Abstract
Background
Chronic respiratory diseases (CRDs) pose a significant global health burden. Antioxidant-rich diets have been associated with improved lung health, but the specific relationship with CRDs remains unclear.
Methods
This study examined the relationship between dietary antioxidant intakes and CRDs using data from the 2001–2018 National Health and Nutrition Examination Survey (NHANES). Information on dietary antioxidant intakes, including vitamins A, C, and E, zinc, selenium, and carotenoid, were collected from the 2 24-h recall interviews to calculate composite dietary antioxidant index (CDAI). CRDs were determined based on self-reported physician diagnoses. To examine the relationship between CDAI and CRDs, multivariate logistic regression was used. To study potential non-linear correlations within these associations, restricted cubic spline (RCS) regression was performed.
Results
The study involved 40 557 individuals. The median CDAI was −0.09 (−2.05, 2.25). We discovered those who were in the fourth quartile of CDAI scores had a 19% lower prevalence than those in the first quartile (OR = 0.81 [0.72–0.91], Ptrend < 0.01) after adjusting for all relevant covariates. The fourth quartile of CDAI was linked with a lower prevalence of emphysema (OR = 0.57 [0.40–0.81], Ptrend < 0.01) and chronic bronchitis (OR = 0.74 [0.62–0.88], Ptrend < 0.01). RCS regression showed that CDAI was non-linearly related to the prevalence of CRDs, with inflection points of 3.20 (P for non-linearity <0.01). The stratified analysis did not identify variables that significantly affected the results.
Conclusion
Higher dietary antioxidant intakes were related with a lower prevalence of CRDs (particularly emphysema and chronic bronchitis) in general adults.
Keywords: CRDs, Antioxidants, Dietary intakes, NHANES
Introduction
Chronic respiratory diseases (CRDs) encompass various conditions such as asthma, emphysema, and chronic bronchitis, which significantly impair lung function and overall respiratory health.1 Asthma is characterized by recurrent episodes of coughing, chest tightness, and shortness of breath.2 Emphysema is a progressive lung disease that damages and destroys the air sacs in the lungs.3 Chronic bronchitis involves airway inflammation and constriction, leading to excessive mucus production and persistent cough.4 These diseases pose a substantial global burden of morbidity and present a significant challenge to the global healthcare system. The development of CRDs is influenced by various factors, including environmental exposures such as air pollution, smoking, occupational hazards, and genetic predispositions.5, 6, 7 Additionally, lifestyle factors like physical inactivity and poor nutrition contribute to the severity of CRDs.8, 9, 10 Epidemiological research suggests that dietary variables, such as antioxidants, may play a vital role in the prevention and management of CRDs.11,12
The Composite Dietary Antioxidant Index (CDAI) is a metric used to assess the total antioxidant capacity of an individual's diet.13 It combines multiple dietary antioxidants, such as vitamins (A, C, E), minerals (selenium, zinc), and phytochemicals (carotenoids), into a single index. By evaluating the collective impact of multiple antioxidants, the CDAI provides a comprehensive measure of the diet's ability to combat oxidative stress.14 Imbalance between free radicals and antioxidants in the body leads to cellular damage.15 Studies have demonstrated that antioxidant-rich diets can reduce the risk of developing chronic diseases, including depression, osteoporosis, cancer, and cardiovascular mortality.16, 17, 18, 19 Antioxidants play a protective role in human health through various mechanisms.20 They can directly scavenge reactive oxygen species (ROS) and prevent them from causing damage to body tissues.21 Additionally, they upregulate endogenous antioxidant enzymes to enhance the body's defenses against oxidative stress.22 By considering both the quantity of antioxidants consumed, the CDAI offers a comprehensive perspective on their potential health benefits.
Several studies have suggested that dietary antioxidants may benefit respiratory health. One meta-analysis reported that increased dietary antioxidant intake was associated with a reduced likelihood of developing chronic obstructive pulmonary disease (COPD).23 Another study focusing on asthmatics found that increasing dietary antioxidant intake may help reduce the frequency of asthma symptoms.24 However, few studies have specifically investigated the relationship between the CDAI and the onset or progression of CRDs. Thus, a comprehensive investigation of this association is warranted. Our study aims to explore the potential protective role of dietary antioxidants against CRDs in adults by analyzing data collected from a representative population. We examined CDAIs obtained from the 2001–2018 National Health and Nutrition Examination Survey (NHANES) to investigate the link between CDAIs and the prevalence of CRDs. By exploring the correlation between dietary antioxidant intake and respiratory health, we aim to identify potential modifiable factors that could contribute to the development of tailored dietary recommendations for individuals susceptible to CRDs.
Materials and methods
Study population
The NHANES is a program conducted by the Centers for Disease Control and Prevention (CDC) in the United States.25 It collects comprehensive health and nutritional data from a nationally representative population. The survey collects information through interviews, medical exams, and laboratory tests, providing valuable data on various aspects of health. The research protocols were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board, and all participants provided informed permission.
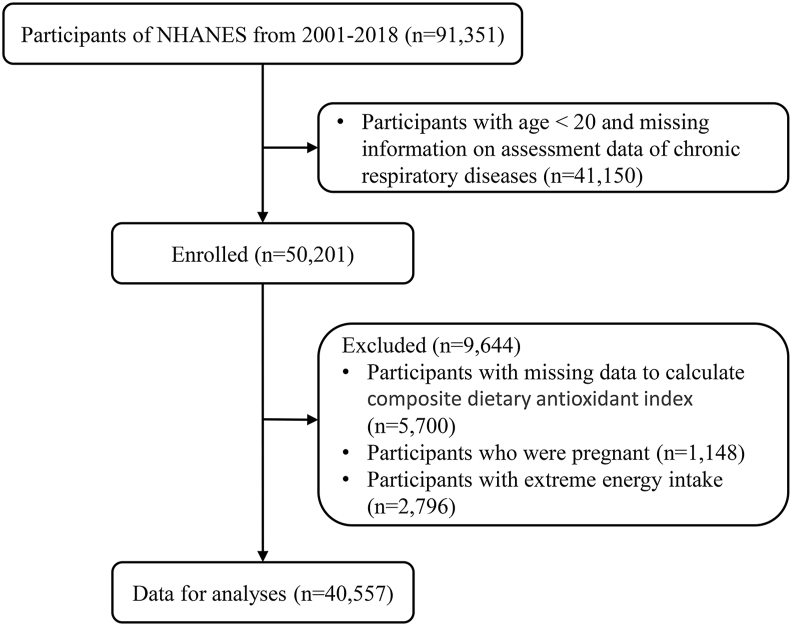
In total, 91 351 people took part in the NHANES from 2001 to 2018. We excluded 41 150 participants with an age <20 and missing information on CRDs. A total of 5700 participants without data to calculate CDAI, 1148 participants who were pregnant and 2796 participants with extreme energy intakes were also ruled out. We eventually included 40 557 participants for analyses (Fig. 1).
