Abstract
Retrotransposons are RNA elements that reverse transcribe their RNA genomes and make a cDNA copy that is inserted back into a new genomic location by the element-encoded integrase protein. Ty1 is a long terminal repeat (LTR) retrotransposon in Saccharomyces cerevisiae that inserts into an ∼700-bp integration window upstream of tRNA genes with a periodicity of ∼80 bp. ATP-dependent chromatin remodeling by Isw2 upstream of tRNA genes leads to changes in chromatin structure and Ty1 integration site selection. We show that the N terminus of Bdp1p, a component of the RNA polymerase III transcription factor TFIIIB, is required for periodic integration of Ty1 into the integration window. Deletion of the Bdp1p N terminus and mutation of ISW2 result in similar disruption of nucleosome positioning upstream of some tRNA genes, and the N-terminal domain of Bdp1p is required for targeting of Isw2 complex to tRNA genes. This study provides the first example for recruitment of an ATP-dependent chromatin-remodeling factor by a general transcription factor in vivo.
Keywords: RNA polymerase III, chromatin, integrase, retrotransposon, transcription factor, tRNA gene
Ty1 is a long terminal repeat (LTR) retrotransposon in Saccharomyces cerevisiae that integrates nonrandomly into the host genome. Previous work has determined that the Ty1 preferentially integrates Ty1 cDNA into ∼700-bp “integration windows” upstream of genes transcribed by RNA polymerase III (pol III) (Ji et al. 1993; Devine and Boeke 1996; Bolton and Boeke 2003). Such insertion depends on the presence of a transcriptionally competent tRNA gene (tDNA) (Devine and Boeke 1996). Simultaneous examination of populations of insertions has shown that insertions occur periodically with maxima ∼80 bp apart upstream of different tDNA copies and types (Bachman et al. 2004).
Targeted integration upstream of tDNAs is a property of diverse retrotransposon types, possibly as a mechanism for avoiding insertion into genes in densely packed host genomes (Kinsey and Sandmeyer 1991; Boeke and Devine 1998; Sandmeyer 1998). Ty3, a LTR-retrotransposon distantly related to Ty1, inserts at the start site of pol III transcription, with integration mediated by an interaction between the pol III factors TFIIIB and TFIIIC and the Ty3 integrase molecule (Yieh et al. 2000, 2002). The Dictyostelium non-LTR transposon TRE-5 has also evolved a mechanism to insert upstream of pol III genes. This transposon inserts an average of 48 bp upstream of the pol III transcription start site, and it has been suggested that the integration machinery identifies the upstream boundary of the TFIIIB preinitiation complex and uses it as a platform for adjacent insertion (Beck et al. 2002; Winckler et al. 2002).
pol III transcribes all tDNAs, the 5S ribosomal RNA genes, and some small nuclear RNA (snRNA) genes, such as U6. All pol III preinitiation complexes contain TFIIIC and TFIIIB (for reviews, see White 1998; Geiduschek and Kassavetis 2001; Schramm and Hernandez 2002). Transcription initiation begins with stable binding of TFIIIC to the internal promoter B box, followed by binding of the subunits of TFIIIB: Brf1p, TBP, and Bdp1p (for review, see Geiduschek and Kassavetis 2001). Addition of Bdp1p to the TFIIIB complex increases complex stability (Kassavetis et al. 1990) and extends its footprint on DNA, likely due to Bdp1p's ability to remodel the complex by changing the configuration of Brf1p on DNA (Kumar et al. 1997; Shah et al. 1999). The Bdp1p itself is reorganized upon DNA binding, with subsequent increase in accessibility of residues 190-210 to hydroxy radical cleavage (Kumar et al. 1997). Binding of Bdp1p is essential to promoter opening, which is likely to coordinate with its induction of a major bend (135°) in the DNA (Kassavetis et al. 1998; Grove et al. 1999). More than half of Bdp1p can be removed by deletion without obvious adverse consequences on viability, transcription activity in vitro, or the DNase I protection footprint of the TFIIIB complex (Kumar et al. 1997).
