Abstract
Thai indigenous roosters are exposed to unsuitable temperatures and humidity, resulting in a lower reproductive potential. Kaempferia parviflora (KP) extract containing methoxyflavones was fed to roosters to improve their reproductive performance. Thirty-two Thai native roosters were orally administered KP extract at 300, 450, and 600 mg, calculated according to their average body weight, for at least 14 d before semen collection and continued supplementation until the end of the experiment. The nonsupplemented group served as the control. Fresh semen in terms of semen volume, sperm concentration, mass movement score, and sperm viability were evaluated. Semen preservation at 5°C and fertility test were examined for total motility (MOT), progressive motility (PMOT), sperm viability, and lipid peroxidation up to 48 h of storage. Testosterone concentrations and testicular function were also determined. The results showed that the highest sperm concentration and sperm motility of fresh semen were observed in KP extract at 600 mg (P < 0.001). KP extract at 600 mg resulted in higher sperm viability than the control and KP extract at 300 mg (P < 0.05), but was not different from KP at 450 mg (P > 0.05). The highest MOT, PMOT, and viability were found in the roosters that received 600 mg oral KP extract (P < 0.05), while those of the roosters that received oral KP extract 300 mg and the control were the lowest (P < 0.05) at all storage times. Lipid peroxidation was significantly lower in the KP extract up to 24 h (P < 0.05). The fertility and hatchability of the KP extract at 600 mg at T48 showed a minor decrease compared to the control at T0. These results might be inferred as a result of good spermatogenesis, as revealed by the results of histological examination and testosterone activity. In summary, oral administration of 600 mg KP extract improved sperm production and successfully preserved rooster semen for a long duration of up to 48 h of storage.
Key words: cooling storage, lipid peroxidation, testosterone concentrations, testicular function
INTRODUCTION
Thai indigenous chickens contributed to more than 23.53% (117,367,900 birds) of the chicken population in Thailand by 2023 (ICT-Thai DLD, 2023). They are popular among Thai consumers, especially Pradu Hang dum (PD), because the meat quality is distinctly tastier and more flavorful than that of broilers with high nutrient content (Charoensin et al., 2021; Tunim et al., 2021). Although indigenous chickens raised in tropical areas are more likely to be tolerant to heat stress than those in temperate regions (Loengbudnark et al., 2023), the temperature and humidity index (THI) values exceeded a threshold of 78 in the rainy and summer seasons for almost 8 mo per yr, which seemed to decrease the sperm production of Thai indigenous roosters (Pimprasert et al., 2023). High sperm production was observed during the winter season owing to low THI. This demonstrates that indigenous Thai roosters were exposed to unsuitable temperatures and humidity, resulting in a lower reproductive potential for almost a year. Similarly, adverse climate conditions during hot climatic conditions severely affect semen production, as semen volume and sperm concentration have been shown in other poultry (Obidi et al., 2008; Harsha et al., 2021; Prabakar et al., 2022). Exposure to high ambient temperatures or heat stress (temperature, 40°C ± 0.5°C; humidity, 63.0–80.0%) seems to induce abnormality in the spermatogenic cells in chicken testes and reduce testosterone production, subsequently decreasing the sperm quality (Chen et al., 2015). Therefore, it is apparent that any improvement in reproductive performance would positively affect the production of indigenous chickens raised in tropical areas.
Kaempferia parviflora (KP), popularly known as "Thai ginger" or “Krachaidum” in Thai, is a medicinal plant in the Zingiberaceae family. It is found in tropical areas, such as Malaysia, Sumatra, and Thailand. Its rhizome has been used in folk medicine for centuries (Wuttidharmmavej, 2002). The phytochemicals of KP mainly contain methoxyflavones, especially 3,5,7,3ʹ,4ʹ-pentamethoxyflavone (PMF), 5,7-dimethoxyflavone (DMF), and 5,7,4ʹ-trimethoxyflavone (TMF) (Mekjaruskul et al., 2012). KP enhances male sexual dysfunction by increasing blood flow to the testis and improving the endothelial cell activity via nitric oxide production, which is necessary for penile erection (Wattanapitayakul et al., 2007; Chaturapanich et al., 2008; Temkitthawon et al., 2011; Lert-Amornpat et al., 2017). The previous study showed that KP improves serum testosterone concentrations, testicular weight, sperm density, and testicular structure in diabetic rats (Fungfuang et al., 2016). Most studies on the application of KP as an additive have been performed in rodents (Fungfuang et al., 2016; Lert-Amornpat et al., 2017), and no research has been conducted on roosters. Therefore, we hypothesized that supplementing roosters with KP would improve sperm production.
In addition to improving sperm production, this study focused on semen storage to maintain the sperm quality over long periods. Generally, rooster-diluted semen is recommended to be stored at low temperatures of 2°C to 5°C. For practical reasons, it is desirable to slow its metabolism without seriously decreasing the semen quality for up to 24 h (Slanina et al., 2015). The stressful circumstances of cold storage have a negative impact on sperm, reducing fertility (Sharafi et al., 2015). However, it is speculated that the good sperm quality of fresh semen may extend sperm storage.
In this study, we investigated the effects of oral KP extract supplementation on testicular function, testosterone levels, and semen quality. In addition, the high sperm quality of fresh semen could lead to an extended storage duration. Fertility capacity was also examined.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee approved the experimental procedures based on the Ethics of Animal Experimentation guidelines of the National Research Council of Thailand (Record no. IACUC-KKU-29/65; Reference no. 660201.2.11/197 (33)).
KP Extraction and Oral Preparation
KP was obtained from the Loei Province, Thailand. Fresh KP was sliced and dried at 60 °C in a hot air oven for 48 h before being ground to a powder. The KP extract was prepared by modifying the procedure from Mekjaruskul et al. (2012). Briefly, the KP powder was macerated with 95% ethanol in a stainless-steel tank for 3 d and evaporated by rotary evaporation. The percentage of yield of KP extract was 4.71%. The KP extract was stored at −20°C for further preparation and formulation. The major compounds of methoxyflavones (PMF, DMF, and TMF) in the KP extract were quantified by high-performance liquid chromatography (HPLC, Agilent 1200 series, Germany) (modified method from Tuntiyasawasdikul et al., 2022). The chromatography separation was performed using an Agilent hypersil ODS column (C18, 3.5 μm particle size, 4.6 mm inner diameter, and 150 mm length) and a controlled temperature of 30°C. The mobile phase comprised a mixture of 0.5% formic acid in acetonitrile and 0.5% formic acid in deionized water (5:95–60:40) at a flow rate of 1.2 mL/min (45 min). The injection sample volume was set at 20 μL with a variable wavelength detector (VWD) detector at a wavelength of 254 nm. The KP ethanolic extract contained 25.19 mg/g, 22.94 mg/g, and 42.54 mg/g for PMF, DMF, and TMF, respectively.