Fig. 1.
Flowchart of the study participants.
Assessment of CDAI and CRDs
In the NHANES, dietary intake data were acquired through a structured 24-h dietary recall interview. The NHANES employs a computer-assisted dietary interview system, which systematically gathers and records details concerning the variety and quantities of ingestible items, encompassing food, beverages (inclusive of water), consumed during the 24 h preceding the interviews. The initial dietary recall interview was administered in a face-to-face setting at the Mobile Examination Center (MEC), while the subsequent interview was conducted via telephone within a period ranging from 3 to 10 days after the first encounter. The assessment of dietary nutrient intake and total energy consumption relied on the University of Texas Food Intake Analysis System in conjunction with data sourced from the United States Department of Agriculture Survey Nutrient Database. To obtain information regarding the intake of six specific dietary antioxidants as well as total energy intake, a process of averaging the results from two separate 24-h recall interviews was undertaken. It is noteworthy that the nutritional assessments did not encompass any nutrients originating from dietary supplements or medicinal compounds.
The CDAI is a calculating method for determining the whole antioxidant amount found in a person's diet.13 It contains 6 dietary antioxidants — vitamins A, C, and E, as well as zinc, selenium, and carotenoids — and assigns weights to each antioxidant based on its potential health benefits.26 To estimate the CDAI, we standardized each of the same six dietary vitamins and minerals by subtracting the global average and dividing by the global standard deviation. We then calculated the CDAI by adding the standard intake of these vitamins. Higher CDAI scores indicate diets that contain more antioxidants and types of antioxidants. In this study, the effect of abnormal values was avoided by excluding participants with abnormal total energy intake (>4200 or <800 kcal/day in male; >3500 or <500 kcal/day in female).
CRDs refer to long-term conditions affecting the airways and lungs, including asthma, emphysema, and chronic bronchitis.5 People with CRDs were defined as affirming the following question: Has a doctor or other health professional ever told you that you have asthma/emphysema/chronic bronchitis?
Covariates
Information on age (years), sex (male or female), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other), education level (below high school, high school, or above high school), total energy intake (kcal/day), and carotenoid supplement use (%) was collected through a standardized approach. The poverty income ratio (PIR) is derived by dividing the household's earnings by a factor unique to the size and composition of the household and categorised into 3 groups (≤1.0, 1.1–3.0, or > 3.0).27 Individual smoking and drinking status were recorded through a standardised questionnaire asking participants about their past and present smoking and drinking status.28 Smoking status was classified as never smokers (<100 cigarettes), current smokers (>100 cigarettes), and former smokers (>100 cigarettes and had quit smoking). Drinking status was classified as nondrinker, low-to-moderate drinker (<1 drink/day for females and <2 drinks/day for males), or heavy drinker (≥1 drink/day for females and ≥2 drinks/day for males). Physical activity was classified as inactive (no leisure-time physical activity), insufficiently active (moderate activity 1–5 times per week with metabolic equivalents [MET] 3–6 or vigorous activity 1–3 times per week with MET >6), and active (moderate activity >5 times per week with MET 3–6 or vigorous activity >3 times per week with MET >6).29,30 Self-reported questionnaires were used to collect information on the prevalence of hypertension and diabetes.
Statistical analysis
The individuals' initial features are expressed as means with standard errors (SEs), medians with interquartile ranges (IQRs), or numbers with percentages. The Student's t-test or Mann-Whitney U test were used to compare continuous variables, while the chi-square test was used to compare categorical variables. The concentration and distribution of CDAI and its components were analysed. The association coefficients between serum and dietary antioxidant micronutrients were determined using pairwise Spearman correlation analysis. The study population was categorised according to CDAI quartiles.
Multifactorial logistic regression was used to analyse the link between CDAI and its components with the prevalence of CRDs, asthma, emphysema and chronic bronchitis in the United States (US) population. Potential non-linear links between CDAI and prevalence of CRDs were analysed using restricted cubic spline (RCS) regression model with 10th, 50th and 90th percentile as nodes. RCS is a flexible and non-linear regression approach that allows for a curved relationship by fitting piecewise polynomials. Stratified analyses were used to explore the effect of different characteristics on the associations of CDAI and its components with the prevalence of CRDs, including age (20–39, 40–59, or ≥ 60 years), sex (female, or male), race (non-Hispanic White, or other), family PIR (≤1.0, 1.1–3.0, or >3.0), smoking status (never, former, or current smoker), drinking status (nondrinker, low-to-moderate drinker, or heavy drinker), physical activity (inactive, insufficiently active, or active), BMI (<25.0, 25.0–29.9, or >29.9 kg/m2), supplement use (no, or yes). All analyses were performed using R (version 4.2.0) and P-values less than 0.05 were considered statistically significant.
Results
Baseline characteristics of the participants
Table 1 shows the NHANES initial features for the general adults from 2001 to 2018. A total of 40 557 adults (7110 participants with CRDs) were included in this analysis. The participants were 48.42% male and 45.23% non-Hispanic white. Except for education level and drinking status (P > 0.05), all baseline variables were significantly different between people with and without CRDs. Patients with CRDs were more likely to be old women (age ≥60 years), female, non-Hispanic white, current smokers, obese, and physically inactive (P < 0.01). They were also more probable to have hypertension and diabetes (P = 0.01). We also looked at the research population's baseline characteristics based on CDAI quartiles (Table S2). We found higher proportions of middle-aged women (age 40–59 years), non-smokers, low-to-moderate drinkers, normal-weight individuals, and physically active individuals among participants in the fourth quartile of the CDAI (P < 0.01). They tended to be less prone to self-reported hypertension and diabetes (P < 0.01).
Table 1.