Our recent work has shown that the region upstream of tDNAs represents a new class of in vivo targets of the ATP-dependent chromatin remodeling complex, Isw2, and that nucleosome positioning activity in this region depended on the presence of a transcriptionally active tDNA (Gelbart et al. 2005). The Isw2 complex is a member of the imitation switch (ISWI) class of ATP-dependent chromatin remodeling complexes that utilizes the energy provided by the phosphate bond in ATP to slide nucleosomes (Fazzio and Tsukiyama 2003). Isw2p ATPase exists in two forms in vivo, as two-subunit (Itc1p-Isw2p) and four-subunit (Itc1p-Isw2p-Dpb4p-Dls1p) complexes (Iida and Araki 2004; McConnell et al. 2004). In vitro, Isw2 can evenly space nucleosomes randomly deposited onto a DNA template (Tsukiyama et al. 1999) and can slide a mononucleosome in cis along a DNA template (Kassabov et al. 2002; Gaillard et al. 2003; Fitzgerald et al. 2004). In vivo, Isw2 functions as a gene-specific transcriptional repressor by sliding nucleosomes to create a nuclease-inaccessible chromatin structure within the promoter regions of its target genes. A sequence-specific DNA-binding protein, Ume6p, recruits the complex to the promoters of early meiotic genes in haploid cells (Goldmark et al. 2000; Fazzio et al. 2001). Similarly, α2p complex is required for targeting Isw2 to MATa-specific gene promoters in α cells. In addition to its regulation of RNA polymerase II (pol II) transcription initiation, Isw2 activity is also needed for efficient pol II transcription termination (Alen et al. 2002). Our recent work showed that mutation of the ATP-dependent chromatin remodeling factor Isw2 disrupted nucleosome positions and Ty1 integration periodicity upstream of tDNAs, suggesting that integration periodicity requires a specific chromatin structure upstream of the tDNA imposed by the Isw2 complex (Gelbart et al. 2005). The activity of Isw2 upstream of tDNAs is intriguing and raises questions about the nature of the connection between nucleosome positioning by Isw2, targeted Ty1 integration, and tDNA activity.
In this work, we present evidence that a domain of Bdp1p between residues 139 and 240 plays a critical role in periodic Ty1 integration upstream of tDNAs in vivo. Loss of the first 240 residues, but not the first 138 residues of Bdp1p, resulted in a disruption of the periodic Ty1 integration pattern. These results are similar to those obtained with isw2 mutants. In both cases, disruption of Ty1 periodic integration was concomitant with changes in chromatin structure in the tDNA upstream region. We show that chromatin structure upstream of several tDNAs is altered similarly in the bdp1-Δ240 mutant and the isw2 mutant, suggesting that defects observed in the bdp1-Δ240 mutants result from loss of Isw2 activity upstream of tDNAs. Finally, we show that targeting of Isw2 to the tDNA upstream region depends on the presence of the first 240 residues of Bdp1p.
Results
Ty1 integration pattern in bdp1 mutant strains
Ty1 integration is periodic upstream of tDNAs in the yeast genome (Bachman et al. 2004). Because the targeting of Ty1 integration upstream of tDNAs depends on the pol III transcription machinery (Devine and Boeke 1996), we examined whether truncated forms of the Bdp1p subunit of TFIIIB, the transcription factor that binds near the downstream boundary of the “integration window,” would affect the integration pattern. This subunit of the TFIIIB complex, like the other components, is essential for viability, and we obtained previously described viable alleles (Ishiguro et al. 2002). We obtained three additional viable alleles with major truncations, Bdp1p 241-594, 1-509, and 241-521 (see Materials and Methods; for a detailed allele list, see Supplementary Table 1). To express each mutant protein, the endogenous copy of BDP1 was replaced with TRP1, and the resultant bdp1::TRP1 knockout allele was complemented with either wild-type BDP1 or its truncation alleles on a centromeric vector, pRS315 (Roberts et al. 1996; Ishiguro et al. 2002).
