Summary
As a developing region, Latin America faces unique cancer control and prevention challenges, which are intensified when considering rare cancers, including sarcomas. Sarcomas are a group of malignancies that arise in the connective tissues of the body—such as muscle, fat, nerves, blood vessels, and bones—accounting for a diverse range of tumours that, although rare, require specialized attention. Sarcoma care and research in Latin America require a comprehensive approach that includes deeper epidemiologic knowledge, diagnostic capacity building, access to innovative treatments, increased patient advocacy, and strengthening of clinical research capacity. This article will review current challenges and opportunities for treating patients with sarcoma in Latin America and outline a pathway toward improvement for regional collaborative groups.
Keywords: Sarcoma, Global health
Introduction
Cancer is a significant public health issue in Latin America and the Caribbean, with an estimated 1.5 million new cases and 710,000 deaths annually.1 Providing excellence in oncologic care is challenging in this region of primarily low- and middle-income countries due to the segmentation of health services, difficulties in accessibility, and social inequality.2 Although the context is diverse in each of the 33 countries within the region, obstacles exceedingly eclipse national borders.2 The COVID-19 pandemic has further contributed to these adversities due to increasing stress on healthcare systems and the resulting global socioeconomic crisis.3
Sarcomas are a heterogeneous group of mesenchymal malignancies of the soft tissue and bone, represented by more than 80 subtypes, the majority of which are categorized as rare cancers based on their incidence.4 Rare cancers—defined as those with an incidence of less than 6 per 100,000 persons per year—can be particularly challenging to diagnose and treat, therefore depending heavily on well-structured networks and timely referral to high-volume centres.5, 6, 7 Consequently, Latin American patients diagnosed with sarcomas can often face more significant barriers to optimal medical care and access to research than those faced by patients with more prevalent malignancies.8 In both Europe and the United States, about 20–25% of all new cancer cases are attributed to rare cancers.9,10 This underscores the importance of understanding the prevalence of these cancers in Latin America to better plan for patient care for a significant proportion of patients diagnosed with malignancies.
It's pertinent to note that while patient care is often organized within countries, a regional-level network plays a critical role in the realm of research. Moreover, as noted by the “Rare Cancer Agenda 2030: Ten Recommendations from the EU Joint Action on Rare Cancers”, “international distant collaboration is all the more crucial” in rare cancers.11 Therefore, a collective regional approach can foster collaboration and resource sharing among nations, which might be especially beneficial for addressing the unique challenges posed by sarcomas in our region.6
Sarcoma care and research require a comprehensive approach that includes diagnostic capacity building, access to innovative treatments, patient advocacy and education, and research. Collaborative efforts among governments, healthcare systems, researchers, and patient advocates are essential to improving outcomes and reducing the region’s sarcoma burden. This article will review current challenges and opportunities for treating patients with sarcoma in Latin America. Further, we aim to pave a pathway forward by offering strategic recommendations for progress, which can assist collaborative groups as they navigate and address these challenges. Ultimately, by addressing the challenges and solutions specific to sarcomas, we can also offer a framework for managing other rare cancers in Latin America.
Search strategy and selection criteria.
An exploration was conducted into "sarcoma in Latin America" and "rare cancer in Latin America" through a review of PubMed, using search terms that included "rare cancer" and "rare tumours", while also considering "clinical research", "clinical practice guidelines", "patients", and "patient advocacy groups", focusing on publications from January 1, 1990 to October 14, 2023. Additionally, websites of recognized research consortia, national sarcoma groups, expert centre networks (such as the French National Cancer Institute and the Scandinavian Sarcoma Group), and networks devoted to rare cancers were examined, including patient advocacy groups active in the rare tumour domain. The review was restricted to documents and web pages that were, at minimum, partly published in English. Authors RCP, BBLD, and VPC performed the initial search and wrote the first draft of the manuscript, which was later reviewed by all co-authors, who included additional references and specific sections based on their professional experience and expertise. The final list of references was selected based on their innovativeness and pertinence to the overarching theme of this manuscript.
Cancer and sarcoma epidemiology in Latin America
Cancer is a growing concern in Latin America due to the increasing incidence of various types of malignancies throughout the region.12,13 Latin America accounts for approximately 8% of new cancer cases worldwide, and cancer has already become the primary reason for premature mortality in nearly 50% of the countries within Latin America and the Caribbean.12 Cancer incidence is expected to increase in the coming years—rising from 1.5 million new cases in 2020 to over 2.4 million by 2040, as a result of distinct factors, including demographic transition.12 Therefore, significant obstacles must be faced in developing well-structured cancer treatment and prevention services for an area where one-third of the population is impoverished.12, 13, 14
Available GLOBOCAN data indicate that, in line with the worldwide cancer pattern, the most frequently identified cancers in both South America and the Caribbean include prostate (15% of total cancers for both sexes), breast (14%), colorectal, lung (7%), and stomach (5%).1,12 Meanwhile, lung cancer remains the primary contributor to cancer-related mortality for both men and women, accounting for 12% of such deaths.1,12 Notably, the incidence of sarcoma is not registered by GLOBOCAN, except for Kaposi’s sarcoma, which accounts for 0.2% (n = 34,270) of all new cancer cases and 0.2% (n = 15,086) of cancer-related deaths worldwide, and 0.2% (n = 2856) of all new cancer cases and 0.1% (n = 571) of cancer-related deaths in Latin America.1,12
As a developing region, Latin America faces unique cancer control and prevention challenges, including limited resources, insufficient infrastructure, and unequal access to healthcare services.13,15 These factors exacerbate the already complex issue of cancer in the region, necessitating a comprehensive understanding of cancer epidemiology to devise effective strategies for prevention, early detection, and treatment.