Baseline characteristics of the general adult population in NHANES 2001–2018.
| Characteristics | Total (n = 40 557) | Chronic respiratory diseases |
P value | |
|---|---|---|---|---|
| No (n = 33 447) | Yes (n = 7110) | |||
| Age, years | <0.01 | |||
| 20-39 | 12 884 (31.77) | 10 654 (35.44) | 2230 (35.43) | |
| 40-59 | 13 281 (32.75) | 11 051 (38.81) | 2230 (35.87) | |
| ≥60 | 14 392 (35.49) | 11 742 (25.75) | 2650 (28.70) | |
| Sex, % | <0.01 | |||
| Female | 20 921 (51.58) | 16 807 (50.93) | 4114 (59.57) | |
| Male | 19 636 (48.42) | 16 640 (49.07) | 2996 (40.43) | |
| Race/ethnicity, % | <0.01 | |||
| Mexican American | 6658 (16.42) | 6001 (8.76) | 657 (4.46) | |
| Other Hispanic | 3385 (8.35) | 2788 (5.34) | 597 (4.97) | |
| Non-Hispanic White | 18 345 (45.23) | 14 606 (68.29) | 3739 (73.19) | |
| Non-Hispanic Black | 8439 (20.81) | 6862 (10.65) | 1577 (11.25) | |
| Other race | 3730 (9.2) | 3190 (6.96) | 540 (6.12) | |
| Education level, % | 0.74 | |||
| Below high school | 10 311 (25.42) | 8594 (15.99) | 1717 (16.43) | |
| High school | 9425 (23.24) | 7750 (23.83) | 1675 (23.98) | |
| Above high school | 20 821 (51.34) | 17 103 (60.17) | 3718 (59.59) | |
| Family PIR, % | <0.01 | |||
| ≤1.0 | 8219 (20.27) | 6521 (13.01) | 1698 (17.18) | |
| 1.1–3.0 | 17 097 (42.16) | 14 032 (35.46) | 3065 (38.11) | |
| >3.0 | 15 241 (37.58) | 12 894 (51.52) | 2347 (44.71) | |
| Smoking status, % | <0.01 | |||
| Never smoker | 22 066 (54.41) | 18 861 (56.31) | 3205 (45.73) | |
| Former smoker | 10 216 (25.19) | 8176 (24.43) | 2040 (28.13) | |
| Current smoker | 8275 (20.4) | 6410 (19.26) | 1865 (26.14) | |
| Drinking status, % | 0.19 | |||
| Nondrinker | 9370 (23.1) | 7789 (18.82) | 1581 (18.74) | |
| Low-to-moderate drinker | 28 020 (69.09) | 23 038 (71.86) | 4982 (72.80) | |
| Heavy drinker | 3167 (7.81) | 2620 (9.32) | 547 (8.46) | |
| Body mass index, % | <0.01 | |||
| <25.0 kg/m2 | 11 674 (28.78) | 9864 (30.91) | 1810 (27.42) | |
| 25.0–29.9 kg/m2 | 13 680 (33.73) | 11 622 (34.26) | 2058 (28.40) | |
| >29.9 kg/m2 | 15 203 (37.49) | 11 961 (34.84) | 3242 (44.18) | |
| Physical activity, % | <0.01 | |||
| Inactive | 10 991 (27.1) | 8877 (21.22) | 2114 (24.93) | |
| Insufficiently active | 14 885 (36.7) | 12 396 (39.79) | 2489 (37.74) | |
| Active | 14 681 (36.2) | 12 174 (38.98) | 2507 (37.34) | |
| Total energy intakes, kcal/day | 0.01 | |||
| Quartile 1 | 10 148 (25.02) | 8212 (21.42) | 1936 (23.65) | |
| Quartile 2 | 10 136 (24.99) | 8351 (24.29) | 1785 (24.34) | |
| Quartile 3 | 10 136 (24.99) | 8472 (26.14) | 1664 (24.76) | |
| Quartile 4 | 10 137 (24.99) | 8412 (28.15) | 1725 (27.26) | |
| Self-reported hypertension, % | <0.01 | |||
| No | 22 955 (56.6) | 19 422 (63.57) | 3533 (55.66) | |
| Yes | 17 602 (43.4) | 14 025 (36.43) | 3577 (44.34) | |
| Self-reported diabetes, % | <0.01 | |||
| No | 35 371 (87.21) | 29 450 (91.33) | 5921 (87.47) | |
| Yes | 5186 (12.79) | 3997 (8.67) | 1189 (12.53) | |
| Supplement use, % | 0.01 | |||
| No | 19 920 (49.12) | 16 618 (46.31) | 3302 (44.19) | |
| Yes | 20 637 (50.88) | 16 829 (53.69) | 3808 (55.81) | |
Abbreviations: PIR, poverty income ratio. Categorical variables are presented as numbers (percentages). Sampling weights were applied for calculation of demographic descriptive statistics; N reflect the study sample while percentages reflect the survey-weighted data
Distributions and concentrations of CDAI among adults with CRDs
Table S1 listed the distribution and concentration of CDAI and its components among adults with CRDs. The mean CDAI was −0.09 (−2.05, 2.25). The average intake of vitamins A, C, and E, and zinc, selenium, and carotenoids in the study population was approximately 481.00 (270.00, 785.00) μg/day, 51.50 (21.50, 111.60) mg/day, 6.58 (4.29, 9.91) mg/day, 9.93 (6.89, 14.08) mg/day, 99.60 (70.10, 136.80) μg/day, and 5344.00 (2002.00, 12162.00) μg/day, respectively. Figure S1. showed the Spearman correlation coefficients among serum and dietary antioxidant micronutrients. Significant correlations were found between both CDAI and individual dietary antioxidant micronutrients (r > 0.55). A strong correlation could be observed between serum zinc and serum arsenic (r = 0.63).
Association between CDAI and CRDs
We found a substantial negative connection between CDAI scores and the frequency of CRDs in all models (Table 2). Higher CDAI scores were linked with a lower prevalence of CRDs. After controlling for all variables, individuals in the fourth quartile of CDAI scores had a 19% lower prevalence than those in the first quartile (OR = 0.81 [0.72–0.91], Ptrend< 0.01). In the unadjusted model and model 2, CDAI was found to be negatively associated with the prevalence of emphysema and chronic bronchitis. Compared to the first quartile, the fourth quartile of CDAI was negatively associated with the prevalence of emphysema (OR = 0.57 [0.40–0.81], Ptrend<0.01) and chronic bronchitis (OR = 0.74 [0.62–0.88], Ptrend<0.01) after adjusting for all confounders. However, no association was observed between CDAI scores and the prevalence of asthma. Furthermore, we investigated the relationship between each dietary antioxidant micronutrient quartile and the occurrence of CRDs in adults (Table S3).