The KP formulation was prepared as described by Mekjaruskul et al. (2012). Briefly, KP was dissolved (total concentration: 150 mg/mL) in a mixture of propylene glycol (28%), polyethylene glycol 400 (35%), ethanol (2%), and deionized water (for adjusting to 100%). A 10 mL syringe was used to administer the KP formulation. Each treatment was orally administered according to body weight.
Animals and Management
The experiment was conducted using 32 Thai native roosters (Pradu Hang Dum, 38 wk of age) were used in the present study. In the open-house system, the roosters were kept in individual cages (60 × 45 × 45 cm). Before starting the experiment, they received 130 g of a commercial diet without KP extract supplementation per day. Water was provided ad libitum throughout the study.
Forty-eight Thai native hens (Pradu Hang Dum) at 42 wk of age with egg production >60% were used for the fertility test. The hens were housed individually, fed approximately 110 g of a commercial diet daily, and provided water ad libitum.
The weather information of daily environmental temperature and relative humidity in the farm area was recorded using an automatic temperature and humidity meter (EL-USB-2, Lascar Electronics, Whiteparish, UK). The temperature and humidity index (THI) was calculated according to the National Oceanic and Atmospheric Administration (1976): THI = (1.8 × temp + 32) − (0.55 − 0.0055 × RH) × (1.8 × temp − 26), where temp is the temperature (°C), and RH is the relative humidity (%). The average temperature was 29.28°C, and the relative humidity was 67.93%. The THI was 79.29 during the conduct of the experiment.
Experimental Design and Sample Collection
The roosters were randomly divided into 4 groups (8 roosters per group) according to the concentration of the KP extract (0, 100, 150, and 200 mg/kg body weight). The mean body weight of the rooster was 3.018 kg. Therefore, the roosters in each treatment group were orally administered the KP extract at doses of 300, 450, and 600 mg. The roosters received KP extract for at least 14 d before semen collection and continued supplementation until the end of the experiment. Fresh semen in terms of semen volume, sperm concentration, mass movement score, and sperm viability were evaluated. The semen was diluted with semen extender and stored at 5°C for 48 h. The total motility, progressive motility, viability, and lipid peroxidation levels were evaluated every 12 h at 0 (after the cooling process), 12, 24, 36, and 48 h of storage. Fertility was evaluated after 0, 24, and 48 h of storage. This experiment was repeated 6 times.
To determine the testosterone concentrations, blood samples were collected twice: on d 0 of the experiment (before KP extract administration) and at the end of the study period (d 120). All roosters were sacrificed by manual cervical dislocation to examine testicular function. The testes were removed from the body, washed with 0.9% NaCl, and weighed before being fixed in 10% formaldehyde for histopathological examination.
Semen Collection, Dilution, and Semen Storage at 5°C
The dorso-abdominal massage technique was used to collect semen twice per week. Individual rooster semen was collected in 1.5 mL microtubes with 0.1 mL of IGGKPh diluent, which was composed of 0.14 g potassium citrate, 1.40 g sodium glutamate, 0.21 g sodium dihydrogen phosphate, 0.98 g disodium hydrogen phosphate, 0.9 g glucose, and 0.9 g inositol in 100 mL of deionized water; pH was 6.95, and osmotic pressure was 380 (mOsm/kg) (Surai and Wishart, 1996). The same person simultaneously collected the samples under the same conditions. Great care was taken to avoid contamination of the sperm with feces, urates, and clear fluid, all of which degrade sperm quality. Within 20 min of collection, semen samples were transferred to the laboratory and the semen quality was determined. The criteria for a standard quality of sperm as follows: 0.2 to 0.6 mL of volume, sperm concentration ≥3 × 109 spermatozoa/mL, and motility ≥80% were pooled to eliminate individual differences and diluted with IGGKPh diluent (1:3). Then, diluted semen was placed in a rack, cooled from 25°C to 5°C for 60 min, and maintained in a refrigerator at 5°C.
Evaluation of Fresh Semen Quality
The volume of ejaculated semen was measured using a 1 mL syringe with an accuracy of 0.02 mL. The sperm concentration was determined using a hemocytometer under a light microscope. One microliter of semen sample was diluted with 999 µL of 4% sodium chloride, and 10 µL semen sample was then loaded into each counting chamber. The sperm concentration is expressed as billions (109) of sperm cells/mL. The mass movements were evaluated. A drop of 5 µL semen was placed on a slide without a coverslip, examined under a compound microscope (100×), and scored on a scale of 0 to 5 (0 = no sperm movement; 5 = very rapid waves and whirlwinds visible, with more than 90% of sperm showing a forward movement). Sperm viability was determined using eosin–nigrosin staining. A 5 µL semen sample was mixed with 10 µL of eosin–nigrosin and smeared on the slide. After drying, 300 sperms were evaluated at 1,000× magnification under a light microscope (Olympus CH30, Tokyo, Japan). Spermatozoa that appeared pink (stained with eosin) were regarded as dead, whereas spermatozoa without any color (no penetration of eosin stain) were regarded as live.
The total motility (MOT) and progressive motility (PMOT) were analyzed using a computer-assisted sperm analysis (CASA) system (version 10 HIM-IVOS; Hamilton Thorne Biosciences, Beverly, MA) with Olympus software to process video material recorded in the “avi” format. For each sample, 2 slides (maintained at 25°C) were filled with 5 µL diluted semen, and 3 fields per slide were recorded for 10 s using a 10× phase-contrast objective (Olympus) in conjunction with a digital camera (Olympus DP 71/25). They operate at 30 fps (60 Hz). A sperm was defined as nonmotile when the average path velocity was less than 5 µm/s. The sperm was considered progressively motile when the average path velocity was greater than 20 µm/s and the straightness index was 80%.
Lipid Peroxidation
Malondialdehyde (MDA) concentration, as an index of lipid peroxidation in the semen samples, was measured using the thiobarbituric acid (TBA) reaction, as previously described (Chuaychu-noo et al., 2021). Semen samples from each treatment were adjusted to 250 × 106 spz/mL, then the semen was mixed with 0.25 mL ferrous sulfate (0.2 mM, Ajex, 0906251) and 0.25 mL ascorbic acid (1 mM, Sigma, A5960). The sample was incubated at 37°C for 1 h after incubation with 1 mL trichloroacetic acid (15%, Sigma, T6399) and 1 mL TBA (0.375%, Sigma, T550) were added and boiled in water for 10 min, after which the samples were cooled to 4°C to stop the reaction. Finally, the samples were centrifuged at a controlled temperature of 4°C at 4,000 × g for 10 min and analyzed using UV‒vis spectrophotometry (Analytikjena Model Specord 250 plus) at 532 nm. All MDA concentrations are expressed as nmol/mL.