However, a significant obstacle in grasping cancer epidemiology within Latin America is the absence of extensive, reliable cancer registries.15 Most countries in this region either do not have population-based registries or possess restricted scope and quality registries.15 High-quality cancer registration extends to a mere 7% of the population in Latin America, whereas the coverage reaches 83% in North America and 60% in Europe.15, 16, 17 This limitation obstructs the precise evaluation of cancer occurrence, frequency, and death rates, complicating the development of tailored approaches for cancer management. Enhancing the standard of cancer registries and data compilation is crucial for a more comprehensive understanding of the cancer burden in Latin America, which will help allocate resources and focus on addressing the most urgent requirements.18
Accordingly, the majority of research on sarcoma epidemiology in Latin America concentrates on distinct cancer forms and frequently relies on data from one institution or hospital-based cancer registries. Examples include research by Dr DelaGarza-Montano examining the epidemiology of musculoskeletal tumours at Mexico City's National Rehabilitation Institute, by Dr Lopes David evaluating sarcoma burden and pathways in hospital-based registries in Brazil, by Dr Chávez describing patients with soft tissue sarcoma treated at the National Institute of Neoplastic Diseases of Peru, and by Dr Figueiredo evaluating public databases in Brazil, among others.8,19, 20, 21 The single publication addressing the incidence of soft tissue sarcomas in the region based on a Population-based Cancer Registry is from the City of São Paulo, Brazil, in which it was estimated to be 3.36/100.000 habitants.21
The well-known database relying on population-based cancer registries Cancer Incidence in Five Continents (CI5) does not account specifically for sarcoma cases, although it does provide data on “bone” and “connective and soft tissue” tumours based on ICD classification of topography, with information from 455 population-based cancer registries in 70 countries for cancers diagnosed from 2013 to 2017.22 The age-standardized incidence of soft tissue malignancies in Latin America and the Caribbean ranges between 0.2/100,000 (females in Jau, Brazil) and 2.9/100,000 (males in Cali, Colombia). For bone tumours, age-standardized incidence ranged from 0.1/100,000 (females in Guadalupe, French territory in the Caribbean) and 2.1/100,000 (males in Cali, Colombia).22 Of note, the use of topography for classification can lead to an underestimation of the incidence of sarcoma, considering that a significant proportion of patients have visceral primary tumours, which can be classified under a different ICD code. For comparison, an analysis from France using data from a pathology review network demonstrates an incidence of sarcomas of 7.1/100,000.23
The elegant analysis by Figueiredo and colleagues highlights the paucity and poor quality of available data in Brazil.21 In this report, sarcomas with an unspecified location or identified as invasive but lacking a precise location constituted 29.7% of the study group. Moreover, tumour grade and clinical staging sub-registrations were noted, with grade X (undefined) and indeterminate clinical stage being the most frequently recorded for these variables, at 91.3% and 79.5% respectively.21 In addition, when a location was specified, the prevalent anatomic site was head and neck (13.6%), which is not in accordance with prior published data. The authors note that anatomical location was determined based on the ICD-10 codes and highlight that the codes for "Malignant neoplasm of connective and soft tissue of head, face and neck" (C49.0) and "Malignant neoplasm of other connective and soft tissue" (C49) are similar. Considering that the original registry required a 3-digit ICD-10 code, authors hypothesize that this might have led to increased notifications under the head-and-neck anatomical site due to errors in adding an extra 0 during the digitalization of official forms.21
Moreover, these registries often face several limitations, such as non-uniform local databases, restricted population coverage to specific areas, and the possibility of double registration for a single case if a patient migrates or receives care from multiple centres.8 Consequently, important parameters like incidence and mortality cannot be accurately estimated based on limited hospital-based registries.24
To better characterize each disease profile, it is crucial to establish comprehensive population-based registries in Latin America.15 High-quality cancer registries and data are essential for mapping rare cancers and understanding their clinical pathways. This information can be used to identify areas for improvement, better direct efforts, and allocate medical resources more effectively.
Challenges and opportunities for the diagnosis and management of sarcoma in Latin America
Patients diagnosed with rare cancers can face exceptional challenges, such as delayed and inaccurate diagnoses, limited access to specialized medical care and suitable treatments, uncertainty in clinical decision-making, inadequate resources—including cancer registries and tissue banks, and the possibility of neglect in drug development.5,7,25, 26, 27
Consequently, the five-year survival rates for rare cancers in Europe are lower than those of common cancers, with 49% and 63% survival rates, respectively.28 Patients with rare cancers may require treatment at specialized centres of expertise or networks, where multidisciplinary teams can concentrate on their unique tumour type, something less frequent for patients with common cancers.6,7,29 Accumulating evidence corroborates the benefit of centralized care specifically in sarcoma to improve survival outcomes.7,30, 31, 32, 33 For illustration, an elegant analysis by Blay and colleagues demonstrated a decrease in relapse and mortality risk of 35% for patients with sarcoma undergoing surgery in sarcoma reference centres.33
Obtaining a tissue diagnosis is crucial for the treatment of sarcoma. However, the likelihood of interpretational errors in regular practice remains high, ranging from 25 to 40 per cent.34,35 The factors that contribute to diagnostic inaccuracies include specialist subjectivity, lack of expertise, intra- and inter-observer variability, and inadequate tumour samples. Therefore, it is important to expand methodologies to improve diagnostic accuracy and minimize errors to guide effective treatment options. This should include ensuring that sufficient diagnostic samples are obtained and properly handled and that biorepository tissues are of the highest quality. For accurate diagnosis and classification of cancers, specialized sarcoma pathologists at reference sarcoma centres in Latin America should analyze high-quality specimens.36 Of note, centralized pathology review has been shown to decrease the expense of managing patients with sarcoma.37
The lack of specific clinical pathways for sarcomas in Latin America leaves clinicians with no clear approach to treating those patients, especially in geographically distant areas. The establishment of sarcoma-specialized networks could guarantee optimal diagnosis and care, irrespective of the location, thereby minimizing healthcare-related personal and social costs.7,33,37 Currently, multidisciplinary team meetings for sarcoma are not mandatory, and there are no established rules governing their implementation in Latin America.