Table 2.
ORs (95% CIs) of the prevalence of chronic respiratory diseases (CRDs) and its components according to quartiles of composite dietary antioxidant index (CDAI) among adults in NHANES 2001–2018.
| Quartiles of CDAI |
|||||
|---|---|---|---|---|---|
| <-2.26 | [-2.26 to −0.37] | [-0.38-1.98] | >1.98 | Ptrend | |
| CRDs | |||||
| Crude | 1 [Reference] | 0.87 (0.79–0.96) | 0.80 (0.72–0.88) | 0.81 (0.73–0.89) | <0.01 |
| Model 1 | 1 [Reference] | 0.90 (0.82–0.99) | 0.82 (0.74–0.90) | 0.83 (0.75–0.91) | <0.01 |
| Model 2 | 1 [Reference] | 0.91 (0.82–1.01) | 0.82 (0.74–0.91) | 0.81 (0.72–0.91) | <0.01 |
| Asthma | |||||
| Crude | 1 [Reference] | 0.90 (0.82–1.00) | 0.87 (0.79–0.96) | 0.90 (0.80–1.02) | 0.09 |
| Model 1 | 1 [Reference] | 0.94 (0.85–1.04) | 0.90 (0.82–1.00) | 0.93 (0.82–1.05) | 0.19 |
| Model 2 | 1 [Reference] | 0.92 (0.82–1.02) | 0.87 (0.79–0.97) | 0.87 (0.76–1.00) | 0.05 |
| Emphysema | |||||
| Crude | 1 [Reference] | 0.86 (0.68–1.09) | 0.59 (0.46–0.75) | 0.44 (0.33–0.58) | <0.01 |
| Model 1 | 1 [Reference] | 0.81 (0.63–1.02) | 0.56 (0.44–0.71) | 0.43 (0.32–0.57) | <0.01 |
| Model 2 | 1 [Reference] | 0.96 (0.74–1.25) | 0.72 (0.53–0.97) | 0.57 (0.40–0.81) | <0.01 |
| Chronic bronchitis | |||||
| Crude | 1 [Reference] | 0.77 (0.67–0.90) | 0.63 (0.54–0.73) | 0.63 (0.55–0.72) | <0.01 |
| Model 1 | 1 [Reference] | 0.80 (0.68–0.93) | 0.64 (0.55–0.75) | 0.64 (0.55–0.74) | <0.01 |
| Model 2 | 1 [Reference] | 0.86 (0.73–1.02) | 0.73 (0.62–0.86) | 0.74 (0.62–0.88) | <0.01 |
Model 1 was adjusted for age (20–39, 40–59, or ≥60), sex (male or female), and race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black or Other).
Model 2 was adjusted for Model 1 plus education level (below high school, high school, or above high school), family income-to-poverty ratio (≤1.0, 1.1–3.0, or >3.0), smoking status (never smoker, former smoker, or current smoker), drinking status (nondrinker, low-to-moderate drinker, or heavy drinker), BMI (<25.0, 25.0–29.9, or >29.9), energy intake levels (in quartiles), physical activity (inactive, insufficiently active, or active), diabetes (yes or no), hypertension (yes or no), and supplement use (yes or no)
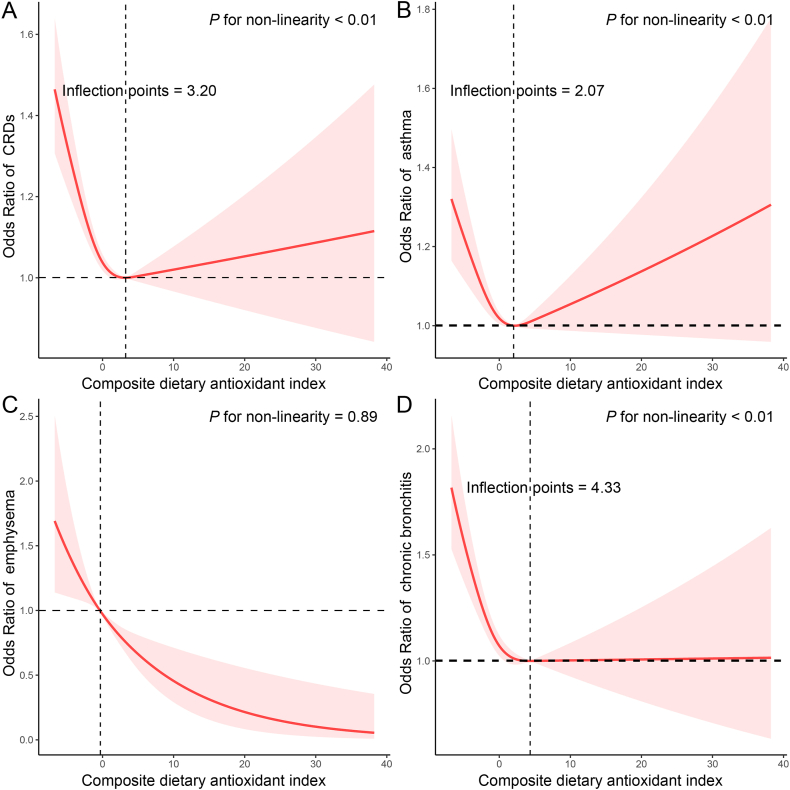
The dose-response relationship between CDAI and CRDs was further analysed using RCS (Fig. 2). We found a non-linear negative correlation between CDAI and the prevalence of CRDs (P for nonlinearity <0.01), with an inflection point of 3.20. This non-linear relationship was also present between CDAI and the prevalence of chronic bronchitis (P for nonlinearity <0.01), with an inflection point of 4.33. However, there was a negative linear association between CDAI and the prevalence of emphysema (P for nonlinearity = 0.89).
Fig. 2.
The exposure-response association of the composite dietary antioxidant index (CDAI) with the prevalence of chronic respiratory diseases (CRDs) by restricted cubic spline (RCS). Analyses were adjusted for covariates age (20–39, 40–59, or ≥60), sex (male or female), race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black or Other), education level (below high school, high school, or above high school), family income-to-poverty ratio (≤1.0, 1.1–3.0, or >3.0), smoking status (never smoker, former smoker, or current smoker), drinking status (nondrinker, low-to-moderate drinker, or heavy drinker), BMI (<25.0, 25.0–29.9, or >29.9), energy intake levels (in quartiles), physical activity (inactive, insufficiently active, or active), diabetes (yes or no), hypertension (yes or no), and supplement use (yes or no).
