and the authors observed that CYP19A1 was one of the targets of the triazole. This prediction was confirmed by our study and also by several colleagues [11], [13], [49].
Interestingly, we showed for the first time that high concentrations of Epox and Tb induced mRNA expression of the aryl hydrocarbon receptor (AHR) gene in human granulosa cells. As we observed a similar pattern in AHR mRNA expression and steroids production in both cell types after triazoles exposure we decided to focus on KGN cells to understand how Tb and Epox could alter the functions of granulosa cells. We developed KGN cell line knock-out for the AHR, which was a good candidate according to the literature [4], [49], [7]. We obtained KGN AHR-/- with reduced level of mRNA and protein expression. We showed that KGN AHR-/- cells had a higher secretion of E2 and Pg than control KGN in response to Tb or Epox exposure suggesting that the AHR signaling pathway could partly be involved in the alteration of triazole in the human granulosa steroidogenesis. However, in in vivo studies of AHR in mice, reduced number of antral follicle and corpus luteum, slower ovarian follicle growth, reduce ability to ovulate, to produce E2, and LH/FSH-response of antral follicles with decrease in FSHR and LHR expression were demonstrated when AHR was invalidated [4], [6], [5], [7], [8]. Therefore, in our study, AHR invalidation would be more related with its xenobiotic sensor role than its folliculogenesis regulator role. In the literature, it exists controversial results about the activation of AHR in response to Epox or Tb exposure. In JEG3 cells, Tb and Epox were not able to activate AHR-dependent pathway [49] whereas in the liver, Tb activated CYP1a1 and CYP1a2 (two enzymes of detoxification) through AHR [35]. Moreover, studies have shown that AHR expression can be negatively regulated by phosphokinase A and positively regulated by FSH in granulosa cells, therefore investigated FSHR/cAMP/PKA pathway by western blot analyses after triazole exposure could be interesting [4], [40]. Further investigations are needed to determine if Tb and Epox are able to activate the AHR-dependent pathway in human granulosa cells.
Several reviews reported a potential involvement of endocrine disruptors in PCOS [18], [47] and metabolic disorders [19]. However, it exists few data about the comparison of endocrine disruptors’s effects between ovarian cells from normal and PCOS or obese patients. In the present study, we studied the triazoles effects on hGCs from Obese (O), PCOS Obese (PO) or not Obese patients (PNW). In our study, we observed that triazoles exposure differently alter hGCs functions in women with a disturbed physiological status and NW patients. Indeed, at 25 µM of Tb, the decrease of the cell viability after 24 h of exposure was higher in the NW group than the other groups, as well as at 100 µM, where NW group had lower viability than PNW group. No difference was found between groups for the viability after Epox exposure. This means that Epox and Tb did not affect cells in the same way. Concerning the steroid secretion, Epox exposure for 48 h altered much more Pg production in O, PNW, PO hGCs as compared to NW hGCs but it can be explained by a lower cell viability in O and PNW groups at this concentration. For the E2 secretion, O, PNW and PO groups were affected at non-cytotoxic concentrations by Epox exposure but not by Tb exposure. Finally, testosterone level was lower in O group after non cytotoxic concentration of Epox (12.5 µM). This is in accordance with the literature. Indeed, obesity impacts the steroidogenesis including the levels of androgens. Moreover, both PNW and PO have more visceral adipose tissue than control women, which was positively correlated with androgen levels. This is illustrated in Zeng et al.’s study where they found that obese PCOS women had higher level of T than normal-weight PCOS [71]. However, in the present study, we observed an age effect in our four groups of patients. Indeed, O patients were older than the other groups suggesting that the demographics of the patient population could also contribute to explain some different sensitivity to the triazoles exposure.