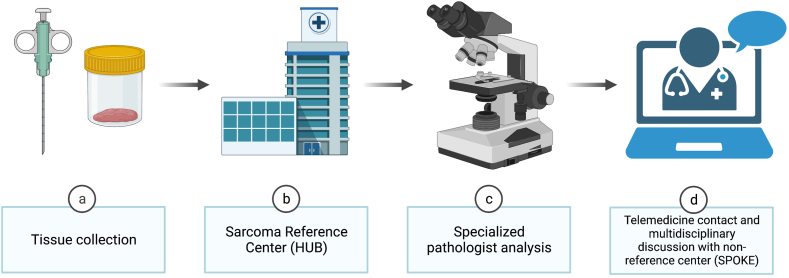
An appropriate structure for these networks is the "hub-and-spoke" model (Fig. 1).6 In this model, collaborating facilities ("spokes") can tap into the expertise of specialized centres ("hubs"). While "hubs" deliver a comprehensive range of services, "spokes" provide a smaller selection but ensure widespread access.6 The "hub-and-spoke" model is highly efficient and effective in situations where there are a moderate to low number of cases requiring specialized knowledge and innovative solutions.6 Hubs should concentrate on offering top-tier expertise, precise clinical, pathological, and biological evaluations of the illness, and well-informed clinical decisions made by a dedicated multidisciplinary team. Furthermore, hubs should encourage research into novel therapies, create patient registries and tissue banks, and direct individuals to available clinical trials. Spokes, on the other hand, are facilities with substantial experience in treating various oncology patients, providing a limited array of services, and are situated as close to the patient's residence as possible. In France, for illustration, the management of sarcoma is orchestrated through a well-established network system (NetSarc), ensuring that all patients receive the highest standard of care.38 This system is twofold: one network is dedicated to clinical care, providing direct treatment and support to patients, while the other focuses on pathology, ensuring accurate diagnoses, and informing treatment strategies.38 For those not treated at reference centres, their cases are meticulously reviewed at multidisciplinary tumour boards.38 Maintaining an ongoing dialogue and strong connection between the hub and spokes is crucial for the network's effectiveness.6 Multidisciplinary evaluations should be an integral part of every care plan from the outset, not just for determining the initial therapeutic strategy but also for subsequent needs when the disease status changes, such as during recurrence or progression. Nevertheless, in some instances, patients may need to travel to the reference center for interventions that the spoke cannot offer, including certain surgical procedures, advanced treatments, and participation in clinical trials.38
Fig. 1.
“Hub-and-spoke” model. This model efficiently extends specialized healthcare services from central "hubs" to more accessible "spokes," optimizing care for conditions that are uncommon and require specialized knowledge.
The relevance of establishing a sarcoma care network in Latin America is highlighted in a recent publication evaluating the clinical pathways of patients with sarcoma in Brazil.8 Patients received care in a total of 312 hospital units, of which only 51% were classified as High-Complexity Oncology Centers (CACON); additionally, only 10 hospitals were classified as a high-volume sarcoma centre, considering a threshold of 70 patients/year per three consecutive years.8 Potentially reflecting difficulties in diagnosis and access to treatment strategies, around 40% of patients waited for longer than 60 days to start therapy after diagnosis.8 Furthermore, in this analysis, treatment at CACON was not associated with decreased time from diagnosis to treatment initiation or with a decreased percentage of cases classified as sarcoma not otherwise specified (NOS) (15%–18%).8
Noteworthy, there isn't a universally accepted definition of a "sarcoma specialist centre" in Latin America, and the cut-off utilized in the above-mentioned manuscript was arbitrary. Defining a high-volume centre is challenging and shouldn't be based solely on the annual count of treated sarcomas. Tumour location and case-specific level of care must also be considered. In this sense, recent data from England highlight the importance of reference centres and tumour boards.39,40 In one study, authors analyzed data from 1878 patients with sarcoma extracted from NHS Digital's National Cancer Registration and Analysis Service, focusing on their treatment centers' volume and expertise.39 Results showed that 60% of patients underwent surgery—76% in specialized sarcoma centres, of which 51% were operated in high-volume specialist centres.39 Survival was highest in high-volume centres, with a significant difference noted in the overall survival rates compared to low-volume centres.39 A second analysis demonstrated high discordance among retroperitoneal sarcoma multidisciplinary team meetings in Great Britain. Notably, based on the specific facility visited, 12 out of 21 patients' conditions were evaluated as either suitable or unsuitable for surgery, while another 10 out of 21 faced a crossroads between receiving curative or palliative-intent treatments.40 Therefore, these data underscore the critical necessity for establishing high-volume centres equipped with multidisciplinary tumour boards to ensure consistent and optimal patient care. Furthermore, continuous follow-up on tumour board recommendations and fostering concordance between high-volume centres is paramount for achieving the best possible outcomes for sarcoma patients.
The Sarcoma European Latin American Network project is a recent initiative that aims to enhance sarcoma treatment through the creation of networks.41,42 Its objective is to improve diagnostic and prognostic outcomes for adult sarcoma patients by establishing networks for pathological diagnoses, multidisciplinary panels, and international registry-based and intercontinental sarcoma biobanks that support clinical and translational research. The project began in 2019 and has since achieved numerous milestones.41,42 As a direct consequence of the integration of professionals by SELNET, local sarcoma groups were established in Brazil (Grupo Brasileiro de Sarcomas—GBS) and Argentina (Grupo Argentino de Sarcomas de Partes Blandas y Oseos—GASPAR), connecting professionals across these countries and on a continental level, ultimately leading to the formation of the Latin American Cooperative Oncology Group (LACOG) Sarcoma Group. Moreover, the first Latin American guidelines for sarcomas were published, and a consensus criterion for sarcoma reference centres was discussed.41,42 Finally, transatlantic tumour boards and research programs have also become a reality, emphasizing the need for and feasibility of international networks for sarcomas and other rare cancers. Ensuring the persistence of these efforts, especially after projects like SELNET conclude, requires in-depth evaluation.