Stratified analysis
We analysed the study population in subgroups according to age, sex, race, family PIR, smoking and drinking status, physical activity, BMI, and supplement use (Table 3). Interestingly, we found that the association between CDAI and prevalence of CRDs was consistent across all subgroups, suggesting that it was not influenced by these demographic factors.
Table 3.
Stratified analyses of the associations between quartiles of composite dietary antioxidant index (CDAI) and the prevalence of chronic respiratory diseases (CRDs) in NHANES 2001–2018.
| Subgroups | N | Quartiles of CDAI |
Pinteraction | |||
|---|---|---|---|---|---|---|
| <-2.26 | [-2.26, −0.37] | [-0.38,1.98] | >1.98 | |||
| Age, years | ||||||
| 20-39 | 12 884 | 1 [Reference] | 0.86 (0.73,1.01) | 0.77 (0.65,0.92) | 0.83 (0.69,0.99) | 0.29 |
| 40-59 | 13 281 | 1 [Reference] | 0.86 (0.72,1.03) | 0.79 (0.65,0.95) | 0.67 (0.55,0.83) | |
| ≥60 | 14 392 | 1 [Reference] | 1.00 (0.85,1.18) | 0.90 (0.73,1.10) | 0.97 (0.79,1.20) | |
| Sex, % | ||||||
| Female | 20 921 | 1 [Reference] | 0.99 (0.84,1.17) | 0.87 (0.73,1.05) | 0.91 (0.75,1.10) | 0.40 |
| Male | 19 636 | 1 [Reference] | 0.86 (0.75,0.97) | 0.79 (0.69,0.89) | 0.75 (0.64,0.86) | |
| Race, % | ||||||
| Non-Hispanic White | 18 345 | 1 [Reference] | 0.93 (0.81,1.07) | 0.84 (0.73,0.97) | 0.82 (0.70,0.96) | 0.77 |
| Other | 22 212 | 1 [Reference] | 0.83 (0.73,0.96) | 0.77 (0.65,0.90) | 0.78 (0.66,0.92) | |
| Family PIR, % | ||||||
| ≤1.0 | 8219 | 1 [Reference] | 0.91 (0.74,1.11) | 0.85 (0.69,1.05) | 0.74 (0.59,0.93) | 0.09 |
| 1.1–3.0 | 17 097 | 1 [Reference] | 0.89 (0.77,1.04) | 0.72 (0.62,0.84) | 0.77 (0.66,0.89) | |
| >3.0 | 15 241 | 1 [Reference] | 0.94 (0.79,1.11) | 0.92 (0.78,1.09) | 0.89 (0.73,1.10) | |
| Smoking status, % | ||||||
| Never smoker | 22 066 | 1 [Reference] | 0.90 (0.78,1.05) | 0.84 (0.72,0.97) | 0.90 (0.76,1.06) | |
| Former smoker | 10 216 | 1 [Reference] | 0.92 (0.76,1.10) | 0.81 (0.65,1.02) | 0.74 (0.59,0.92) | 0.32 |
| Current smoker | 8275 | 1 [Reference] | 0.92 (0.76,1.13) | 0.84 (0.69,1.03) | 0.74 (0.59,0.92) | |
| Drinking status, % | ||||||
| Nondrinker | 9370 | 1 [Reference] | 1.03 (0.83,1.29) | 0.99 (0.77,1.26) | 0.88 (0.69,1.12) | |
| Low-to-moderate drinker | 28 020 | 1 [Reference] | 0.87 (0.76,0.98) | 0.75 (0.66,0.86) | 0.76 (0.65,0.88) | 0.43 |
| Heavy drinker | 3167 | 1 [Reference] | 0.92 (0.65,1.29) | 1.03 (0.71,1.50) | 1.06 (0.70,1.61) | |
| Physical activity, % | ||||||
| Inactive | 10 991 | 1 [Reference] | 0.98 (0.82,1.17) | 0.80 (0.65,0.99) | 0.96 (0.78,1.19) | |
| Insufficiently active | 14 885 | 1 [Reference] | 0.87 (0.75,1.02) | 0.79 (0.68,0.92) | 0.73 (0.62,0.86) | 0.65 |
| Active | 14 681 | 1 [Reference] | 0.90 (0.73,1.10) | 0.85 (0.70,1.03) | 0.81 (0.65,1.01) | |
| Body mass index, % | ||||||
| <25.0 kg/m2 | 11 674 | 1 [Reference] | 0.96 (0.77,1.20) | 0.90 (0.75,1.08) | 0.82 (0.66,1.01) | |
| 25.0–29.9 kg/m2 | 13 680 | 1 [Reference] | 0.95 (0.79,1.14) | 0.82 (0.67,1.00) | 0.81 (0.66,0.99) | 0.80 |
| >29.9 kg/m2 | 15 203 | 1 [Reference] | 0.86 (0.74,0.99) | 0.78 (0.67,0.92) | 0.82 (0.69,0.97) | |
| Supplement use, % | ||||||
| No | 19 920 | 1 [Reference] | 0.88 (0.76,1.01) | 0.80 (0.70,0.92) | 0.77 (0.64,0.93) | 0.99 |
| Yes | 20 637 | 1 [Reference] | 0.93 (0.82,1.06) | 0.84 (0.72,0.98) | 0.85 (0.72,0.99) | |
Analyses were adjusted for covariates age (20–39, 40–59, or ≥60), sex (male or female), race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black or Other), education level (below high school, high school, or above high school), family income-to-poverty ratio (≤1.0, 1.1–3.0, or >3.0), smoking status (never smoker, former smoker, or current smoker), drinking status (nondrinker, low-to-moderate drinker, or heavy drinker), BMI (<25.0, 25.0–29.9, or >29.9), energy intake levels (in quartiles), physical activity (inactive, insufficiently active, or active), diabetes (yes or no), hypertension (yes or no), and supplement use (yes or no) when they were not the strata variables
Discussion
We conducted a study with a total of 40 557 participants to investigate the potential connection between CDAI scores and the prevalence of CRDs. Our findings revealed that individuals in the fourth quartile of CDAI scores exhibited a 19% lower prevalence of CRDs compared to those in the first quartile (OR = 0.81 [0.72–0.91], Ptrend< 0.01). Among the 3 CRDs examined, CDAI showed a negative association with the prevalence of chronic bronchitis and emphysema, but no significant association was observed with asthma. Notably, this association between CDAI and CRDs remained consistent across all subgroups analysed. This pioneering study is the first to demonstrate a link between CDAI and CRD prevalence in the general population.