Fertility
Forty-eight hens were randomly assigned to 4 groups (12 hens/group) with 6 replications. All hens were inseminated with 0.1 mL (150 × 106 spermatozoa/dose) of chilled semen once a week. Insemination was performed between 03.00 and 05.00 pm. Eggs were obtained between d 2 and 8 after insemination. Candling the eggs on d 7 of incubation determined fertility. The hatchability rate was determined by the hatching of fertile eggs approximately 21 d after the start of incubation.
Testosterone Evaluation
The blood samples were collected from the brachial vein twice: on d 0 of the experiment (before KP extract administration) and at the end of the study (d 120). The serum was separated by centrifugation at 2,200 × g for 15 min at room temperature, stored at −20°C, and was maintained until testosterone analysis.
The testosterone concentrations were quantified using enzyme immunoassay (EIAs). The 96-well plates were precoated with a secondary antibody diluted in coating buffer:150 μL (10 μg/mL) goat anti-rabbit IgG (Arbor Assays) for testosterone EIAs. The coated plates were prepared by incubating at RT for 15 to 24 h. The wells were emptied and blotted dry, followed by adding 250 μL blocking buffer (100 mM phosphate, 150 mM sodium chloride, 1% Tween20, 0.09% sodium azide, 10% sucrose, pH 7.5) and incubating for 15 to 24 h at RT. After incubation, the wells were emptied, blotted, and dried in a Sanpla Dry Keeper (Sanpla Corp., Auto A-3, Japan) with a loose desiccant at the bottom. After drying (humidity <20%), the plates were heat sealed in a foil bag with a 1-g desiccant packet and stored at 4 ◦C until use. Rooster serum 50 μL (diluted 1:1 with assay buffer) was added to appropriate wells. Subsequently, 25 μL of steroid horseradish peroxidase conjugate (HRP; 1:20,000 dilution) was immediately added to each well, followed by 25 μL of primary antibody (R156/7; 1:110,000 dilution) added to all but nonspecific-binding-wells and incubated at room temperature for 60 min. The plates were then washed 4 times with wash buffer, and 100 μL of TMB substrate solution was added, followed by incubation for 45 to 60 min at room temperature without shaking. The assay was validated for rooster serum by demonstrating parallelism between serial dilutions of serum and the testosterone standard curve based on Pearson's correlation coefficient analyses (r2 = 0.9549). The absorbance was measured at 450 nm. Assay sensitivity (based on 90% binding) was 0.08 ng/mL, and the intra- and interassay CVs were <10% (Khonmee et al., 2019).
Histological Evaluation
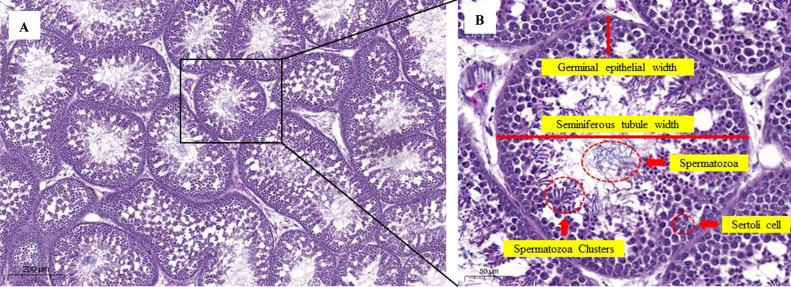
Testicular tissue was infused with 10% formalin for 24 h. Subsequently, the tissues were processed according to the standard histological protocol for paraffin embedding and cut into 3-µm-thick slices as previously described (Sun et al., 2019). Two sections were prepared for each rooster. Thirty seminiferous tubules were randomly examined from each section. The sections were stained with hematoxylin and eosin. The sections were viewed under a light microscope. The Johnsen score, a parameter used to categorize the levels of spermatogenesis based on the presence of the main cell types in the seminiferous tubules, was determined by assigning a score from 10 to 1 as previously described (Johnsen, 1970). The numbers of Sertoli cells and sperm clusters were quantified as described by González-Morán et al. (2008) using digital slide scanners (3DHISTECH Ltd., Budapest, Hungary). The slides were imaged using a 40× microscope objective. The viewable field at this setting measured 320 μm × 80 μm providing a total viewing area of 25,600 μm2. Ten microscopic fields were measured for each testicle, and the average per field was calculated. The diameter of the seminiferous tubules and germinal epithelial width were measured using a digital slide scanner (3DHISTECH Ltd., Budapest, Hungary) at 400× magnification. Figure 1 shows the histological examination of the seminiferous tubules, which consisted of the parameter evaluation in the present study.
Figure 1.
Histological structures of rooster testis stained with hematoxylin and eosin at 100× magnification (A). Parameters of evaluated histology (B). Scale bar = 200 µm (A) and 50 µm (B).
Statistical Analysis
Before conducting statistical analysis in each experiment, the data were tested for normal distribution using the Shapiro–Wilk test. The homogeneity of the residual variances was eliminated using the Levene's test and outlier data. In the fresh semen experiment, data were analyzed using a completely randomized design, and the treatment groups (KP extract supplement) were compared using Tukey's post hoc test. In the cold-semen experiment, a completely randomized design using 6 replicates with a split-plot over time was analyzed. Two factors were statistically analyzed: the first factor was 4 levels of treatment (KP extract supplement) at 0, 300, 450, and 600 mg of KP extract, and the second factor was 5 levels of time to semen storage at 0, 12, 24, 36, and 48 h after the cooling process. Finally, the testosterone and testicular function experiments used the same experimental design as the cool-semen experiment for statistical analysis. The only difference was the time of serum testosterone level detection, which was compared before and after testing. The effects of treatment (KP extract supplementation), time to storage, and their interaction mean values for each parameter were compared using Tukey's post hoc test. They were considered statistically significant at P < 0.05, and the results are expressed as mean values ± mean square error (MSE).
RESULTS
Effects of KP Extract on Fresh Semen Quality
The effects of oral administration of KP extract on the quality of fresh semen are shown in Table 1. The highest concentration and mass movement were observed with KP extract 600 mg (P < 0.001); meanwhile, those of the roosters that received oral KP extract of 300 and 450 mg were similar to those of the control (P > 0.05). Oral KP extract of 600 mg resulted in higher sperm viability than the control and KP extract at 300 mg (P < 0.05). There was no significant difference in the volume of fresh semen among the treatment groups (P > 0.05).
Table 1.