Considering the difference between groups at the basal condition and after the exposure to Epox and Tb in term of E2, Pg and testosterone secretion, we decided to analyze if there was a difference of the AMH expression. Indeed, the AMH secreted by granulosa cells plays a crucial role in secondary, preantral and small antral follicle [3]. In good agreement with the literature, we showed that serum AMH and AMH mRNA were higher in PCOS groups [57]. Interestingly when exposed to 100 µM of Tb or Epox (cytotoxic concentration), hGCs from PCOS groups had the biggest inhibition of AMH mRNA compared to the other groups. Two hypotheses can be raised: i) Epox and Tb changed the nature of granulosa cells from PCOS antral follicles to PCOS mature follicle expressing less AMH. Zhang et al.’ study showed that the stem cell factor important for the recruitment and the development of primordial follicle was negatively regulated with the concentration of AMH [74]. ii) PCOS groups were more sensitive to Tb and Epox and the expression was more dysregulated by the exposure to the fungicide than in the other groups. This last hypothesis is more convincing since steroids levels in the medium were decreased. Finally, we analyzed the expression of AHR mRNA in granulosa cells and under basal condition, as expected, no differences were found between groups. Interestingly, for 100 µM of Epox and Tb, an higher upregulation of AHR mRNA was observed for O, PN and PO groups. This can be related to the fact that these groups were also more impacted than the control group in steroidogenesis and confirmed that AHR has definitively a role in the Tb and Epox effects on granulosa cells.
The present study has some limitations. Firstly, it will be very interesting to measure the triazole concentration in the human follicular fluid (FF) as some chemicals has been already detected in [18], [46]. Studies about in vivo exposure routes of Tb and Epox have shown that after oral administration, these triazoles are distributed within metabolic organs such as liver and kidneys [22], [24]. In liver, for example, Epox is metabolized in 47 different metabolites through oxidation, hydroxylation and conjugation [75]. Therefore, the analysis of triazoles and their main metabolites in FF could give us better indications about the degree of ovarian cells exposure. Secondly, detoxification enzymes such as CYP1A1 and CYP1B1, targeted by the xenobiotic-AHR couple, are present in granulosa cells [14], [29] therefore, their implication in triazole exposure effect needs to be investigated, for example by an enzymatic activity study. Thirdly, to investigate if these triazoles or metabolites concentrations interferes with obesity and PCOS, it will be important to determine if it exists an association between FF triazole concentration and plasma glucose and lipid. Indeed, it has been recently shown that total phthalate metabolites urine concentrations were positively associated with BMI, lipid accumulation product and significantly linked with fasting plasma glucose [41]. In a case-control study, Zhan et al. showed that environmental exposure to bisphenol analogs was associated with increased odds of PCOS and this association was stronger among obese and overweight women [72]. Moreover, in this study, we only analysed individual pesticide effect which is not relevant regarding realistic exposure scenarios. For example, Choubbane et al. have found several residues of pesticides in vegetables and fruits eaten by humans [16] and it has been shown that pesticides can get synergic effects on ovaries [42]. As mentioned above, the pesticide concentrations used here are well above those found in the environment. We have only studied the acute effect over a short period of time, more related to a state of intoxication, compared to low doses used over a long period which would be more related to chronic exposure to pesticides. To investigate lower concentrations of pesticides mixtures found in the Centre-Val de Loire region on granulosa cells function will complete risk assessment for fertility in women. A last limitation is the size and also a better understanding of our population. For example a questionnaire about the sector of their profession, the environment of the place of residence (town or country), the diet (organic or not), and finally whether they smoke or not could be asked for each patient. Finally, to increase the clinical relevance of our study, a larger and more diverse patient cohorts are needed for generalizability to broader populations.