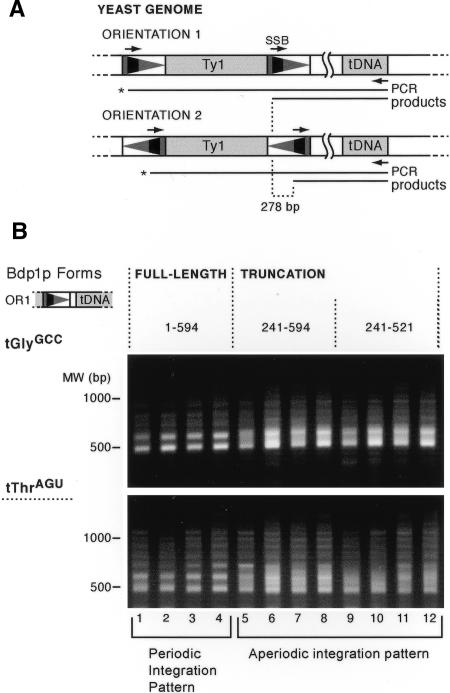
We employed a previously established assay (Bachman et al. 2004) to detect de novo Ty1 integration events in the presence of truncation alleles of Bdp1p. We induced transposition of a marked Ty1 element carrying a unique primer-binding site (the “SSB”) in the LTR. After integration into the genome, insertions in both orientations can be amplified using either the SSB primer (orientation 1: OR1) or its reverse complement (orientation 2: OR2) in combination with a primer complementary to the 3′ end of the tDNA in the genome (Fig. 1A). Because of the asymmetrical position of the SSB sequence in the LTR, the PCR products from amplification of opposite orientation insertions differ in length by 278 bp (Bachman et al. 2004). We amplified insertion events using tDNA primers complementary to the tGlyGCC (with 16 members) or tThrAGU (with 11 members) families of tDNA in yeast. Therefore, the resulting PCR products represent a population of insertions upstream of multiple tDNAs.
Figure 1.
PCR assay and pattern of Ty1 integration in bdp1 N-terminal deletion mutants. (A) Schematic of the PCR assay used in this work. Galactose-induction of a plasmid-borne transposon pVIT41 containing a unique sequence marker (“SSB,” narrow black rectangle) in the LTR (triangle) results in integration into the yeast genome. PCR using the SSB primer and a primer complementary to the tDNA results in amplification of a population of insertion events upstream of genes within the same tDNA family. Use of a primer complementary to a unique region downstream of a specific tDNA amplifies insertions upstream of a single tDNA target. Insertions in both orientations can be amplified using either the SSB primer or its reverse complement. Because of the asymmetric position of the SSB in the LTR, PCR products generated with the orientation 1 (OR1) primer will be 278 bp larger than those using the reverse complement, orientation 2 (OR2). (B) Removal of the first 240 residues of Bdp1p results in altered patterns of Ty1 insertions upstream of tGlyGCC (16 copies in the yeast genome) and tThrAGU genes (11 copies). Strains expressing either the wild-type Bdp1p (1-594) or the truncation alleles (241-594 and 241-521) were transformed with pVIT41, and four independent transformants were induced for transposition. Insertions upstream of tGlyGCC (top) and tThrAGU (bottom) were analyzed by PCR using the orientation 1 primer.