The CDAI is an indicator used to quantify total dietary antioxidant intake,13 taking into account antioxidants from various dietary sources such as fruits, vegetables, tea, and coffee.31 Previous studies have indicated that higher CDAI scores (indicating greater antioxidant intake) are associated with a reduced risk of developing diabetic nephropathy.32 Oxidative stress in the body is believed to contribute to chronic diseases like liver disease, cardiovascular disease, certain types of cancer, and neurodegenerative diseases.33, 34, 35 Antioxidant-rich foods have shown positive effects on cardiovascular health, cancer prevention, and cognitive function.36, 37, 38 By neutralizing free radicals and reducing oxidative stress, a diet high in antioxidants may help prevent the progression of chronic diseases.39 In line with these findings, our study revealed a negative correlation between antioxidant intake and the prevalence of CRDs. Specifically, individuals with higher total dietary antioxidant intake exhibited a lower prevalence of emphysema and chronic bronchitis. These results lend support to the notion that antioxidants may play a protective role in the development of CRDs.
Antioxidants play a crucial role in maintaining the delicate balance between harmful ROS and protective antioxidants in the body.40 Research indicates that increasing antioxidant consumption in the diet can reduce the incidence of respiratory disorders.41 Notably, vitamins A, C, and E are known for their potent antioxidant properties and immune-enhancing effects.42 These vitamins play a vital role in safeguarding the respiratory tract from inflammation, which is a common underlying factor in diseases like asthma and COPD.43 Additionally, zinc and selenium, as trace minerals, act as cofactors for antioxidant enzymes in the body. They contribute to normal immune system functioning by reducing inflammation and promoting tissue healing in the respiratory system.44,45 Deficiencies in these minerals are associated with impaired respiratory function and heightened susceptibility to respiratory infections.46 Carotenoids, found in various foods, are pigments with antioxidant properties. They have demonstrated protective effects on the respiratory system, including a reduced risk of asthma and COPD.47 Overall, our findings suggest that higher CDAI scores (indicating multiple antioxidant consumption) are associated with a decreased likelihood of respiratory diseases when compared to single antioxidant intake. It is crucial to highlight that the link between CDAI and respiratory health is complex and influenced by other factors such as genetics, lifestyle, and environmental exposures.
The protective effects of antioxidants can be attributed to several mechanisms. Firstly, oxidative stress has been linked to the development of CRDs, leading to airway inflammation and tissue damage.48 Antioxidants work to neutralize free radicals and protect cells and tissues from oxidative damage.49 Therefore, a higher dietary antioxidant intake may reduce oxidative stress, alleviating symptoms and halting the progression of CRDs. Secondly, antioxidants have been found to influence immunological activity, a critical characteristic of many CRDs.50,51 Antioxidants, such as vitamin C and vitamin E, possess immunomodulatory properties that promote a balanced immune response, modulate the production of pro-inflammatory mediators, and reduce allergic reactions.52,53 This immunomodulatory action could help explain the apparent protective relationship between dietary antioxidant intake and CRDs. Furthermore, antioxidants have been linked to enhanced lung function.54 Oxidative stress-induced lung tissue damage can lead to impaired respiratory function.55 Antioxidant-rich diets, particularly those high in fruits and vegetables, have been associated with improved lung function indicators.56 These findings suggest that increasing dietary antioxidant consumption may promote lung health and lower the prevalence of CRDs.
Our study identified several key risk factors associated with CRDs, including older age (≥60 years), female gender, non-Hispanic white ethnicity, current smoking status, obesity, and physical inactivity. These findings underscore the multifactorial nature of CRDs and the importance of addressing the complex interplay of factors that contribute to their development. Inflammation is a central player in the pathogenesis of CRDs. Current smokers and obese individuals are known to have higher rates of inflammation due to increased production of pro-inflammatory cytokines such as TNF α and IL-6 from adipose tissue, as well as a lower production of anti-inflammatory cytokines.57 This heightened inflammatory state can contribute to the progression of CRDs. It is noteworthy that our results also indicated a higher prevalence of hypertension and diabetes among individuals with CRDs. Both of these conditions are closely linked with inflammation, and in particular, weight status plays a pivotal role in this relationship. Obesity is recognized as a state of chronic low-grade inflammation, which can exacerbate the risk of hypertension and diabetes.57 Furthermore, our findings revealed that CRD patients were more likely to be over the age of 60 years and female. This aligns with the observation that postmenopausal women, experiencing lower estrogen levels, face an increased risk of several chronic diseases. Changes in fat distribution in the body during menopause contribute to a pro-inflammatory environment,58 further underscoring the role of inflammation in the pathogenesis of CRDs.
The study's strength is that it uses data from an extensive number of participants from the nine cycles of the 2001–2018 NHANES, which increases the confidence of the study's findings. Second, compared with previous metrics, the CDAI is a novel approach to assessing dietary antioxidant intake that takes into account the intake of key antioxidants, including vitamins A, C, and E, and zinc, selenium, and carotenoids. Third, we not only explored the effects of multiple confounders, but also assessed the association between CDAI and the prevalence of CRDs through stratified analyses.
However, this study has several drawbacks. First, this is a cross-sectional study, which does not allow for causal inference of correlations. Second, CRDs include not only asthma, emphysema and chronic bronchitis but also other diseases, so the conclusions drawn from this study are limited and need to be validated by including more types of CRDs. Third, the intakes of various dietary nutrients were derived from the average of 2 24-h dietary reviews, so they may not be representative of daily dietary changes. Additionally, dietary intakes may vary among individuals due to personal preferences and cultural factors, leading to potential confounding effects.
Conclusion
CDAI is negatively associated with the prevalence of CRDs (especially emphysema and chronic bronchitis) in the adult population. These findings highlight a potential benefit to diets high in antioxidant-rich foods with regards to lung function and onset of CRD. Further studies need to be done to determine whether higher dietary intake of antioxidant-rich foods is in fact protective especially in populations at higher risk of developing CRDs (ie, obese, postmenopausal women, or physically inactive individuals). Further studies on the underlying mechanisms are necessary.