5. Conclusion and perspectives
To our knowledge, our study is the first to show that 1. Tb and Epox are able to reduce steroid production by human granulosa cells through partly an increase in AHR mRNA expression. This reduction in steroid production could be associated with a lower fertility and/or some reproductive disorders. However, effects at environmentally relevant concentrations need more analysis for potential sublethal effects. Furthermore, a deeper exploration of the specific pathways through which fungicides exert effects remains to be investigated. 2. Granulosa cells from women with metabolic and PCOS disorders (obese, PCOS or both) have a greater alteration in steroid secretion in response to Tb and Epox exposure than cells from normal weight women without PCOS. Even if the Tb and Epox exposure levels used in vitro on granulosa cells are largely higher than those observed in soil and waters where the patients are located. Future studies will be conducted in order to investigate the levels of Tb and Epox in follicular fluid from control and PCOS and/or obese patients living in the area of Centre-Val de Loire.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Funding
The authors thank to OFB (Office Français de la Biodiversité) and Région Centre Val de Loire for their funding (HAPOFERTI grant number 32000858, and PESTIFERTI grant number 32001127).
CRediT authorship contribution statement
Froment Pascal: Investigation, Methodology, Validation. serra Loise: Formal analysis, Investigation, Methodology, Validation, Writing – original draft. Guerif Fabrice: Investigation, Methodology, Validation. amalric Laurence: Formal analysis, Investigation, Methodology, Validation. Henriot Abel: Investigation, Methodology, Validation. Jolivet Claudy: Formal analysis, Investigation, Methodology, Validation. Frogel Claire: Formal analysis, Investigation, Methodology, Validation, Writing – original draft. Caria Giovanni: Formal analysis, Investigation, Methodology, Validation, Writing – original draft. rame Christelle: Formal analysis, Investigation, Methodology, Supervision, Validation. bongrani alice: Formal analysis, Investigation, Methodology, Validation, Writing – original draft. dupont joelle: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. Estienne Anthony: Formal analysis, Investigation, Methodology, Validation, Writing – original draft. corbin emilie: Formal analysis, Investigation, Methodology, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof. L.H. Lash
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.12.009.
We assumed that 1 kg = 1 L so (2.3 *10-3 g/L /329.76 g/mol = 7 µM)
We assumed that 1 kg = 1 L so (0.02 g/kg *10-3/307.82 g/mol)= 65 µM
We assumed that 1 kg = 1 L so (0.025 g/kg *10-3/307.82 g/mol)= 81 µM
We assumed that 1 kg = 1 L so (0.02 g/kg *10-3/329.76 g/mol)= 61 µM
We assumed that 1 kg = 1 L so (0.04 g/kg *10-3/329.76 g/mol)= 121 µM
(9 *10-6 g/L / 307.82 g/mol)= 30 nM
(5 *10-6 g/L / 307.82 g/mol)= 16 nM
(0.27 *10-6 g/L / 329.76 g/mol)= 0.8 nM
(0.45 *10-6 g/L / 329.76 g/mol)= 1.4 nM
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Data will be made available on request.
References
- 1.American Society for Reproductive Medicine Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Angelier F., Prouteau L., Brischoux F., Chastel O., Devier M.-H., Le Menach K., Martin S., Mohring B., Pardon P., Budzinski H. High contamination of a sentinel vertebrate species by azoles in vineyards: a study of common blackbirds (Turdus merula) in multiple habitats in western France. Environ. Pollut. 2023;316 doi: 10.1016/j.envpol.2022.120655. [DOI] [PubMed] [Google Scholar]