The pattern of PCR products from insertions in the BDP1+ wild-type strain shows the periodic banding pattern characteristic of Ty1 insertion events upstream of tDNAs (Fig. 1B, lanes 1-4; Bachman et al. 2004). Because the orientation 1 primer was used, a 404-bp band would occur if Ty1 had inserted at the start site of tRNA transcription. Instead, the smallest band is close to 500 bp, indicating that the Ty1 integration window is positioned 80-90 bp upstream of the tRNA start site, and the bands are separated by 80-90 bp, reflecting periodic integration within the window, as seen previously (Devine and Boeke 1996; Bachman et al. 2004). In contrast, the Bdp1p truncations lacking the first 240 residues of the protein show a more diffuse, blurred pattern compared with the wild type (Fig. 1B, lanes 5-12). The “blurring” effect is strongest at integration positions close to the tDNA but is observable up to a significant distance from the tDNA, extending to at least -600 from the start site of transcription. The same phenomenon is seen upstream of both tGlyGCC and tThrAGU, suggesting a general effect on tDNA targets. The size of the smallest PCR products is also similar in all samples, indicating that the mutant Bdp1 proteins do not alter the tDNA-proximal boundary of the integration window.
Bdp1p 139-240 is required for periodic integration
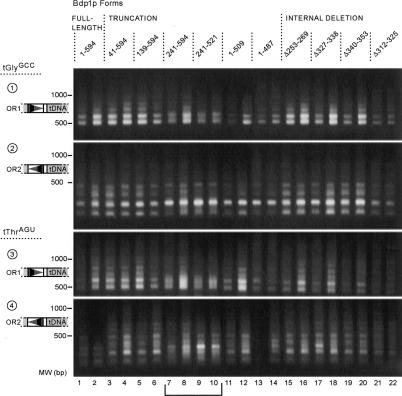
In order to further define the regions of Bdp1p necessary for periodic Ty1 integration, we analyzed insertion events in other bdp1 mutant strains. Most of the alleles show the same pattern of bands as the wild-type strain (Fig. 2). Deletion of the first 138 residues (Fig. 2, lanes 5,6) had no effect on the Ty1 integration pattern, whereas deleting up to position 240 did (Fig. 2, lanes 7-10), suggesting that the region between residues 139 and 240 of Bdp1p contains the sequences responsible for determining the periodic pattern of targeted Ty1 integration. Removal of the C terminus (1-509, Fig. 2, lanes 11,12; 1-487, Fig. 2, lanes 13,14) did not change the pattern. The internal deletions tested also did not show any defect in Ty1 periodic integration.
Figure 2.
Pattern of integration in all bdp1 mutants. Residues between 139 and 241 of Bdp1p are responsible for the altered pattern of Ty1 insertion. Strains expressing each bdp1 allele were transformed with pVIT41, and two independent transformants were induced for transposition. Genomic DNA was prepared from the population of induced cells and insertions were analyzed by PCR with all four primer pairs. (Top two panels) tGlyGCC-specific primer with orientation 1 primer (panel 1) and orientation 2 primer (panel 2). (Bottom two panels) tThrAGU-specific PCR with orientation 1 primer (panel 3) and orientation 2 primer (panel 4). PCR failed in lane 13 of panel 4. Residues present in the Bdp1p forms are noted at the top. (Lanes 7-10) A bracket denotes the lanes of PCR products from the N-terminal bdp1 deletion strains that show defects in periodic integration.
The effect on Ty1 integration does not correlate with the transcriptional competence of the Bdp1p form, as N-terminal deletions up to residue 263 and C-terminal truncations to residue 464 were all able to efficiently transcribe SUP4 and SNR6 RNA in vitro (Kumar et al. 1997). None of the internal deletions tested in this assay were defective for in vitro transcription (Kumar et al. 1997).