Effects of treatments on fresh semen quality in a rooster (mean ± SEM).
| Treatments | Concentration (×109/mL) | Volume (mL) | Mass movement (score 1–5) | Viability (%) |
|---|---|---|---|---|
| Control | 3.44 ± 0.11b | 0.49 ± 0.03 | 4.51 ± 0.04b | 92.27 ± 0.60c |
| KP 300 mg | 3.42 ± 0.11b | 0.51 ± 0.03 | 4.49 ± 0.02b | 92.94 ± 0.19bc |
| KP 450 mg | 3.64 ± 1.10b | 0.53 ± 0.04 | 4.44 ± 0.03b | 94.22 ± 0.57ab |
| KP 600 mg | 4.21 ± 0.80a | 0.49 ± 0.04 | 4.70 ± 0.06a | 95.66 ± 0.14a |
| P value | <0.001 | 0.208 | <0.001 | <0.001 |
Values within a column with different superscript letters (a,b,c) indicate significant differences between treatments.
Boldface indicates p < 0.05.
Effects of KP Extract on Cold Semen Storage
The effects of the KP extract on semen quality after storage at 5°C for 48 h are presented in Table 2. The interaction between treatment and storage time was significant for sperm quality and lipid peroxidation (P < 0.05). Sperm quality (MOT, PMOT, and viability) continued to decrease with increasing storage time (P < 0.05), whereas MDA concentration increased with increasing storage time (P < 0.05). The highest MOT, PMOT, and viability were found in the roosters that received 600 mg of oral KP extract (P < 0.05), while those of the roosters that received oral KP extract 100 mg and the control were the lowest (P < 0.05) at all storage times. A significant difference in MDA levels was observed at 0, 12, and 24 h (P < 0.05). However, no significant differences were observed after 36 and 48 h of storage (P > 0.05).
Table 2.
Effects of treatments with different times to storage during 0, 12, 24, 36, and 48 h on rooster semen quality (mean ± SEM) incubation at 5°C.
| Treatment |
P value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Time to storage (h) | Control | KP 300 mg | KP 450 mg | KP 600 mg | SEM | Treatment | Time to storage | Interaction |
| MOT (%) | ||||||||
| 0 | 92.98a,v | 92.67a,v | 93.65a,v | 94.79a,v | 0.41 | <0.001 | <0.001 | <0.001 |
| 12 | 78.77d,w | 82.25c,w | 86.96b,w | 88.66a,w | 0.45 | |||
| 24 | 70.12c,x | 71.19c,x | 80.58b,x | 83.02a,x | 0.35 | |||
| 36 | 50.77c,y | 54.00b,y | 52.77b,y | 55.52a,y | 0.36 | |||
| 48 | 46.35c,z | 46.89c,z | 49.16b,z | 52.12a,z | 0.29 | |||
| PMOT (%) | ||||||||
| 0 | 81.52c,v | 82.29bc,v | 83.31b,v | 86.91a,v | 0.46 | <0.001 | <0.001 | <0.001 |
| 12 | 70.74c,w | 70.28c,w | 74.04b,w | 81.04a,w | 0.90 | |||
| 24 | 64.10d,x | 66.30c,x | 69.83b,x | 76.35a,x | 0.98 | |||
| 36 | 37.70c,y | 39.92b,y | 43.45b,y | 45.24a,z | 0.49 | |||
| 48 | 36.67b,z | 37.43b,y | 37.32b,z | 41.44a,y | 0.65 | |||
| Viability (%) | ||||||||
| 0 | 93.69c,v | 92.89bc,v | 94.15ab,v | 95.58a,v | 0.34 | <0.001 | <0.001 | <0.001 |
| 12 | 87.53c,w | 85.67c,w | 89.04b,w | 90.87a,w | 0.29 | |||
| 24 | 83.15c,x | 81.11c,x | 83.28b,x | 87.15a,x | 0.56 | |||
| 36 | 68.90d,y | 73.85c,y | 75.82b,y | 78.73a,y | 0.79 | |||
| 48 | 64.77c,z | 68.00b,z | 68.54b,z | 73.18a,z | 0.71 | |||
| MDA (250 × 106 spz/nmol/mL) | ||||||||
| 0 | 1.33b,v | 1.20ab,v | 1.13a,v | 1.07a,v | 0.03 | <0.001 | <0.001 | <0.001 |
| 12 | 1.75b,w | 1.76b,w | 1.54ab,w | 1.46a,w | 0.04 | |||
| 24 | 2.32b,x | 2.38ab,x | 2.27ab,x | 2.23a,x | 0.05 | |||
| 36 | 2.46a,x | 2.48a,x | 2.45a,x | 2.44a,y | 0.05 | |||
| 48 | 2.80a,y | 2.79a,y | 2.75a,y | 2.68a,z | 0.03 | |||
Percentage of total motility (MOT); progressive motility (PMOT); malondialdehyde (MDA); values within a row with different superscript letters (a,b,c,d) indicate significant differences between treatments; values within a column with different superscript letters (v,w,x,y,z) indicate significant differences between storage times.
Boldface indicates p < 0.05.
Effects of KP Extract on Fertility
The effects of the KP extract on semen quality after storage at 5°C for 48 h are presented in Table 3. The interaction effect between treatment and storage time was significant for the percent of fertility (P < 0.05) but not for the percent of hatchability (P > 0.05). The percentage of fertility and hatchability was decreased when semen was used with increasing storage time (P < 0.05).
Table 3.
Effects of treatments with different times to storage during 0, 24, and 48 h on the percent fertility and hatchability (mean ± SEM).
| Treatment |
P value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Time to storage (h) | Control | KP 300 mg | KP 450 mg | KP 600 mg | SEM | Treatment | Time to storage | Interaction |
| Fertility (%) | ||||||||
| 0 | 71.45d,x | 76.67c,x | 82.28b,x | 86.58a,x | 1.17 | <0.001 | <0.001 | 0.007 |
| 24 | 66.18c,y | 68.62bc,y | 71.98ab,y | 74.67a,y | 0.35 | |||
| 48 | 60.24b,z | 60.84b,z | 66.68a,z | 69.96a,z | 0.29 | |||
| Hatchability (%) | ||||||||
| 0 | 64.00b,x | 63.90b,x | 65.89b,x | 68.71a,x | 0.63 | <0.001 | <0.001 | 0.513 |
| 24 | 57.46b,y | 58.94b,y | 58.82b,y | 63.10a,y | 0.69 | |||
| 48 | 53.30b,z | 53.73b,z | 54.60b,z | 57.96a,z | 0.57 | |||
Values within a row with different superscript letters (a,b,c,d) indicate significant differences between treatments; values within a column with different superscript letters (x,y,z) indicate significant differences between storage times.
Boldface indicates p < 0.05.
At T0, the highest fertility was observed in eggs fertilized by the semen of the roosters that received oral KP extract of 600 mg (P < 0.05), while those fertilized by the semen of the roosters in the control were the lowest (P < 0.05). At T24 and T48, fertility was comparable between eggs fertilized with the semen of the roosters that received oral KP extract 600 mg and 450 mg (P > 0.05), but higher than that of the ones fertilized by the semen of roosters that received 300 mg oral KP extract and the control (P < 0.05). Notably, 600 mg KP extract (74.67 vs. 69.96%) showed a higher and minor decrease, respectively, compared to the control at T0 (71.45%).