Clinical trials for sarcoma in Latin America
Developing clinical trials for cancer, particularly interventional clinical trials for rare cancers—including sarcomas—is challenging in Latin America.43,44 The region faces numerous obstacles that hinder the successful execution of these trials, including limited infrastructure, inadequate funding, and a lack of regulatory harmonization.43,44 The limited number of clinical trials conducted in this region reflects these difficulties. In 2022, out of 75,084 cancer treatment trials registered on clinicaltrials.gov, only 4167 trials (5.5%) were conducted in Latin American and Caribbean nations.43 Most of these trials are phases 3–4 (69%), and the vast majority are industry-sponsored (83%).43
Inadequate funding poses a significant obstacle to developing clinical trials for cancer in Latin America. The region suffers from a lack of investment in research and development, leading to insufficient financial support for both public and private institutions conducting clinical trials. In 2020, Latin American countries allocated 0.59% of their GDP to research and development, with Brazil spending 1.15%, Argentina 0.54%, Mexico 0.30%, Chile 0.33%, Colombia 0.29%, and Peru 0.17%.45 This expenditure is 3–4 times lower than that of high-income nations - for comparison, Japan, the USA, and Germany spent 3.27%, 3.47%, and 3.13%, respectively.45 In 2009–2010, a significant percentage of medical oncologists in Latin America and the Caribbean, approximately 6.1%, reported being the primary sponsor of their research proposals.44 One study found that Mexican physicians had the highest rate of self-support, with up to 41.2% indicating that they used their own resources to support their projects, while 25% of Peruvian and 22% of Colombian doctors reported the same.44 The high cost associated with interventional clinical trials for rare cancers further exacerbates this problem, as these trials often require advanced technology, cutting-edge treatments, and specialized care. Limited funding often results in fewer trials, hindering research progress and the development of novel therapies for sarcomas, and restricting patients’ access to potentially life-saving medications/technologies.
Another significant challenge in Latin America is the limited healthcare infrastructure, which often restricts access to essential resources and specialized care.44 Many countries in the region lack the necessary facilities, equipment, and trained personnel to conduct complex clinical trials. The reduced quantity of researchers pursuing academic careers in the field of science in Latin American nations is also a contributing factor.46 Data from the Organization for Economic Co-operation and Development from 2021 shows that European Union Countries have 9.4 researchers per 1000 employees.47 In contrast, Argentina has 3.1 and Chile 1.3.47 This is particularly problematic for rare cancers, which often demand highly specialized expertise, resources, and care integration. This often limited infrastructure and personnel impacts the quality of the trials and potentially reduces the number of participants.
The lack of regulatory harmonization across Latin American countries is another obstacle to the development of clinical trials for cancer.48 Each country has its own regulatory framework and requirements, which can create substantial challenges when conducting multi-country clinical trials. These inconsistencies can lead to delays in trial approvals, increased costs, and potential inconsistencies in implementing clinical trial protocols. One analysis evaluating the time to set up a global phase III oncology trial demonstrated a considerably longer time for research approval in South America (median: 236 days) compared to Europe (52 days), North America (26 days), and the Asia–Pacific region (62 days).49 This is especially problematic for sarcomas—and rare cancers in general—as multi-country collaborations are often necessary to achieve the required sample size.
The limited availability of patient registries and comprehensive epidemiological data in Latin America further complicates the development of clinical trials for sarcomas. Accurate and accessible data is essential to identify patient populations and design appropriate trial protocols. Without these resources, it is difficult to identify a target group or tailor therapies and interventions. This is particularly problematic in rare cancers—including sarcomas—as it limits the capability of predicting enrollment and limits the capability of conducting prospective clinical trials in the region.
In the last decades, international collaborative groups have focused on expanding worldwide clinical trial options for rare cancers. Launched in 2011, the International Rare Cancers Initiative (IRCI) represents a collaborative effort among the National Institute for Health Research Cancer Research Network, Cancer Research UK, the National Cancer Institute, and the European Organization for Research and Treatment of Cancer.25 This initiative aims to expedite the creation of international clinical trials for patients with rare cancers, thus accelerating the development of novel treatments for these individuals.
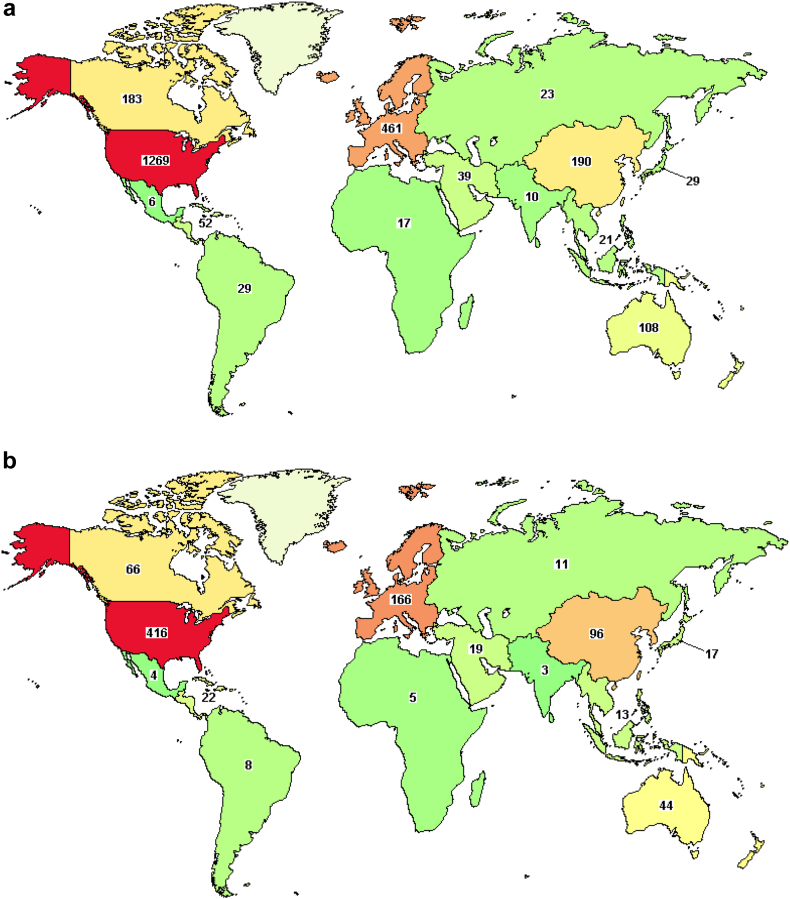
Despite these international partnerships, in accordance with the low percentage of oncology trials that are overall available in Latin America, trial options for patients with sarcoma are low in the region. A survey of interventional studies, regardless of status, performed on April 2nd, 2023 on clinicaltrials.gov, demonstrated that only 29 of a total of 1941 trials were available in South America, and only 6 in Mexico (Fig. 2a). Considering only trials that are recruiting or active, 8 out of 632 are available in South America, and 4 in Mexico (Fig. 2b). Addressing these challenges is crucial to improving the quality of cancer care and advancing the development of new therapies for patients with sarcoma in Latin America.