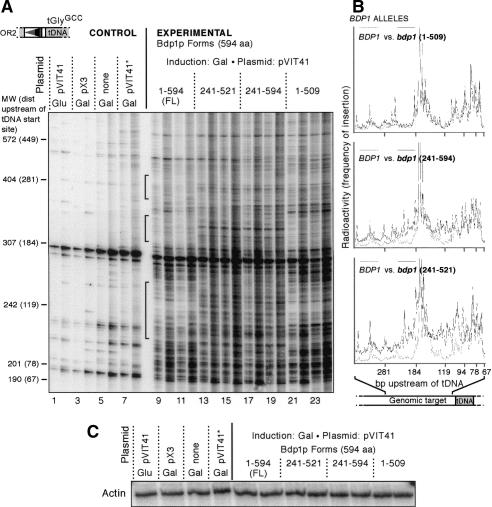
High-resolution analysis of the Ty1 targeting defect in bdp1 241-594
To increase the resolution of the targeting assay, we 32P-labeled the SSB oligo, performed the PCR reactions, and ran the resulting products on 4% denaturing polyacrylamide gels. We amplified insertions upstream of tGlyGCC genes and used the orientation 2 primer because it generated products small enough to resolve on the gel. Two PCR reactions were performed on each sample, with either 28 or 30 cycles of PCR to evaluate whether the pattern of insertion was constant with increasing cycles of PCR, and that the change in pattern was not due to increase in the bulk amount of PCR product. The OR2 primer was previously shown to have some nonspecific binding activity (Bachman et al. 2004), but the background bands can easily be seen using this method. There is a clear difference between the pattern of bands in the negative controls (Fig. 3A, lanes 1-8) and the experimental lanes (Fig. 3A, lanes 9-24). The smallest band on the gel is ∼200 bp, indicating that, as observed previously, the first integration event occurred 80-90 bp upstream of the tDNA start. Using the OR2 primer, the smallest band would be 126 bp if insertion occurred at the start site of tRNA transcription. The wild-type lanes show many bands as well as a periodic pattern of band intensity, consistent with the agarose gel patterns. These high-resolution gels show regions inaccessible to integration in the presence of full-length Bdp1p that become more accessible in the strains lacking the N-terminal 240 residues (Fig. 3A, lanes 13-20, see brackets). This cannot be explained by a general increase in transposition, as the overall transposition frequency in the truncation mutants was similar to that of the wild type (data not shown). The positions of the differences can also been seen clearly on the traces of the gels (Fig. 3B). The trace shows that loss of the Bdp1p C terminus alone (Bdp1p 1-509) does not result in major changes to the pattern of integration compared with the pattern in the presence of the full-length protein (Fig. 3B, top panel), though subtle differences can be observed. When bdp1-Δ240 is compared with wild type (Fig. 3B, middle panel), the targeting efficiency is similar at positions close to the tDNA (-67 to 110) but more integration is observed around -119, -174, and further upstream in the mutant. Additional loss of the C terminus (bdp1 241-521) (Fig. 3B, bottom panel) results in a pattern similar to that of bdp1-Δ240 (Fig. 3B, middle panel), with an increase in signal intensity. This could suggest that although the C terminus does not strongly affect integration pattern alone, in the absence of the N terminus, loss of the C-terminal residues can affect integration pattern and perhaps intensity as well. To ensure that the observed differences in intensity were not due to differences in the amount of input DNA, we performed PCR reactions for the actin gene (Fig. 3C). Actin PCRs were in the linear range of the PCR reaction (data not shown). All samples show equal amounts of actin PCR product, indicating that the same amount of genomic DNA was used in each PCR reaction.
Figure 3.
High-resolution analysis of PCR products from Ty1 integration events in bdp1 mutants. (A) Two transformants per negative control (lanes 1-8) or Bdp1p form (lanes 9-24) were analyzed by PCR. The orientation 2 primer was end-labeled with 32P, and insertions upstream of tGlyGCC genes were analyzed by PCR and run on a 4% polyacrylamide denaturing gel. For each transformant, 28 cycles of PCR (odd-numbered lanes) and 30 cycles of PCR (even-numbered lanes) are shown. Black brackets indicate regions where insertions are seen in the Bdp1p 241-594 and 241-521 mutants but not the full-length Bdp1p. (Glu) No induction on galactose; (pX3) Ty plasmid with no SSB marker in the LTR; (none) no plasmid; (pVIT41★) pVIT41 donor plasmid with a Ty1 element expressing a defective integrase protein; (MW) molecular-weight markers for comparison to size of PCR products on gel. Number in parentheses indicates the relative position upstream of the tDNA transcription start site, which was determined by subtracting the length of the tDNA and the LTR amplified by the OR2 primer (123 bp) from the size of the molecular-weight marker. (B) Traces of the polyacrylamide gel. Radioactivity is plotted vs. distance upstream of the tDNA start site. The trace from full-length Bdp1p (dashed line, trace of lane 9) is compared with Bdp1p 1-509 (top panel, solid line, trace of lane 21), Bdp1p 241-594 (middle panel, solid line, trace of lane 19), and Bdp1p 241-521 (bottom panel, solid line, trace of lane 13). The amount of radioactivity represents the abundance of a PCR product of a given length, which can also be interpreted as the frequency of insertion into a certain position. (C) Loading control: PCR of actin gene using the genomic DNA used in the integration PCR.