The highest hatchability was observed in eggs fertilized with the semen of roosters that received 600 mg of oral KP extract at all storage times (P < 0.05). The hatchability percentages were not significantly different among the other 3 groups at the same storage time (P > 0.05).
Effects of KP Extract on Testis Weight, Testis Size, and Testicular Function
The effects of KP extract on testis weight and size are presented in Table 4. There were significant differences in testis weight among the treatments. The weight of the testes was higher in the roosters that received 600 mg and 450 mg of oral KP extract, whereas it was lower in the roosters that received oral KP extract 300 mg and the control (P < 0.05). However, they did not show a significant difference in testis size.
Table 4.
Effects of treatments on testes weight and testes size (mean ± SEM).
| Treatment | Testes weight (g) |
Testes size (cm) |
|||||
|---|---|---|---|---|---|---|---|
| Right | Left | Total | Right |
Left |
|||
| Width | Length | Width | Length | ||||
| Control | 20.20 ± 0.81b | 18.00 ± 0.66b | 38.20 ± 0.92b | 2.76 ± 0.22 | 5.38 ± 0.07 | 2.74 ± 0.07 | 5.12 ± 0.13 |
| KP 300 mg | 19.93 ± 1.58b | 18.00 ± 1.12b | 37.33 ± 1.42b | 2.80 ± 0.15 | 5.16 ± 0.19 | 2.63 ± 0.08 | 4.98 ± 0.16 |
| KP 450 mg | 23.50 ± 1.18a | 21.67 ± 1.09a | 45.17 ± 1.18a | 2.98 ± 0.17 | 5.40 ± 0.15 | 2.93 ± 0.18 | 4.95 ± 0.42 |
| KP 600 mg | 25.20 ± 0.81a | 24.60 ± 0.81a | 49.80 ± 0.86a | 3.12 ± 0.10 | 5.50 ± 0.14 | 3.08 ± 0.17 | 5.54 ± 0.15 |
| P value | <0.001 | <0.001 | 0.286 | 0.448 | 0.112 | 0.456 | |
Values within a column with different superscript letters (a,b) mean significant differences between treatments.
Boldface indicates p < 0.05.
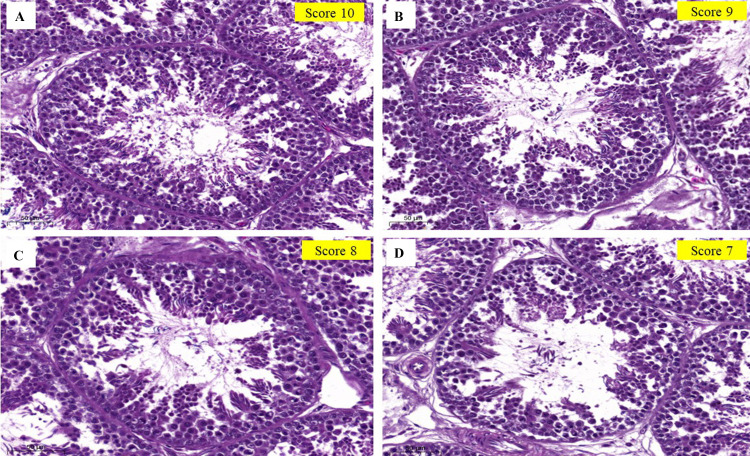
The effects of oral KP extract on the histological parameters are presented in Table 5. Administration of KP extract to roosters improved testicular structure (Johnsen score; Figure 2) and increased the number of Sertoli cells, spermatozoa clusters, seminiferous size, and germinal epithelium width (P < 0.05). The highest Johnsen score, number of Sertoli, Spermatozoa Clusters, seminiferous size, and the germinal epithelium width were observed in KP extract 600 mg (P < 0.05).
Table 5.
Effects of treatments on Johnsen score, number of Sertoli cells, spermatozoa clusters, seminiferous size, and germinal epithelial width (mean ± SEM).
| Treatment | Parameters |
||||
|---|---|---|---|---|---|
| Johnsen score | Number of Sertoli cells/Fields | Spermatozoa Clusters/ Fields | Seminiferous size (μm) | Germinal Epithelial Width (μm) | |
| Control | 8.20 ± 0.67c | 11.45 ± 0.39b | 29.50 ± 0.84c | 249 ± 3.31b | 78.34 ± 0.65b |
| KP 300 mg | 8.28 ± 0.66bc | 11.36 ± 0.35b | 30.54 ± 0.58bc | 264 ± 5.95b | 79.03 ± 0.64b |
| KP 450 mg | 8.98 ± 0.25ab | 12.16 ± 0.39ab | 32.22 ± 0.36ab | 264 ± 4.37b | 82.61 ± 0.91ab |
| KP 600 mg | 9.40 ± 0.55a | 13.25 ± 0.30a | 33.30 ± 0.58a | 286 ± 4.87a | 85.75 ± 0.46a |
| P value | 0.002 | 0.008 | 0.002 | 0.004 | 0.023 |
Values within a column with different superscript letters (a,b) mean significant differences between treatments.
Boldface indicates p < 0.05.
Figure 2.
Standardization of Johnsen scores in the seminiferous tubule cross-sections of roosters stained with hematoxylin and eosin (H&E). (A) Johnsen score of 10 (complete spermatogenesis and perfect tubules); (B) Johnsen score of 9 (many spermatozoa present and disorganized spermatogenesis); (C) Johnsen score of 8 (only a few spermatozoa present); (D) Johnsen score of 7 (no spermatozoa, but many spermatids present).
Effects of KP Extract on Testosterone Concentrations
The effects of KP extract on serum testosterone concentrations are shown in Table 6. The interaction between treatment and time was significant for testosterone concentrations (P < 0.05). Testosterone concentrations increased at the end of the study compared to those at the beginning of the treatment (P < 0.05). Before treatment, testosterone concentrations were not significantly different between the groups (P > 0.05). After treatment or at the end of the study, roosters receiving oral KP extract of 450 and 600 mg had significantly higher plasma testosterone concentrations than the other groups (P < 0.05).
Table 6.
Effects of treatment on serum testosterone levels. Data are presented as the mean ± SEM. The different characters indicated significant differences (P < 0.05).
| Treatment | Serum testosterone level (ng/mL) |
P value |
|||
|---|---|---|---|---|---|
| Before | After | Treatment | Time | Interaction | |
| Control | 0.79 ± 0.27a,y | 1.23 ± 0.33b,x | 0.028 | <0.0001 | 0.005 |
| KP 300 mg | 0.70 ± 0.35a,y | 1.27 ± 0.11b,x | |||
| KP 450 mg | 0.72 ± 0.23a,y | 1.89 ± 0.28a,x | |||
| KP 600 mg | 0.75 ± 0.27a,y | 2.24 ± 0.44a,x | |||
Values within a column with different superscript letters (a,b) indicate significant differences between treatments; values within a row with different superscript letters (x,y) indicate significant differences between time.