Fig. 2.
Number of oncology trials for sarcoma available in Latin America. A search conducted on April 2nd, 2023, on clinicaltrials.gov for interventional sarcoma studies showed that out of 1941 trials, only 29 are accessible in South America and 6 in Mexico (a). When considering only the actively recruiting or ongoing trials, 8 out of a total of 632 are in South America and 4 in Mexico (b).
Recommendations for progress
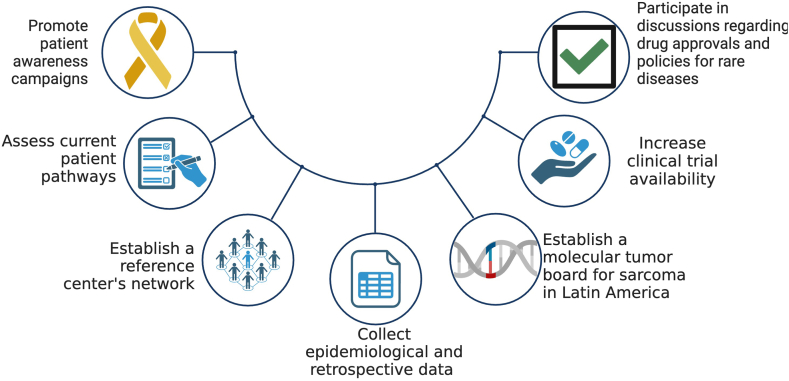
Addressing the complex challenges posed by sarcoma requires a collaborative approach that extends beyond the scope of a single collaborative group. While organizations like LACOG can play a pivotal role in improving sarcoma care in our region—conducting clinical trials and convening dedicated specialists—a broader, more inclusive strategy is needed. As we delve into this multifaceted landscape, we call for the collective participation of various stakeholders across the region, each contributing with their unique capacities to a comprehensive, shared response. Our recommendations for progress are rooted in this principle of collaboration and are summarized below (Fig. 3).
Fig. 3.
LACOG Sarcoma group proposed recommendations for progress. Tackling sarcoma effectively demands a unified, region-wide effort that surpasses the capacity of individual groups. A comprehensive strategy involving diverse regional stakeholders is essential for a coordinated response to the disease.
Promote patient awareness campaigns
Patient awareness campaigns are key as part of a strategy to share information with the general population about signs and symptoms which could lead to a diagnosis of sarcoma. Ultimately, this could make individuals seek timely medical attention that can translate into earlier diagnosis. Moreover, the drive to enhance sarcoma care and research must highlight the viewpoints of patients. Therefore, patient involvement in setting the agenda for clinical studies and service delivery is of utmost importance.50 Recent data from the Netherlands corroborate this need, demonstrating that patients diagnosed with rare cancers, for example, were more willing to travel as long as necessary to receive specialized care than common cancer patients.29
In addition, such campaigns are tools to create a supportive environment for patients with a diagnosis of sarcoma, encouraging a sense of community and solidarity. Patient awareness campaigns can also empower individuals to be in charge of their health and participate actively in their treatment journey. Encouraging self-advocacy and informed decision-making can result in better adherence to treatment plans and improved communication between patients and healthcare providers. Previous data has highlighted the importance of social media platforms for achieving patient engagement.50,51 For illustration, utilizing hashtags in campaigns on platforms such as X (formerly Twitter) can serve as a potent method to raise awareness of interdisciplinary and enable knowledge-sharing on a regional scale.52
Revise current clinical pathways
To optimize the care of patients with sarcoma in Latin America, it is essential to revise the current clinical pathways, which, as mentioned above, are not currently well-characterized.8 This evaluation is key for stakeholders to identify areas that require additional resources and training, thus optimizing resource allocation. Ultimately, this can contribute to improved patient outcomes by creating a more robust oncology infrastructure for the care of individuals with a diagnosis of sarcoma in our region.
One simple step to evaluate current treatment pathways is to elaborate a comprehensive questionnaire to explore various aspects of sarcoma care in hospitals within a particular collaborative group. This questionnaire should discuss local multidisciplinary team coordination and available treatment options, among other information, and may serve as a valuable tool in evaluating and improving sarcoma care, fostering advancements in the field, and facilitating impactful changes in healthcare practices.
Establishment of a Reference Center Network
Creating a reference centre network for the care of sarcoma patients in Latin America is a vital step in enhancing the standard of care for this population.7,30 This network can facilitate the sharing of knowledge, resources, and best practices by connecting leading institutions and experts in the field. Formal recognition of sarcoma reference centres in Latin America is fundamental to establishing this network and thus optimizing care and resources. The NETSARC network established in France has demonstrated the transformational role of centralized care in the outcomes of patients with sarcoma.31,33
Collection of epidemiological and retrospective clinical data
In addition to assessing current clinical pathways, improved resource allocation can also be achieved as a result of a deeper understanding of the occurrence, prevalence, and overall impact of sarcomas in Latin America. This knowledge can be obtained through high-quality real-world data compilation and the establishment of rare cancer registries.