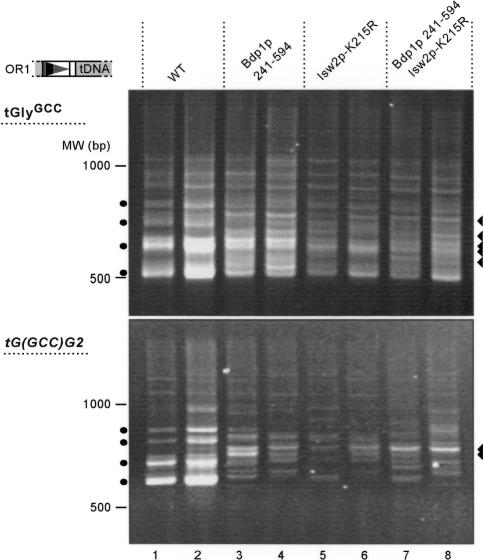
bdp1-Δ240 and Δisw2 mutants show similar disruption of periodic Ty1 integration
The PCR banding pattern observed in the bdp1-Δ240 mutants is very similar to that observed in Δisw2 mutants (Gelbart et al. 2005). We directly compared the Ty1 integration profile in strains expressing either Bdp1p 241-594 or catalytically-inactive Isw2p-K215R, as well as the double mutant (Fig. 4). PCR analysis of the Ty1 integration pattern upstream of tGlyGCC genes in the genome of the wild-type strain shows the characteristic periodic banding pattern (denoted by circles). In contrast, many more intervening bands are observed in both the isw2 and bdp1 single and double mutant strains (triangles), with minor differences between the single and double mutants. Disruption of the periodic Ty1 integration pattern in the catalytically-inactive Isw2p-K215R mutant suggests that it is Isw2 chromatin remodeling activity, and not merely its physical presence, that affects Ty1 integration site selection. Furthermore, these results indicate that in general the Bdp1p N-terminal region and catalytically active Isw2p have similar effects on Ty1 integration. The subtle differences observed between the single and double mutants suggest that, although the effects of Isw2p and the Bdp1p N-terminal region are similar, each may have its own weak independent activity upstream of tDNAs. Similar conclusions were reached from the analysis of integration events upstream of a single tDNA target, tG(GCC)G2 (Fig. 4B). A periodic banding pattern is observed in the wild-type strain (denoted by circles), whereas the pattern is altered in all the mutant strains, with integration events occurring into more positions (new bands denoted by triangles). For example, there is an area between the second and third band of the periodic pattern in the wild type that contains at least one prominent band in the mutants, indicating new integration positions that become available in this region in the mutant strains. The similar, though not identical, integration patterns in the isw2 and bdp1 single and double mutants suggest the possibility that Bdp1p and Isw2p function in the same pathway to regulate the Ty1 integration pattern.
Figure 4.