Boldface indicates p < 0.05.
DISCUSSION
Sperm production in roosters is affected by various factors, including breed, age, nutrition, and environment. In tropical regions, high environmental temperature and humidity cause heat stress in poultry, subsequently decreasing the growth rate (Boonkum et al., 2021) and resulting in detrimental effects on semen production (Obidi et al., 2008; Harsha et al., 2021; Prabakar et al., 2022). Therefore, there is a need to investigate whether any improvement in the reproductive performance positively affects the production of indigenous chicken raised in tropical areas. In this study, the levels of KP extract supplements influenced the reproductive function of roosters, in which the oral administration of 600 mg KP extract was satisfactory in almost all sperm parameters. The KP extract improved fresh semen quality by increasing sperm concentration, mass movement, viability, and decreasing lipid peroxidation. Although the semen quality decreased as the storage time increased, the KP extract supplement at 600 mg could enhance the semen quality after cold storage at 5°C for 48 h compared to the others. Furthermore, the fertility and hatchability of the KP extract 600 mg at T48 showed a minor decrease compared to the control at T0. These results might be inferred as a result of good spermatogenesis, as revealed by the results of histological examination and testosterone concentrations.
The quality of fresh semen is required to produce higher sperm doses and extend fertility until it is used for artificial insemination to reach maximum fertility. However, increasing environmental temperature and relative humidity with the threshold points of THI at 78 seem to decrease sperm production, especially sperm concentration, in Thai native roosters and pigeons (Wannaratana et al., 2021; Pimprasert et al., 2023). Exposure to heat stress can induce abnormalities in spermatogenesis and decrease testosterone production (Chen et al., 2015; Xiong et al., 2020). In the present study, roosters were raised in an open-house system with an average THI of 79.29, considering exposure to heat stress. Therefore, sperm concentration was obviously affected (Table 1); however, the oral administration of 600 mg could improve the fresh semen quality by increasing sperm concentration, and other sperm parameters. A previous study on diabetic rats reported that KP extract supplementation increased sperm density, testosterone concentrations, and histological features of the testes (Fungfuang et al., 2016).
The present study examined the histological characteristics and testosterone concentrations to elucidate how KP enhances fresh semen quality. The oral administration of KP extract (600 mg) had a positive effect on the testicular structure by increasing testicular functions (Table 5) and testosterone concentrations (Table 6). It has been reported that spermatic blood flow was markedly increased after treatment with KP extract; in other words, KP has a vasodilatory effect (Chaturapanich et al., 2008). KP extract contains several flavonoids, such as PMF, which relax the human corpus cavernosum and improve blood flow (Jansakul et al., 2012). Meanwhile, the DMF induced dilatation of the arteries (Temkitthawon et al., 2011) and enhanced testosterone production through cAMP in rat testicular tumor cells (Horigome et al., 2014). Therefore, it might be implied that increasing blood flow to the testis would stimulate testosterone production and secretion (Damber and Janson, 1978) by acting directly on the Sertoli cells to stimulate spermatogenesis (O'Donnell et al., 1994), subsequently impacting testicular function and sperm development (Prabakar et al., 2022). Furthermore, testicular blood flow was related to testicular weight (Wang et al., 1983); thus, an increase in the testicular weight was also observed in the present study (Table 4). Therefore, we inferred that the oral administration of 600 mg KP to roosters leads to increased testosterone concentrations and subsequently enhances spermatogenesis by improving sperm production.
The percentage of good sperm in cold-storage semen depends mainly on fresh semen quality. However, lipid peroxidation is an important aspect of the oxidative stress that occurs during dilution and cooling incubation, which shortens the lifespan of cold-stored semen (Sharafi et al., 2015). Besides, the poultry sperm cell structure contains a high amount of polyunsaturated fatty acids, which are sensitive to lipid peroxidation in the presence of reactive oxygen species (ROS), resulting in sperm DNA damage and decreased sperm motility (Cerolini et al., 2006; Gholami et al., 2010; Partyka et al., 2012), which is a consequence of plasma membrane dysfunction, leading to decreased fertilizing ability. Therefore, antioxidants that act as free radical scavengers, have been proposed as semen extender additives to improve semen preservation. In the present study, 600 mg of KP extract reduced lipid peroxidation for up to 24 h. Accordingly, the sperm quality of cooled stored semen was enhanced (Table 2). This might be highly beneficial as an antioxidant in KP. It was reported that the TMP, one of the major methoxyflavone components in KP extraction, can reduce the production of intravascular ROS (Horigome et al., 2014). However, the antioxidant properties were no longer observed after 36 h of storage. This may be due to insufficient antioxidants in the semen or excessive ROS formation, leading to higher MDA values over time (Agarwal et al., 2003). In addition, heat stress mainly caused a decrease in the antioxidant enzyme activity and disrupted the balance between oxidative and antioxidative status in seminal plasma (Xiong et al., 2020), suggesting that high environmental temperatures could accelerate lipid peroxidation, resulting in high ROS in semen storage.
Successful fertilization is related to the quality of the cool storage of semen. Little information is available on the use of cold-stored semen after 24 h, especially regarding its fertility capacity. Notably, the fertility rates of the roosters that received 600 mg of oral KP extract and stored for up to 48 h showed a higher and minor decrease compared with control at T0 (Table 3). This result was superior to previous studies that supplemented creatine as an antioxidant to diluent with a maximum fertility rate in Vietnamese native chickens at 48 h after storage of approximately 60% (Bui et al., 2021). However, our previous study, in which a solid storage medium was supplemented with serine as an antioxidant, showed a greater fertility capacity when compared to the same storage time (Kheawkanha et al., 2023). A difference in the extender and antioxidant supplementation may be noted in this case. Therefore, considering the results of sperm preservation and fertility, supplementation with antioxidants as semen additives in the extender should be considered to improve cold semen preservation.
In the present study, roosters that received 600 mg of KP extract orally showed the most remarkable performance in terms of sperm production, semen preservation, and fertility capacity. However, it seems plausible that a high dose of oral KP in the rooster diet might have a greater impact on these parameters. However, it is important to note that KP is extracted with alcohol to produce the active components of methoxyflavones (Sutthanut et al., 2007). Despite the alcohol being removed from the KP extract prior to consumption, the taste and flavor are undesirable for the rooster, subsequently declining to eat. Hence, in this experiment, it is necessary to administer the KP dose using a syringe to achieve more accurate quantification. Meanwhile, the oral bioavailability of methoxyflavones is extremely poor, and they are lipophilic molecules immiscible in water (Mekjaruskul et al., 2012). Several techniques have been developed to enhance the solubility and bioavailability of methoxyflavones. Mekjaruskul et al. (2013) reported that a complex of cyclodextrin and KP successfully increased the dissolution rate, improved drug permeability in Caco-2 cells, and enhanced the oral bioavailability of methoxyflavones. Unfortunately, cyclodextrin tends to be expensive; therefore, it is unsuitable for producing animal feed. In addition, Chairuk et al. (2020) developed a self-nano-emulsifying drug delivery system (SNEDDS) of KP that can increase the area under the curve (AUC), the concentration of a drug in the bloodstream over time after it has been taken, by 5.38 times compared to the KP extract. However, high concentrations of surfactants can trigger gastrointestinal irritation. Therefore, additional product development by improving oral bioavailability may be required to enhance taste and flavor, which are appropriate for animal supplements.