Abbreviations
CRDs, Chronic respiratory diseases; NHANES, National Health and Nutrition Examination Survey; CDAI, composite dietary antioxidant index; RCS, restricted cubic spline; ROS, reactive oxygen species; COPD, chronic obstructive pulmonary disease; NCHS, National Center for Health Statistics; PIR, poverty income ratio; MET, metabolic equivalents; SEs, standard errors; IQRs, interquartile ranges.
Funding
None.
Data availability
NHANES data described in this manuscript are available at https://wwwn.cdc.gov/nchs/nhanes/.
Author contributions
Shidong Wang: Conceptualization, Methodology, Software, Formal analysis, Writing-original draft, Visualization. Hong Teng: Writing-review and editing. Lin Zhang: Writing-review and editing. Liang Wu: Conceptualization, Methodology, Project administration, Writing-review and editing, Supervision.
Ethics approval and consent to participant
All participants provided written informed consent and study procedures were approved by the National Center for Health Statistics Research Ethics Review Board (Protocol Number: Protocol #98-12, Protocol #2005–06, Protocol #2011–17, and Protocol #2018–01).
Consent to participate
The manuscript is approved by all authors for publication.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
We appreciate the people who contributed to the NHANES data we studied.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2023.100851.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Collaborators GBDCRD Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porsbjerg C., Melen E., Lehtimaki L., Shaw D. Asthma. Lancet. 2023;401(10379):858–873. doi: 10.1016/S0140-6736(22)02125-0. [DOI] [PubMed] [Google Scholar]
- 3.Singh D., Agusti A., Anzueto A., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5) doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 4.Mejza F., Gnatiuc L., Buist A.S., et al. Prevalence and burden of chronic bronchitis symptoms: results from the BOLD study. Eur Respir J. 2017;50(5) doi: 10.1183/13993003.00621-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Han X., Li J., et al. Associations between the compositional patterns of blood volatile organic compounds and chronic respiratory diseases and ages at onset in NHANES 2003-2012. Chemosphere. 2023;327 doi: 10.1016/j.chemosphere.2023.138425. [DOI] [PubMed] [Google Scholar]
- 6.Marchetti P., Miotti J., Locatelli F., et al. Long-term residential exposure to air pollution and risk of chronic respiratory diseases in Italy: the BIGEPI study. Sci Total Environ. 2023;884 doi: 10.1016/j.scitotenv.2023.163802. [DOI] [PubMed] [Google Scholar]
- 7.Knox-Brown B., Patel J., Potts J., et al. Small airways obstruction and its risk factors in the Burden of Obstructive Lung Disease (BOLD) study: a multinational cross-sectional study. Lancet Global Health. 2023;11(1):e69–e82. doi: 10.1016/S2214-109X(22)00456-9. [DOI] [PubMed] [Google Scholar]
- 8.Xiong T., Bai X., Wei X., et al. Exercise rehabilitation and chronic respiratory diseases: effects, mechanisms, and therapeutic benefits. Int J Chronic Obstr Pulm Dis. 2023;18:1251–1266. doi: 10.2147/COPD.S408325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raad S., Smith C., Allen K. Nutrition status and chronic obstructive pulmonary disease: can we move beyond the body mass index? Nutr Clin Pract. 2019;34(3):330–339. doi: 10.1002/ncp.10306. [DOI] [PubMed] [Google Scholar]
- 10.Arigliani M., Spinelli A.M., Liguoro I., Cogo P. Nutrition and lung growth. Nutrients. 2018;10(7) doi: 10.3390/nu10070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scoditti E., Massaro M., Garbarino S., Toraldo D.M. Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients. 2019;11(6) doi: 10.3390/nu11061357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sierra-Vargas M.P., Montero-Vargas J.M., Debray-Garcia Y., Vizuet-de-Rueda J.C., Loaeza-Roman A., Teran L.M. Oxidative stress and air pollution: its impact on chronic respiratory diseases. Int J Mol Sci. 2023;(1):24. doi: 10.3390/ijms24010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright M.E., Mayne S.T., Stolzenberg-Solomon R.Z., et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004;160(1):68–76. doi: 10.1093/aje/kwh173. [DOI] [PubMed] [Google Scholar]
- 14.Luu H.N., Wen W., Li H., et al. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxidants Redox Signal. 2015;22(11):951–959. doi: 10.1089/ars.2014.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Meo S., Venditti P. Evolution of the knowledge of free radicals and other oxidants. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/9829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L., Sun Y., Cao R., Wu X., Huang T., Peng W. Non-linear association between composite dietary antioxidant index and depression. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.988727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Tang W., Li H., Lv J., Chang L., Chen S. Composite dietary antioxidant index negatively correlates with osteoporosis among middle-aged and older US populations. Am J Transl Res. 2023;15(2):1300–1308. [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y.C., Paragomi P., Wang R., et al. Composite dietary antioxidant index and the risk of colorectal cancer: findings from the Singapore Chinese Health Study. Int J Cancer. 2022;150(10):1599–1608. doi: 10.1002/ijc.33925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Yi Z. Association of the Composite dietary antioxidant index with all-cause and cardiovascular mortality: a prospective cohort study. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.993930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginter E., Simko V., Panakova V. Antioxidants in health and disease. Bratisl Lek Listy. 2014;115(10):603–606. doi: 10.4149/bll_2014_116. [DOI] [PubMed] [Google Scholar]
- 21.Arulselvan P., Fard M.T., Tan W.S., et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi J.H., Dong F.X. The relevant targets of anti-oxidative stress: a review. J Drug Target. 2021;29(7):677–686. doi: 10.1080/1061186X.2020.1870987. [DOI] [PubMed] [Google Scholar]
- 23.Seyedrezazadeh E., Moghaddam M.P., Ansarin K., et al. Dietary factors and risk of chronic obstructive pulmonary disease: a systemic review and meta-analysis. Tanaffos. 2019;18(4):294–309. [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Larsen V., Del Giacco S.R., Moreira A., et al. Asthma and dietary intake: an overview of systematic reviews. Allergy. 2016;71(4):433–442. doi: 10.1111/all.12800. [DOI] [PubMed] [Google Scholar]
- 25.Gu W., Tian Z., Tian W., et al. Association of rest-activity circadian rhythm with chronic respiratory diseases, a cross-section survey from NHANES 2011-2014. Respir Med. 2023;209 doi: 10.1016/j.rmed.2023.107147. [DOI] [PubMed] [Google Scholar]