- 3.Baarends W.M., Uilenbroek J.T., Kramer P., Hoogerbrugge J.W., van Leeuwen E.C., Themmen A.P., Grootegoed J.A. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–4962. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- 4.Baba T., Mimura J., Nakamura N., Harada N., Yamamoto M., Morohashi K., Fujii-Kuriyama Y. Intrinsic function of the aryl hydrocarbon (Dioxin) receptor as a key factor in female reproduction. Mol. Cell Biol. 2005;25:10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett K.R., Tomic D., Gupta R.K., Babus J.K., Roby K.F., Terranova P.F., Flaws J.A. The aryl hydrocarbon receptor is required for normal gonadotropin responsiveness in the mouse ovary. Toxicol. Appl. Pharmacol. 2007;223:66–72. doi: 10.1016/j.taap.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett K.R., Tomic D., Gupta R.K., Miller K.P., Meachum S., Paulose T., Flaws J.A. The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol. Reprod. 2007;76:1062–1070. doi: 10.1095/biolreprod.106.057687. [DOI] [PubMed] [Google Scholar]
- 7.Benedict J.C. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol. Sci. 2000;56:382–388. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- 8.Benedict J.C., Miller K.P., Lin T.-M., Greenfeld C., Babus J.K., Peterson R.E., Flaws J.A. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol. Reprod. 2003;68:1511–1517. doi: 10.1095/biolreprod.102.007492. [DOI] [PubMed] [Google Scholar]
- 9.Canépa S., Lainé A., Bluteau A., Fagu C., Flon C., Monniaux D. Validation d’une méthode immunoenzymatique pour le dosage de la progestérone dans le plasma des ovins et des bovins. Cah. Des. Tech. De. l’INRA. 2008;64:19–30. [Google Scholar]
- 10.Canipari R. Oocyte--granulosa cell interactions. Hum. Reprod. Update. 2000;6:279–289. doi: 10.1093/humupd/6.3.279. [DOI] [PubMed] [Google Scholar]
- 11.Cao S., Ye L., Wu Y., Mao B., Chen L., Wang X., Huang P., Su Y., Ge R.-S. The effects of fungicides on human 3β-hydroxysteroid dehydrogenase 1 and aromatase in human placental cell line JEG-3. Pharmacology. 2017;100:139–147. doi: 10.1159/000475531. [DOI] [PubMed] [Google Scholar]
- 12.Chabbert-Buffeta N., Skinner D.C., Caraty A., Bouchard P. Neuroendocrine effects of progesterone. Steroids. 2000;65:613–620. doi: 10.1016/s0039-128x(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 13.Chambers J.E., Greim H., Kendall R.J., Segner H., Sharpe R.M., Van Der Kraak G. Human and ecological risk assessment of a crop protection chemical: a case study with the azole fungicide epoxiconazole. Crit. Rev. Toxicol. 2014;44:176–210. doi: 10.3109/10408444.2013.855163. [DOI] [PubMed] [Google Scholar]
- 14.Chen H.-S., Chiang P.-H., Wang Y.-C., Kao M.-C., Shieh T.-H., Tsai C.-F., Tsai E.-M. Benzyl butyl phthalate induces necrosis by AhR mediation of CYP1B1 expression in human granulosa cells. Reprod. Toxicol. 2012;33:67–75. doi: 10.1016/j.reprotox.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Chen X., Zhu Q., li X., Huang T., Wang S., Wang Y., Chen X., Lin Z., Ge R. Pubertal exposure to tebuconazole increases testosterone production via inhibiting testicular aromatase activity in rats. Chemosphere. 2019;230:519–526. doi: 10.1016/j.chemosphere.2019.05.122. [DOI] [PubMed] [Google Scholar]