CONCLUSIONS
In conclusion, this is the first report on the use of KP extract in livestock. Roosters that received 600 mg of oral KP extract showed the most remarkable performance in terms of sperm production, semen preservation, and fertility. However, it seems plausible that a high dose of oral KP in the rooster diet might have a greater impact on these parameters. Therefore, additional product development is required to improve the taste and flavor of animal supplements.
ACKNOWLEDGMENTS
The Network Center for Animal Breeding and Omics Research, Khon Kaen University, and the Center for Research and Development of Herbal Health Products, Khon Kaen University, financially supported this study. The Graduate School of Khon Kaen University supported Supakorn Authaida through the Research Fund for Supporting Lecturers to Admit High-Potential Students for Study and Research in His Expert Program Year 2021 (grant no. 641JT103). The authors are grateful to Kittikorn Boonsri for his assistance in histological evaluation and to Patcharapa Towiboon for her technical assistance in blood sample processing and laboratory analyses of testosterone concentrations.
DISCLOSURES
This manuscript has not been published or submitted for publication elsewhere. The content does not impose any conflict of interest and all the authors are agreeable in the manuscript content.
REFERENCES
- Agarwal A., Said T.M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Update. 2003;9:331–345. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- Boonkum W., Duangjinda M., Kananit S., Chankitisakul V., Kenchaiwong W. Genetic effect and growth curve parameter estimation under heat stress in slow-growing Thai native chickens. Vet. Sci. 2021;8:297. doi: 10.3390/vetsci8120297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui H.Y.T., Dang H.L., Phuong N.T.H. Effects of creatine on the quality and fertility of chicken semen during liquid storage. ISSAAS. 2021;27:27–37. [Google Scholar]
- Cerolini S., Zaniboni L., Maldjian A., Gliozzi T. Effect of docosahexaenoic acid and α-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology. 2006;66:877–886. doi: 10.1016/j.theriogenology.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Chairuk P., Tubtimsri S., Jansakul C., Sriamornsak P., Weerapol Y. Enhancing oral absorption of poorly water-soluble herb (Kaempferia parviflora) extract using self-nanoemulsifying formulation. Pharm. Dev. Technol. 2020;25:340–350. doi: 10.1080/10837450.2019.1703134. [DOI] [PubMed] [Google Scholar]
- Charoensin S., Laopaiboon B., Boonkum W., Phetcharaburanin J., Villareal M.O., Isoda H., Duangjinda M. Thai native chicken as a potential functional meat source rich in anserine, anserine/carnosine, and antioxidant substances. Animals. 2021;11:902. doi: 10.3390/ani11030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturapanich G., Chaiyakul S., Verawatnapakul V., Pholpramool C. Effects of Kaempferia parviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction. 2008;136:515–522. doi: 10.1530/REP-08-0069. [DOI] [PubMed] [Google Scholar]
- Chen Z., Zhang J.R., Zhou Y.W., Liang C., Jiang Y.Y. Effect of heat stress on the pituitary and testicular development of Wenchang chicks. Arch. Anim. Breed. 2015;58:373–378. [Google Scholar]
- Chuaychu-Noo N., Thananurak P., Boonkum W., Vongpralub T., Chankitisakul V. Effect of organic selenium dietary supplementation on quality and fertility of cryopreserved chicken sperm. Cryobiology. 2021;98:57–62. doi: 10.1016/j.cryobiol.2020.12.008. [DOI] [PubMed] [Google Scholar]
- Damber J.E., Janson P. The effects of LH, adrenaline and noradrenaline on testicular blood flow and plasma testosterone concentrations in anaesthetized rats. Acta Endocrinol. (Copenh). 1978;88:390–396. doi: 10.1530/acta.0.0880390. [DOI] [PubMed] [Google Scholar]
- Fungfuang W., Lert-Amornpat T., Maketon C. Effects of Black ginger (Kaempferia parviflora) on the testicular function in streptozotocin-induced diabetic male rats. Vet. Integr. Sci. 2016;14:95–107. [Google Scholar]
- Gholami H., Chamani M., Towhidi A., Fazeli M.H. Effect of feeding a docosahexaenoic acid-enriched nutriceutical on the quality of fresh and frozen-thawed semen in Holstein bulls. Theriogenology. 2010;74:1548–1558. doi: 10.1016/j.theriogenology.2010.06.025. [DOI] [PubMed] [Google Scholar]
- González-Morán M.G., Guerra-Araiza C., Campos M.G., Camacho-Arroyo I. Histological and sex steroid hormone receptor changes in testes of immature, mature, and aged chickens. Domest. Anim. Endocrinol. 2008;35:371–379. doi: 10.1016/j.domaniend.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Harsha M., Swathi B., Neeradi R., Kannaki T.R., Venkata Rama Rao S., Shanmugam M., Pranay Kumar K., Vidyasagar Reddy A. Effects of heat stress on semen quality of Gramapriya male line roosters. Pharm. Innov. 2021;10:1413–1417. [Google Scholar]
- Horigome S., Yoshida I., Tsuda A., Harada T., Yamaguchi A., Yamazaki K., Inohana S., Isagawa S., Kibune N., Satoyama T., Katsuda S., Suzuki S., Watai M., Hirose N., Mitsue T., Shirakawa H., Komai M. Identification and evaluation of anti-inflammatory compounds from Kaempferia parviflora. Biosci. Biotechnol. Biochem. 2014;78:851–860. doi: 10.1080/09168451.2014.905177. [DOI] [PubMed] [Google Scholar]
- Information and Communication Technology Center of Thai Department of Livestock Development (ICT-Thai DLD). Available online chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://ict.dld.go.th/webnew/images/stories/stat_web/yearly/2565/country/6-chick.pdf
- Jansakul C., Tachanaparuksa K., Mulvany M.J., Sukpondma Y. Relaxant mechanisms of 3,5,7,3ʹ,4ʹ-pentamethoxyflavone on isolated human cavernosum. Pharmocology. 2012;691:235–244. doi: 10.1016/j.ejphar.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Johnsen S.G. Testicular biopsy score count - a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- Kheawkanha T., Chankitisakul V., Thananurak P., Pimprasert M., Boonkum W., Vongpralub T. Solid storage supplemented with serine of rooster semen enhances higher sperm quality and fertility potential during storage at 5°C for up to 120 h. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khonmee J., Brown J.L., Li M.Y., Somgird C., Boonprasert K., Norkaew T., Punyapornwithaya V., Lee W.M., Thitaram C. Effect of time and temperature on stability of progestagens, testosterone and cortisol in Asian elephant blood stored with and without anticoagulant. Conserv. Physiol. 2019;7:coz031. doi: 10.1093/conphys/coz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lert-Amornpat T., Maketon C., Fungfuang W. Effect of Kaempferia parviflora on sexual performance in streptozotocin-induced diabetic male rats. Andrologia. 2017;49:e12770. doi: 10.1111/and.12770. [DOI] [PubMed] [Google Scholar]