In addition, retrospective clinical data can gather valuable insights into the effectiveness of different treatment approaches in real-world scenarios. This is of particular importance given the under-representation of Latin American patients in the pivotal clinical trials that led to the approval of several medications that are currently part of the standard of care for sarcomas. Moreover, data collected from well-established, high-volume centres can be particularly valuable in managing ultra-rare subtypes of sarcomas.53,54
Establishment of molecular tumour boards for sarcoma in Latin America
The implementation of molecular tumour boards dedicated to sarcoma can greatly elevate the quality of care offered to patients in Latin America.55 These tumour boards have the potential to bring together a multidisciplinary team of experts specialized in surgical oncology, medical oncology, radiation oncology, pathology, and genomics. The molecular tumour board's interdisciplinary approach allows for a comprehensive assessment of the patient's condition and facilitates the selection of the most suitable and effective treatment options available. The MASTER trial results suggest the benefit of comprehensive genomic and transcriptomic profiling of rare cancers and subsequent discussion in molecular tumour boards for efficient diagnosis and personalized treatments.56 In addition, data from the Tata Memorial Hospital, a tertiary cancer centre in India, highlight the feasibility of molecular tumour boards in low- and middle-income countries.57
Increase clinical trial availability
Increasing the availability of clinical trials for sarcoma patients in Latin America is crucial for advancing patient treatment options, and enabling access to promising novel therapies. Inclusion in clinical trials allows patients to benefit from innovative treatments while contributing to the global understanding of sarcoma and developing new therapeutic strategies. To enhance clinical trial availability, it is essential to strengthen collaborations between research institutions, healthcare providers, pharmaceutical companies and funding agencies. Optimization of the regulatory process is also fundamental to attracting protocols to the region.43
Participate in discussions regarding drug approvals and policies for rare diseases
Participation of researchers and patient advocates in discussions regarding drug approvals and policies for rare diseases, such as sarcoma, is vital for ensuring that patients in Latin America have access to the latest and most effective treatments. Flexibilization of trial design and criteria for new technologies approvals should be individualized for each context, not in the model of common cancers, as a one-size-fits-all. The involvement of regional researchers and patient representatives in these discussions can help raise awareness of the unmet needs of sarcoma patients in Latin America and advocate for the prioritization of rare disease research and drug development. Collaborative efforts can also help to streamline regulatory processes and foster partnerships between public and private entities, ensuring that promising new therapies become available to patients in a timely and accessible manner.
Additionally, active participation in policy discussions can contribute to developing compassionate use programs, expanded access initiatives, and orphan drug incentives that can ultimately improve the lives of patients diagnosed with sarcoma across the region.
Conclusion
In conclusion, the LACOG Sarcoma Group recognizes the limited availability of clinical trials and the significant challenges faced by patients with sarcoma in Latin America. Patients face critical obstacles such as delayed diagnosis, inadequate access to specialized care, and socioeconomic disparities. To address these issues, we propose an ambitious set of recommendations for progress that emphasizes strengthening regional collaboration, expanding clinical trial opportunities, and fostering the development of multidisciplinary sarcoma care teams.
Contributors
Conceptualization: RCP and RJG; visualization: RCP; writing—original draft: RCP, BBLD, and VCP; writing—review & editing: RCP, BBLD, VPC, RRM, CALM, MLGD, JCHV, MLZ, CLCM, MC, RS, and RJG; investigation: RCP, BBLD, VPC, RRM, CALM, MLGD, JCHV, MLZ, CLCM, MC, RS, and RJG; supervision: RJG.
Editor’s note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
RCP: has received consulting fees from Bayer, Servier, Astellas for participation in advisory boards. He also received payment or honoraria for lectures, presentations, speakers bureaus for Bayer, Servier, Pfizer, Amgen, BMS, Merck, and Knight Therapeutics. He also received Support for attending meetings and/or travel from Astrazeneca. In addition, he serves as Chair of the Latin American Cooperative Oncology Group (LACOG) Sarcoma group and Member of the Sociedade Brasileira de Oncologia Clínica (SBOC) Sarcoma Committee. BBLD: has received Consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from DECIPHERA PHARMACEUTICALS. She also reports support for attending meetings and/or travel from PTC THERAPEUTICS and participation on a Data Safety Monitoring Board or Advisory Board from BOEHRINGER INGELHEIM. She also reports Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from: ESMO GLOBAL POLICY COMMITTEE; BRAZILIAN SOCIETY OF MEDICAL ONCOLOGY—SARCOMA BRANCH; EUROPEAN CANCER ORGANIZATION—YOUNG PROFESSIONALS GROUP. VP: none. RRM: has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Knight Therapeutics. He also reports Participation on a Data Safety Monitoring Board or Advisory Board from Boehringer (Advisory role). In addition, he is the President/Founder of Grupo Brasileiro de Sarcomas. CALM: has received Consuling fees from BOEHRINGER INGELHEIM, SERVIER, and ADDIUM. He also reports Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from SERVIER and ADDIUM (SPEAKER HONORARIA). In addition, he reports support for attending meetings and/or travel from BOEHRINGER INGELHEIM, and participation on a Data Safety Monitoring Board or Advisory Board from BOEHRINGER INGELHEIM, SERVIER and ADDIUM. MLGD: has received grants or contracts from any entity from ASCO-PFIZER GRANT (PRINCIPAL INVESTIGATOR). JCHV: none. MLZ: has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, Astra Zeneca, Bayer, Biotoscana, BMS, Boheringer, Eli Lilly, Janssen, MSD, Merck-Serono, Novartis, Roche, Sanofi. He also reports support for attending meetings and/or travel from Astra Zeneca, Bayer, Biotoscana, BMS, Boheringer, Eli Lilly, Janssen, MSD, Merck-Serono. CLCM: has received consulting fees, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from MSD and ASTRAZENECA, reports Participation on a Data Safety Monitoring Board or Advisory Board from MSD and ASTRAZENECA, and has received consulting fees from MSD and ASTRAZENECA. He also reports Support for attending meetings and/or travel from Astrazeneca. MC: has received Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, BMS, elea phoenix, MSD. He also reports Support for attending meetings and/or travel from Bayer. RS: none. RJG: none.