- 16.Choubbane H., Ouakhssase A., Chahid A., Taourirte M., Aamouche A. Pesticides in fruits and vegetables from the Souss Massa region, Morocco. Food Addit. Contam.: Part B. 2022;15:79–88. doi: 10.1080/19393210.2022.2028196. [DOI] [PubMed] [Google Scholar]
- 17.Cloix L., Reverchon M., Cornuau M., Froment P., Ramé C., Costa C., Froment G., Lecomte P., Chen W., Royère D., et al. Expression and regulation of INTELECTIN1 in human granulosa-lutein cells: role in IGF-1-induced steroidogenesis through NAMPT1. Biol. Reprod. 2014;91 doi: 10.1095/biolreprod.114.120410. [DOI] [PubMed] [Google Scholar]
- 18.Craig Z.R., Wang W., Flaws J.A. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142:633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- 19.Darbre P.D. Endocrine disruptors and obesity. Curr. Obes. Rep. 2017;6:18–27. doi: 10.1007/s13679-017-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Castro V.L.S.S., Maia A.H. Prenatal epoxiconazole exposure effects on rat postnatal development: prenatal rat’s exposure to epoxiconazole. Birth Defects Res. B. 2012;95:123–129. doi: 10.1002/bdrb.20345. [DOI] [PubMed] [Google Scholar]
- 21.Deng Y., Zhang Y., Li S., Zhou W., Ye L., Wang L., Tao T., Gu J., Yang Z., Zhao D., et al. Steroid hormone profiling in obese and nonobese women with polycystic ovary syndrome. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.EFSA Conclusion regarding the peer review of the pesticide risk assessment of the active substance epoxiconazole. EFS2. 2008;138:1–80. doi: 10.2903/j.efsa.2008.138r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EFSA Scientific opinion on risk assessment for a selected group of pesticides from the triazole group to test possible methodologies to assess cumulative effects from exposure through food from these pesticides on human health. EFSA J. 2009;7(9):1167. 2009. [Google Scholar]
- 24.EFSA Conclusion on the peer review of the pesticide risk assessment of the active substance tebuconazole. EFS2. 2014;12:3485. [Google Scholar]
- 25.European Commission Règlement (UE) no 253/2011 de la Commission du 15 mars 2011 modifiant le règlement (CE) no 1907/2006 du Parlement européen et du Conseil concernant l’enregistrement, l’évaluation et l’autorisation des substances chimiques, ainsi que les restrictions applicables à ces substances (REACH), en ce qui concerne l’annexe XIIITexte présentant de l’intérêt pour l’EEE. J. Off. De. l’Union Eur. 2011 RÈGLEMENT (UE) No 253/2011 DE LA COMMISSION, 69/7-69/12. [Google Scholar]
- 26.Fernández M., Bourguignon N., Lux-Lantos V., Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ. Health Perspect. 2010;118:1217–1222. doi: 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fustinoni S., Mercadante R., Polledri E., Rubino F.M., Mandic-Rajcevic S., Vianello G., Colosio C., Moretto A. Biological monitoring of exposure to tebuconazole in winegrowers. J. Expo. Sci. Environ. Epidemiol. 2014;24:643–649. doi: 10.1038/jes.2014.14. [DOI] [PubMed] [Google Scholar]
- 28.Heise T., Schmidt F., Knebel C., Rieke S., Haider W., Pfeil R., Kneuer C., Niemann L., Marx-Stoelting P. Hepatotoxic effects of (tri)azole fungicides in a broad dose range. Arch. Toxicol. 2015;89:2105–2117. doi: 10.1007/s00204-014-1336-1. [DOI] [PubMed] [Google Scholar]
- 29.Horling K., Santos A.N., Fischer B. The AhR is constitutively activated and affects granulosa cell features in the human cell line KGN. Mol. Hum. Reprod. 2011;17:104–114. doi: 10.1093/molehr/gaq074. [DOI] [PubMed] [Google Scholar]