- Loengbudnark W., Chankitisakul V., Boonkum W. The genetic impact of heat stress on the egg production of Thai native chickens (Pradu Hang dum) PLoS One. 2023;18 doi: 10.1371/journal.pone.0281328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekjaruskul C., Jay M., Sripanidkulchai B. Modulatory effects of Kaempferia parviflora extract on mouse hepatic cytochrome P450 enzymes. Ethnopharmacology. 2012;141:831–839. doi: 10.1016/j.jep.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Mekjaruskul C., Yang Y.T., Leed M.G., Sadgrove M.P., Jay M., Sripanidkulchai B. Novel formulation strategies for enhancing oral delivery of methoxyflavones in Kaempferia parviflora by SMEDDS or complexation with 2-hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 2013;445:1–11. doi: 10.1016/j.ijpharm.2013.01.052. [DOI] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration . US Govt. Printing Office; Washington, DC: 1976. Livestock hot weather stress. US Dept. Commerce, Natl. Weather Serv. Central Reg., Reg. Operations Manual Lett. C-31-76. [Google Scholar]
- Obidi J.A., Onyeanusi B.I., Rekwot P.I., Ayo J.O. Seasonal variations in seminal characteristics of Shikabrown breeder cocks. Poult. Sci. 2008;7:1219–1223. [Google Scholar]
- O'Donnell R.J., Springfield D.S., Motwani H.K., Ready J.E., Gebhardt M.C., Mankin H.J. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. Bone Joint Surg. Am. 1994;76:1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- Partyka A., Łukaszewicz E., Niżański W. Effect of cryopreservation on sperm parameters, lipid peroxidation and antioxidant enzymes activity in fowl semen. Theriogenology. 2012;77:1497–1504. doi: 10.1016/j.theriogenology.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Pimprasert M., Kheawkanha T., Boonkum W., Chankitisakul V. Influence of semen collection frequency and seasonal variations on fresh and frozen semen quality in Thai native roosters. Animals. 2023;13:573. doi: 10.3390/ani13040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakar G., Gopi M., Kolluri G., Rokade J.J., Pavulraj S., Pearlin B.V., Sudamrao Khillare G., Madhupriya V., Singh J., Tyagi Mohan J. Seasonal variations on semen quality attributes in turkey and egg type chicken male breeders. Biometeorology. 2022;66:1547–1560. doi: 10.1007/s00484-022-02299-x. [DOI] [PubMed] [Google Scholar]
- Sharafi M.M., Zhandi A., Shahverdi M. Shakeri Beneficial effects of nitric oxide induced mild oxidative stress on post-thawed bull semen quality. Fertil. Steril. 2015;9:230–237. doi: 10.22074/ijfs.2015.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanina T., Petrovičová L., Miškeje M., Kňížat L., Mirda J., Lukáč N., Trandžík J., Petrovičová I., Massányi P. The effect of diluent, temperature and age on turkey spermatozoa motility in vitro. Appl. Anim. Res. 2015;43:131–136. [Google Scholar]
- Sun Y., Xue F., Li Y., Fu L., Bai H., Ma H., Xu S., Chen J. Differences in semen quality, testicular histomorphology, fertility, reproductive hormone levels, and expression of candidate genes according to sperm motility in Beijing-You chickens. Poult. Sci. 2019;98:4182–4189. doi: 10.3382/ps/pez208. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Wishart G.J. Poultry artificial insemination technology in the countries of the former USSR. World Poult. Sci. J. 1996;52:27–43. [Google Scholar]
- Sutthanut K., Sripanidkulchai B., Yenjai C., Jay M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A. 2007;1143:227–233. doi: 10.1016/j.chroma.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Temkitthawon P., Hinds T.R., Beavo J.A., Viyoch J., Suwanborirux K., Pongamornkul W., Sawasdee P., Ingkaninan K. Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. Ethnopharmacology. 2011;137:1437–1441. doi: 10.1016/j.jep.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunim S., Phasuk Y., Aggrey S.E., Duangjinda M. Increasing fat deposition via upregulates the transcription of peroxisome proliferator-activated receptor gamma in native crossbred chickens. Animals. 2021;11:90. doi: 10.3390/ani11010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuntiyasawasdikul S., Sripanidkulchai B. Development and clinical trials on anti-inflammatory effect of transdermal patch containing a combination of Kaempferia parviflora and Curcuma longa extracts. J. Drug Deliv. Sci. Technol. 2022;68 [Google Scholar]
- Wang J., Galil K.A., Setchell B.P. Changes in testicular blood flow and testosterone production during aspermatogenesis after irradiation. Endocrinology. 1983;98:35–46. doi: 10.1677/joe.0.0980035. [DOI] [PubMed] [Google Scholar]
- Wannaratana S., Olanratmanee E.O., Charoenmuang K., Boriharnthanawuth T., Tangtrongwanich B., Jongpattana T., Sukhor Y., Kongthip A., Sananmuang T. Seasonal effect on semen availability and quality of racing pigeon in Thailand. Vet. World. 2021;14:1459–1464. doi: 10.14202/vetworld.2021.1459-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanapitayakul S.K., Suwatronnakorn M., Chularojmontri L., Herunsalee A., Niumsakul S., Charuchongkolwongse S., Chansuvanich N. Kaempferia parviflora ethanolic extract promoted nitric oxide production in human umbilical vein endothelial cells. J. Ethnopharmacol. 2007;110:559–562. doi: 10.1016/j.jep.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Wuttidharmmavej W. Rattanakosin Pharmaceutical Scripture. SILPA SIAM P23ACKAGING & PRINTING CO., LTD; Bangkok, Thailand: 2002. [Google Scholar]
- Xiong Y., Yin Q., Li J., He S. Oxidative stress and endoplasmic reticulum stress are involved in the protective effect of alpha lipoic acid against heat damage in chicken testes. Animals. 2020;10:384. doi: 10.3390/ani10030384. [DOI] [PMC free article] [PubMed] [Google Scholar]