Acknowledgements
The authors acknowledge the use of ChatGPT, a large language model trained by OpenAI, based on the GPT-3.5 and GPT-4 architectures, to assist us in writing this article, with a specific focus on improving readability and language.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Werutsky G., Barrios C.H., Cardona A.F., et al. Perspectives on emerging technologies, personalised medicine, and clinical research for cancer control in Latin America and the Caribbean. Lancet Oncol. 2021;22:e488–e500. doi: 10.1016/S1470-2045(21)00523-4. [DOI] [PubMed] [Google Scholar]
- 3.Araujo S.E.A., Leal A., Centrone A.F.Y., et al. Impact of COVID-19 pandemic on care of oncological patients: experience of a cancer center in a Latin American pandemic epicenter. Einstein (Sao Paulo) 2020;19 doi: 10.31744/einstein_journal/2021AO6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Pinieux G., Karanian M., Le Loarer F., et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trama A., Marcos-Gragera R., Sánchez Pérez M.J., et al. Data quality in rare cancers registration: the report of the RARECARE data quality study. Tumori. 2017;103:22–32. doi: 10.5301/tj.5000559. [DOI] [PubMed] [Google Scholar]
- 6.Frezza A.M., Trama A., Blay J.Y., Casali P.G. Networking in rare cancers: what was done, what’s next. Eur J Surg Oncol. 2019;45:16–18. doi: 10.1016/j.ejso.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Blay J.Y., Casali P., Bouvier C., et al. European Reference Network for rare adult solid cancers, statement and integration to health care systems of member states: a position paper of the ERN EURACAN. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David B.B.L., Abdon Mello C., Santos Thuler L.C., de Melo A.C. Overview of adult sarcoma burden and clinical pathways in Brazil. JCO Glob Oncol. 2022;8 doi: 10.1200/GO.21.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatta G., van der Zwan J.M., Casali P.G., et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–2511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 10.DeSantis C.E., Kramer J.L., Jemal A. The burden of rare cancers in the United States. CA Cancer J Clin. 2017;67:261–272. doi: 10.3322/caac.21400. [DOI] [PubMed] [Google Scholar]
- 11.JARC – Joint Action on Rare Cancers. Rare cancer agenda 2030: ten recommendations from the EU Joint Action on Rare Cancers. 2019 [Google Scholar]
- 12.Piñeros M., Laversanne M., Barrios E., et al. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg Health Am. 2022;13 doi: 10.1016/j.lana.2022.100294. None. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrios C.H., Werutsky G., Mohar A., et al. Cancer control in Latin America and the Caribbean: recent advances and opportunities to move forward. Lancet Oncol. 2021;22:e474–e487. doi: 10.1016/S1470-2045(21)00492-7. [DOI] [PubMed] [Google Scholar]
- 14.Cazap E., de Almeida L.M., Arrossi S., et al. Latin America and the Caribbean code against cancer: developing evidence-based recommendations to reduce the risk of cancer in Latin America and the Caribbean. J Glob Oncol. 2019;5:1–3. doi: 10.1200/JGO.19.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piñeros M., Abriata M.G., Mery L., Bray F. Cancer registration for cancer control in Latin America: a status and progress report. Rev Panam Salud Publica. 2017;41 doi: 10.26633/RPSP.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siesling S., Louwman W.J., Kwast A., et al. Uses of cancer registries for public health and clinical research in Europe: results of the European Network of Cancer registries survey among 161 population-based cancer registries during 2010-2012. Eur J Cancer. 2015;51:1039–1049. doi: 10.1016/j.ejca.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Forsea A.M. Cancer registries in Europe-going forward is the only option. Ecancermedicalscience. 2016;10:641. doi: 10.3332/ecancer.2016.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werutsky G., Gössling G., Pellegrini R.A., Ampuero G.A.S., Rebelatto T. Socioeconomic impact of cancer in Latin America and the Caribbean. Arch Med Res. 2022;53:818–825. doi: 10.1016/j.arcmed.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 19.DelaGarza-Montano P., Estrada-Villasenor E., Dominguez Rubio R., et al. Epidemiological aspects of osteosarcoma, giant cell tumor and chondrosarcoma musculoskeletal tumors--experience of the National Rehabilitation Institute, Mexico City. Asian Pac J Cancer Prev. 2015;16:6451–6455. doi: 10.7314/apjcp.2015.16.15.6451. [DOI] [PubMed] [Google Scholar]
- 20.Chávez M., Ziegler G., Cotrina J., Galarreta J., de la Cruz M., Mantilla R. Current situation of soft tissue sarcomas: registry of a Latin American cancer institute. Cir Esp. 2019;97:203–212. doi: 10.1016/j.ciresp.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 21.de Figueiredo L.O., Júnior A.A.G., de Assis Acurcio F., et al. A demographic and clinical panorama of a sixteen-year cohort of soft tissue sarcoma patients in Brazil. Sci Rep. 2021;11 doi: 10.1038/s41598-021-02032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray F. International Agency for Research on Cancer; Lyon: 2023. Cancer incidence in five continents, Vol. XII (IARC CancerBase No. 19) [Google Scholar]
- 23.Ducimetière F., Lurkin A., Ranchère-Vince D., et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripathee S., MacLennan S.J., Poobalan A., Omar M.I., Guntupalli A.M. The role of Hospital-Based Cancer Registries (HBCRs) as information systems in the delivery of evidence-based integrated cancer care: a scoping review. Health Systems. 2023:1–15. doi: 10.1080/20476965.2023.2216749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keat N., Law K., Seymour M., et al. International rare cancers initiative. Lancet Oncol. 2013;14:109–110. doi: 10.1016/S1470-2045(12)70570-3. [DOI] [PubMed] [Google Scholar]
- 26.Casali P.G. Do rare cancers deserve specific strategies for cancer research? Lancet Oncol. 2010;11:506–507. doi: 10.1016/S1470-2045(10)70099-1. [DOI] [PubMed] [Google Scholar]
- 27.Gatta G., Trama A., Capocaccia R., RARECARENet Working Group Epidemiology of rare cancers and inequalities in oncologic outcomes. Eur J Surg Oncol. 2019;45:3–11. doi: 10.1016/j.ejso.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Gatta G., Capocaccia R., Botta L., et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18:1022–1039. doi: 10.1016/S1470-2045(17)30445-X. [DOI] [PubMed] [Google Scholar]
- 29.de Heus E., Engelen V., Dingemans I., et al. Differences in health care experiences between rare cancer and common cancer patients: results from a national cross-sectional survey. Orphanet J Rare Dis. 2021;16:249. doi: 10.1186/s13023-021-01886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loong H.H., Blay J.Y., Munhoz R.R. International collaborations and regional challenges in providing specialist multidisciplinary sarcoma care. Am Soc Clin Oncol Educ Book. 2019;39:616–623. doi: 10.1200/EDBK_239131. [DOI] [PubMed] [Google Scholar]