- 30.Huang B., Ren X., Wu L., Zhu L., Xu B., Li Y., Ai J., Jin L. Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. PLoS One. 2016;11 doi: 10.1371/journal.pone.0145895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.J. Vandesompele K. De Preter F. Pattyn B. Poppe N. Van Roy A. De Paepe F. Speleman Accurate Norm. Realt. Quant. RT-PCR data Geom. averaging Mult. Intern. Control Genes 3 7 2022.0034.1-0034.11. [DOI] [PMC free article] [PubMed]
- 32.Kahle M., Buerge I.J., Hauser A., Müller M.D., Poiger T. Azole fungicides: occurrence and fate in wastewater and surface waters. Environ. Sci. Technol. 2008;42:7193–7200. doi: 10.1021/es8009309. [DOI] [PubMed] [Google Scholar]
- 33.Keye W.R., JR., Jaffe R.B. Modulation of pituitary gonadotropin response to gonadotropin-releasing hormone by estradiol. J. Clin. Endocrinol. Metab. 1974;38:805–810. doi: 10.1210/jcem-38-5-805. [DOI] [PubMed] [Google Scholar]
- 34.Kjærstad M.B., Taxvig C., Nellemann C., Vinggaard A.M., Andersen H.R. Endocrine disrupting effects in vitro of conazole antifungals used as pesticides and pharmaceuticals. Reprod. Toxicol. 2010;30:573–582. doi: 10.1016/j.reprotox.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Knebel C., Heise T., Zanger U.M., Lampen A., Marx-Stoelting P., Braeuning A. The azole fungicide tebuconazole affects human CYP1A1 and CYP1A2 expression by an aryl hydrocarbon receptor-dependent pathway. Food Chem. Toxicol. 2019;123:481–491. doi: 10.1016/j.fct.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 36.Kongsbak K., Vinggaard Anne Marie, Hadrup Niels, Audouze Karine. A computational approach to mechanistic and predictive toxicology of pesticides. ALTEX. 2014;31:11–22. doi: 10.14573/altex.1304241. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Zhang M., Li S., Lv R., Chen P., Liu R., Liang G., Yin L. The use of the nematode caenorhabditis elegans to evaluate the adverse effects of epoxiconazole exposure on spermatogenesis. IJERPH. 2016;13:993. doi: 10.3390/ijerph13100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma F., Li Y., Yu Y., Li Z., Lin L., Chen Q., Xu Q., Pan P., Wang Y., Ge R. Gestational exposure to tebuconazole affects the development of rat fetal Leydig cells. Chemosphere. 2021;262 doi: 10.1016/j.chemosphere.2020.127792. [DOI] [PubMed] [Google Scholar]
- 39.Machado S.C., Souza B.M., de Aguiar Marciano L.P., Souza Pereira A.F., de Carvalho D.T., Martins I. A sensitive and accurate vortex-assisted liquid-liquid microextraction-gas chromatography-mass spectrometry method for urinary triazoles. J. Chromatogr. A. 2019;1586:9–17. doi: 10.1016/j.chroma.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 40.Matvere A., Teino I., Varik I., Kuuse S., Tiido T., Kristjuhan A., Maimets T. FSH/LH-dependent upregulation of Ahr in murine granulosa cells is controlled by PKA signaling and involves epigenetic regulation. IJMS. 2019;20:3068. doi: 10.3390/ijms20123068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milankov A., Milanović M., Milošević N., Sudji J., Pejaković S., Milić N., Bjelica A., Medić Stojanoska M. The effects of phthalate exposure on metabolic parameters in polycystic ovary syndrome. Clin. Chim. Acta. 2023;540 doi: 10.1016/j.cca.2023.117225. [DOI] [PubMed] [Google Scholar]
- 42.Mourikes V.E., Flaws J.A. Reproductive toxicology: effects of chemical mixtures on the ovary. Reproduction. 2021;162:F91–F100. doi: 10.1530/REP-20-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Othmène Y.B., Salem I.B., Hamdi H., Annabi E., Abid-Essefi S. Tebuconazole induced cytotoxic and genotoxic effects in HCT116 cells through ROS generation. Pestic. Biochem. Physiol. 2021;174 doi: 10.1016/j.pestbp.2021.104797. [DOI] [PubMed] [Google Scholar]