- 26.Maugeri A., Hruskova J., Jakubik J., et al. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: a cross-sectional assessment in the Kardiovize study. Free Radic Biol Med. 2019;131:274–281. doi: 10.1016/j.freeradbiomed.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Fadeyev K., Nagao-Sato S., Reicks M. Nutrient and food group intakes among U.S. Children (2-5 Years) differ by family income to poverty ratio, NHANES 2011-2018. Int J Environ Res Publ Health. 2021;18(22) doi: 10.3390/ijerph182211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu Z., Chen X., Geng T., et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: results from NHANES. Diabetes Care. 2022;45(6):1453–1461. doi: 10.2337/dc21-2371. [DOI] [PubMed] [Google Scholar]
- 29.Pate R.R., Pratt M., Blair S.N., et al. Physical activity and public health. A recommendation from the Centers for disease Control and prevention and the American college of sports medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 30.Thivel D., Tremblay A., Genin P.M., Panahi S., Rivière D., Duclos M. Physical activity, inactivity, and sedentary behaviors: definitions and implications in occupational health. Front Public Health. 2018;6:288. doi: 10.3389/fpubh.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu W., Shi Y., Wang R., et al. Antioxidant activity and healthy benefits of natural pigments in fruits: a review. Int J Mol Sci. 2021;22(9) doi: 10.3390/ijms22094945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J., Chen Y., Zou L., et al. Dose-response relationship between dietary antioxidant intake and diabetic kidney disease in the US adults with diabetes. Acta Diabetol. 2023 doi: 10.1007/s00592-023-02125-9. [DOI] [PubMed] [Google Scholar]
- 33.Seen S. Chronic liver disease and oxidative stress - a narrative review. Expet Rev Gastroenterol Hepatol. 2021;15(9):1021–1035. doi: 10.1080/17474124.2021.1949289. [DOI] [PubMed] [Google Scholar]
- 34.Liguori I., Russo G., Curcio F., et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 36.Kim K., Vance T.M., Chun O.K. Greater total antioxidant capacity from diet and supplements is associated with a less atherogenic blood profile in U.S. Adults. Nutrients. 2016;8(1) doi: 10.3390/nu8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J., Kim H., Lee J., Choi I.J., Kim Y.I., Kim J. Antioxidant-rich diet, GSTP1 rs1871042 polymorphism, and gastric cancer risk in a hospital-based case-control study. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.596355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park H.A., Ellis A.C. Dietary antioxidants and Parkinson's disease. Antioxidants. 2020;9(7) doi: 10.3390/antiox9070570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrari C.K., Percario S., Silva J.C., da Silva Torres E.A. An apple plus a Brazil nut a day keeps the doctors away: antioxidant capacity of foods and their health benefits. Curr Pharmaceut Des. 2016;22(2):189–195. doi: 10.2174/1381612822666151117122715. [DOI] [PubMed] [Google Scholar]
- 40.Poljsak B., Suput D., Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Larsen V., Potts J.F., Omenaas E., et al. Dietary antioxidants and 10-year lung function decline in adults from the ECRHS survey. Eur Respir J. 2017;50(6) doi: 10.1183/13993003.02286-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins M.R., Izadi A., Kaviani M. Antioxidants and exercise performance: with a focus on vitamin E and C supplementation. Int J Environ Res Publ Health. 2020;17(22) doi: 10.3390/ijerph17228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salo P.M., Mendy A., Wilkerson J., et al. Serum antioxidant vitamins and respiratory morbidity and mortality: a pooled analysis. Respir Res. 2022;23(1):150. doi: 10.1186/s12931-022-02059-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riccioni G., D'Orazio N. The role of selenium, zinc and antioxidant vitamin supplementation in the treatment of bronchial asthma: adjuvant therapy or not? Expet Opin Invest Drugs. 2005;14(9):1145–1155. doi: 10.1517/13543784.14.9.1145. [DOI] [PubMed] [Google Scholar]
- 45.Weyh C., Kruger K., Peeling P., Castell L. The role of minerals in the optimal functioning of the immune system. Nutrients. 2022;14(3) doi: 10.3390/nu14030644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isbaniah F., Wiyono W.H., Yunus F., Setiawati A., Totzke U., Verbruggen M.A. Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: results from a randomized controlled trial. J Clin Pharm Therapeut. 2011;36(5):568–576. doi: 10.1111/j.1365-2710.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 47.Manochkumar J., Singh A., Efferth T., Ramamoorthy S. Untapping the protective role of carotenoids against respiratory diseases. Phytomedicine. 2022;104 doi: 10.1016/j.phymed.2022.154286. [DOI] [PubMed] [Google Scholar]
- 48.Liu K., Hua S., Song L. PM2.5 exposure and asthma development: the key role of oxidative stress. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/3618806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma G.N., Gupta G., Sharma P. A comprehensive review of free radicals, antioxidants, and their relationship with human ailments. Crit Rev Eukaryot Gene Expr. 2018;28(2):139–154. doi: 10.1615/CritRevEukaryotGeneExpr.2018022258. [DOI] [PubMed] [Google Scholar]
- 50.Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9(12) doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11) doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spoelstra-de Man A.M.E., Elbers P.W.G., Oudemans-Van Straaten H.M. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24(4):248–255. doi: 10.1097/MCC.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee G.Y., Han S.N. The role of vitamin E in immunity. Nutrients. 2018;10(11) doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sdona E., Hallberg J., Andersson N., et al. Dietary antioxidant intake in school age and lung function development up to adolescence. Eur Respir J. 2020;55(2) doi: 10.1183/13993003.00990-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicks M.E., O'Brien M.M., Bowler R.P. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD. 2011;8(4):264–269. doi: 10.3109/15412555.2011.579202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siedlinski M., Boer J.M., Smit H.A., Postma D.S., Boezen H.M. Dietary factors and lung function in the general population: wine and resveratrol intake. Eur Respir J. 2012;39(2):385–391. doi: 10.1183/09031936.00184110. [DOI] [PubMed] [Google Scholar]
- 57.Raghubeer S: The influence of epigenetics and inflammation on cardiometabolic risks. Semin Cell Dev Biol 2024, 154(Pt C):175-184. 10.1016/j.semcdb.2023.02.006. [DOI] [PubMed]
- 58.Christensen A., Pike C.J. Menopause, obesity and inflammation: interactive risk factors for Alzheimer's disease. Front Aging Neurosci. 2015;7:130. doi: 10.3389/fnagi.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NHANES data described in this manuscript are available at https://wwwn.cdc.gov/nchs/nhanes/.