- 31.Derbel O., Heudel P.E., Cropet C., et al. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive population-based cohort) PLoS One. 2017;12 doi: 10.1371/journal.pone.0158406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heudel P.E., Cousin P., Lurkin A., et al. Territorial inequalities in management and conformity to clinical guidelines for sarcoma patients: an exhaustive population-based cohort analysis in the Rhône-Alpes region. Int J Clin Oncol. 2014;19:744–752. doi: 10.1007/s10147-013-0601-2. [DOI] [PubMed] [Google Scholar]
- 33.Blay J.-Y., Honoré C., Stoeckle E., et al. Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol. 2019;30:1143–1153. doi: 10.1093/annonc/mdz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray-Coquard I., Montesco M.C., Coindre J.M., et al. Sarcoma: concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann Oncol. 2012;23:2442–2449. doi: 10.1093/annonc/mdr610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lurkin A., Ducimetière F., Vince D.R., et al. Epidemiological evaluation of concordance between initial diagnosis and central pathology review in a comprehensive and prospective series of sarcoma patients in the Rhone-Alpes region. BMC Cancer. 2010;10:150. doi: 10.1186/1471-2407-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer R.M.S.S.B., de Camargo V.P., Silva M.L.G., et al. Brazilian consensus on the diagnosis and treatment of extremities soft tissue sarcomas. J Surg Oncol. 2020;121:743–758. doi: 10.1002/jso.25847. [DOI] [PubMed] [Google Scholar]
- 37.Perrier L., Rascle P., Morelle M., et al. The cost-saving effect of centralized histological reviews with soft tissue and visceral sarcomas, GIST, and desmoid tumors: the experiences of the pathologists of the French Sarcoma Group. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honoré C., Méeus P., Stoeckle E., Bonvalot S. Soft tissue sarcoma in France in 2015: epidemiology, classification and organization of clinical care. J Visc Surg. 2015;152:223–230. doi: 10.1016/j.jviscsurg.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Tirotta F., Bacon A., Collins S., et al. Primary retroperitoneal sarcoma: a comparison of survival outcomes in specialist and non-specialist sarcoma centres. Eur J Cancer. 2023;188:20–28. doi: 10.1016/j.ejca.2023.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Tirotta F., Hodson J., Alcorn D., et al. Assessment of inter-centre agreement across multidisciplinary team meetings for patients with retroperitoneal sarcoma. Br J Surg. 2023;110:1189–1196. doi: 10.1093/bjs/znad157. [DOI] [PubMed] [Google Scholar]
- 41.Blay J.Y., Palmerini E., Bollard J., et al. SELNET clinical practice guidelines for bone sarcoma. Crit Rev Oncol Hematol. 2022;174 doi: 10.1016/j.critrevonc.2022.103685. [DOI] [PubMed] [Google Scholar]
- 42.Blay J.Y., Hindi N., Bollard J., et al. SELNET clinical practice guidelines for soft tissue sarcoma and GIST. Cancer Treat Rev. 2022;102 doi: 10.1016/j.ctrv.2021.102312. [DOI] [PubMed] [Google Scholar]
- 43.Gössling G., Rebelatto T.F., Villarreal-Garza C., et al. Current scenario of clinical cancer research in Latin America and the Caribbean. Curr Oncol. 2023;30:653–662. doi: 10.3390/curroncol30010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gómez H.L., Pinto J.A., Castañeda C., Vallejos C.S. Current barriers for developing clinical research in Latin America: a cross-sectional survey of medical oncologists. Clin Res Trials. 2015;1 doi: 10.15761/CRT.1000108. [DOI] [Google Scholar]
- 45.Statistics . UNESCO Institute for Statistics; Montreal, QC, Canada: 2021. UIf. Research and development expenditure (% of GDP)—Latin America & Caribbean.http://data.uis.unesco.org/index.aspx?queryid=3684 [Google Scholar]
- 46.Guzman M., Canedo-Marroquín G., Jimenez-Vargas N.N. Challenges in gastrointestinal research in Latin America. Nat Rev Gastroenterol Hepatol. 2023;20:199–200. doi: 10.1038/s41575-023-00748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.OECD . 2023. ‘Researchers’ (indicator) [DOI] [Google Scholar]
- 48.Rolfo C., Caglevic C., Bretel D., et al. Cancer clinical research in Latin America: current situation and opportunities. Expert opinion from the first ESMO workshop on clinical trials, Lima, 2015. ESMO Open. 2016;1 doi: 10.1136/esmoopen-2016-000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metzger-Filho O., de Azambuja E., Bradbury I., et al. Analysis of regional timelines to set up a global phase III clinical trial in breast cancer: the adjuvant lapatinib and/or trastuzumab treatment optimization experience. Oncologist. 2013;18:134–140. doi: 10.1634/theoncologist.2012-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan G., Subbiah V. Rare disease research powered by empowered patients: solving the zebra puzzle through social media. Cancer. 2023 doi: 10.1002/cncr.34765. [DOI] [PubMed] [Google Scholar]
- 51.Patsos M. MSJAMA: the internet and medicine: building a community for patients with rare diseases. JAMA. 2001;285:805. [PubMed] [Google Scholar]
- 52.Nawaz F.A., Barr A.A., Desai M.Y., et al. Promoting research, awareness, and discussion on AI in medicine using #MedTwitterAI: a longitudinal twitter hashtag analysis. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.856571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stacchiotti S., Frezza A.M., Blay J.Y., et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127:2934–2942. doi: 10.1002/cncr.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stacchiotti S., Maria Frezza A., Demetri G.D., et al. Retrospective observational studies in ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society (CTOS) community of experts on the minimum requirements for the evaluation of activity of systemic treatments. Cancer Treat Rev. 2022;110 doi: 10.1016/j.ctrv.2022.102455. [DOI] [PubMed] [Google Scholar]
- 55.Luchini C., Lawlor R.T., Milella M., Scarpa A. Molecular tumor boards in clinical practice. Trends Cancer. 2020;6:738–744. doi: 10.1016/j.trecan.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Horak P., Heining C., Kreutzfeldt S., et al. Comprehensive genomic and transcriptomic analysis for guiding therapeutic decisions in patients with rare cancers. Cancer Discov. 2021;11:2780–2795. doi: 10.1158/2159-8290.CD-21-0126. [DOI] [PubMed] [Google Scholar]
- 57.Behel V., Noronha V., Choughule A., et al. Impact of molecular tumor board on the clinical management of patients with cancer. JCO Glob Oncol. 2022;8 doi: 10.1200/GO.22.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]