- 44.Ozcan Dag Z., Dilbaz B. Impact of obesity on infertility in women. J. Turk. Ger. Gynecol. Assoc. 2015;16:111–117. doi: 10.5152/jtgga.2015.15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasquali R., Gambineri A., Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113:1148–1159. doi: 10.1111/j.1471-0528.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 46.Petro E.M.L., D’Hollander W., Covaci A., Bervoets L., Fransen E., De Neubourg D., De Pauw I., Leroy J.L.M.R., Jorssen E.P.A., Bols P.E.J. Perfluoroalkyl acid contamination of follicular fluid and its consequence for in vitro oocyte developmental competence. Sci. Total Environ. 2014;496:282–288. doi: 10.1016/j.scitotenv.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 47.Piazza M.J., Urbanetz A.A. Environmental toxins and the impact of other endocrine disrupting chemicals in women’s reproductive health. JBRA Assist. Reprod. 2019;23(2):154–164. doi: 10.5935/1518-0557.20190016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polledri E., Mercadante R., Fustinoni S. Determination of tebuconazole and penconazole fungicides in rat and human hair by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2018;32:1243–1249. doi: 10.1002/rcm.8153. [DOI] [PubMed] [Google Scholar]
- 49.Rieke S., Koehn S., Hirsch-Ernst K., Pfeil R., Kneuer C., Marx-Stoelting P. Combination effects of (Tri)Azole fungicides on hormone production and xenobiotic metabolism in a human placental cell line. IJERPH. 2014;11:9660–9679. doi: 10.3390/ijerph110909660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider S., Hofmann T., Stinchcombe S., Moreno M.C.R., Fegert I., Strauss V., Gröters S., Fabian E., Thiaener J., Fussell K.C., et al. Species differences in developmental toxicity of epoxiconazole and its relevance to humans: epoxiconazole toxicity-species differences. Birth Defects Res. B. 2013;98:230–246. doi: 10.1002/bdrb.21058. [DOI] [PubMed] [Google Scholar]
- 51.Schummer C., Salquèbre G., Briand O., Millet M., Appenzeller B.M.R. Determination of farm workers’ exposure to pesticides by hair analysis. Toxicol. Lett. 2012;210:203–210. doi: 10.1016/j.toxlet.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Serra L., Estienne A., Caria G., Ramé C., Jolivet C., Froger C., Henriot A., Amalric L., Guérif F., Froment P., et al. In vitro exposure to triazoles used as fungicides impairs human granulosa cells steroidogenesis. Environ. Toxicol. Pharmacol. 2023;104 doi: 10.1016/j.etap.2023.104295. [DOI] [PubMed] [Google Scholar]
- 53.Silva V., Mol H.G.J., Zomer P., Tienstra M., Ritsema C.J., Geissen V. Pesticide residues in European agricultural soils – A hidden reality unfolded. Sci. Total Environ. 2019;653:1532–1545. doi: 10.1016/j.scitotenv.2018.10.441. [DOI] [PubMed] [Google Scholar]
- 54.Spritzer P.M., Lecke S.B., Satler F., Morsch D.M. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149:R219–R227. doi: 10.1530/REP-14-0435. [DOI] [PubMed] [Google Scholar]
- 55.Stinchcombe S., Schneider S., Fegert I., Rey Moreno M.C., Strauss V., Gröters S., Fabian E., Fussell K.C., Pigott G.H., van Ravenzwaay B. Effects of estrogen coadministration on epoxiconazole toxicity in rats: epoxiconazole toxicity-estrogen deficiency. Birth Defects Res B. 2013;98:247–259. doi: 10.1002/bdrb.21059. [DOI] [PubMed] [Google Scholar]
- 56.Syngenta (2023). Une nouvelle triazole seule homologuée face aux maladies des blés. Syngenta France.
- 57.Tata B., Mimouni N.E.H., Barbotin A.-L., Malone S.A., Loyens A., Pigny P., Dewailly D., Catteau-Jonard S., Sundström-Poromaa I., Piltonen T.T., et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018;24:834–846. doi: 10.1038/s41591-018-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tauchnitz N., Kurzius F., Rupp H., Schmidt G., Hauser B., Schrödter M., Meissner R. Assessment of pesticide inputs into surface waters by agricultural and urban sources - a case study in the Querne/Weida catchment, central Germany. Environ. Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115186. [DOI] [PubMed] [Google Scholar]
- 59.Taxvig C., Vinggaard A.M., Hass U., Axelstad M., Metzdorff S., Nellemann C. Endocrine-disrupting properties in vivo of widely used azole fungicides. Int. J. Androl. 2008;31:170–177. doi: 10.1111/j.1365-2605.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- 60.Taxvig C., Hass U., Axelstad M., Dalgaard M., Boberg J., Andeasen H.R., Vinggaard A.M. Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol. Sci. 2007;100:464–473. doi: 10.1093/toxsci/kfm227. [DOI] [PubMed] [Google Scholar]
- 61.The Lancet Regional Health – Europe Polycystic ovary syndrome: What more can be done for patients? Lancet Reg. Health - Eur. 2022;21 doi: 10.1016/j.lanepe.2022.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Huang R., Li X., Zhu Q., Liao Y., Tao T., Kang X., Liu W., Li S., Sun Y. High concentration of chemerin caused by ovarian hyperandrogenism may lead to poor IVF outcome in polycystic ovary syndrome: a pilot study. Gynecol. Endocrinol. 2019;35:1072–1077. doi: 10.1080/09513590.2019.1622087. [DOI] [PubMed] [Google Scholar]
- 63.WHO . World Health Organization; 2021. Obesity and Overweight. [Google Scholar]
- 64.Winckel A., Ollagnier S., Gabillard S. Managing groundwater resources using a national reference database: the French ADES concept. SN Appl. Sci. 2022;4 [Google Scholar]
- 65.WMA General Assembly (2022). WMA - The World Medical Association-WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. World Medical Association.
- 66.Wu L.L.-Y., Dunning K.R., Yang X., Russell D.L., Lane M., Norman R.J., Robker R.L. High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- 67.Y. Nishi T. Yanase Y.-M. Mu K. Oba I. Ichino M. Saito M. Nomura C. Mukasa T. Okabe K. Goto et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor 142 2001 9. [DOI] [PubMed]
- 68.Yakin K., Hela F., Oktem O. Progesterone signaling in the regulation of luteal steroidogenesis. Mol. Hum. Reprod. 2023;29 doi: 10.1093/molehr/gaad022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yong E.L., Baird D.T., Yates R., Reichert L.E., Hillier S.G. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J. Clin. Endocrinol. Metab. 1992;74:842–849. doi: 10.1210/jcem.74.4.1548349. [DOI] [PubMed] [Google Scholar]
- 70.Zarn J.A., Brüschweiler B.J., Schlatter J.R. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ. Health Perspect. 2003;111:255–261. doi: 10.1289/ehp.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng X., Xie Y., Liu Y., Long S., Mo Z. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta. 2020;502:214–221. doi: 10.1016/j.cca.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Zhan W., Tang W., Shen X., Xu H., Zhang J. Exposure to bisphenol A and its analogs and polycystic ovarian syndrome in women of childbearing age: A multicenter case-control study. Chemosphere. 2023;313 doi: 10.1016/j.chemosphere.2022.137463. [DOI] [PubMed] [Google Scholar]
- 73.Zhang H., Lu S., Xu R., Tang Y., Liu J., Li C., Wei J., Yao R., Zhao X., Wei Q., et al. Mechanisms of estradiol-induced EGF-like factor expression and oocyte maturation via G protein-coupled estrogen receptor. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa190. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, J.-F., Yu, C.-M., YAN, L.-L. and Ma, J.-L. (2018). Effect of anti-mullerian hormone on stem cell factor in serum, follicular fluid and ovarian granular cells of polycystic ovarian syndrome patients. 22, 7877–7882. [DOI] [PubMed]
- 75.Zhang Z., Gao B., He Z., Li L., Shi H., Wang M. Enantioselective metabolism of four chiral triazole fungicides in rat liver microsomes. Chemosphere. 2019;224:77–84. doi: 10.1016/j.chemosphere.2019.02.119. [DOI] [PubMed] [Google Scholar]
- 76.Zhou D., Li S., Li W., Yin T., Xu W., Zhang J., Yang J. Increased expression of PGRN protein in follicular fluid and mRNA in granulosa cells in overweight patients with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;218:106–112. doi: 10.1016/j.ejogrb.2017.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.