Ty1 integration pattern upstream of a single and multiple copies of tGlyGCC in the bdp1-Δ240 and isw2-K215R single mutants and the bdp1-Δ240isw2-K215R double mutant. (Top) The orientation 1 primer was combined with the tGlyGCC primer to amplify insertions upstream of the family of tGlyGCC genes. (Bottom) Insertions upstream of a single tGlyGCC, tG(GCC)G2, were amplified using the orientation 1 primer and a primer complementary to unique sequence downstream of the tRNA gene. The resulting products are therefore larger than those generated in the top panel. Two independent transformants of each strain are shown. Circles denote the four bands characteristic of periodic Ty1 integration, and triangles denote the major bands present in the mutant strains but not in the wild type.
bdp1-Δ240 and isw2 mutants show similar chromatin alterations upstream of tDNAs
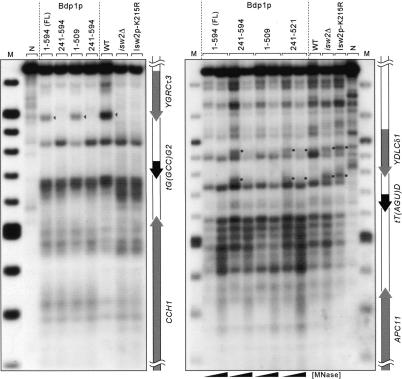
Analysis of chromatin structure upstream of tDNAs in Δisw2 mutants showed that in the absence of functional Isw2p, nucleosome positions were altered in the same regions that Ty1 periodic integration was disrupted in the Δisw2 mutants (Gelbart et al. 2005). Because of the similarity in Ty1 integration pattern in the bdp1-Δ240 mutants and the Δisw2 mutants, we examined the chromatin structure upstream of several tDNAs in the bdp1-Δ240 mutants by micrococcal nuclease (MNase) digestion followed by indirect end-labeling. Figure 5 shows the chromatin analysis upstream of two Ty1 integration targets, tG(GCC)G2 (one of two tGlyGCC genes on chromosome 7) and tT(AGU)D (tThrAGU on chromosome 4), in both the bdp1-Δ240 and Δisw2 mutants. There is a strong MNase cut site upstream of tG(GCC)G2 that is present in the wild-type strains but absent in both the bdp1-Δ240 and Δisw2 mutants (Fig. 5, left panel, marked by a triangle). The digestion pattern downstream of tG(GCC)G2 is similar in wild type and mutants. Similarities in chromatin structure between isw2 and bdp1-Δ240 mutants were also observed upstream of tT(AGU)D (Fig. 5, right panel). The MNase digestion patterns are consistent with the presence of a nucleosome over YDLCδ1 in wild-type that is shifted in a subpopulation of isw2 and bdp1-Δ240 mutant cells (Fig. 5, right panel, marked by a circle). The differences in these regions correspond to the -90 and the -180 positions, major areas in which periodic integration is disrupted in the bdp1-Δ240 mutant (Fig. 3A) and isw2 mutants (Fig. 4; Gelbart et al. 2005).
Figure 5.
Nucleosome mapping by MNase digestion in the bdp1 mutants compared with isw2 mutants at two different tRNA genes, tG(GCC)G2 and tT(AGU)D. (N) Naked DNA digestion; (Isw2p-K215R) catalytically inactive mutant of Isw2p; (FL) full-length; (M) molecular weight markers. Circles to the right of a lane denote cut sites absent in the wild-type, full-length Bdp1p, and Bdp1p 1-509 but present in the other mutant strains. Triangles denote sites present in wild-type, full-length Bdp1p and Bdp1p 1-509 but absent in the other mutant strains.
Residues 1-240 of Bdp1p are required for Isw2p targeting to tDNAs
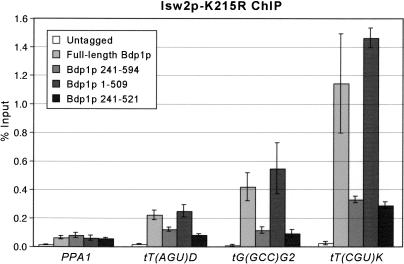
The similarity of the MNase digestion pattern upstream of the tDNAs in the bdp1-Δ240 and isw2 mutants suggests that the N terminus of Bdp1p may be required to recruit Isw2 chromatin remodeling activity to the regions upstream of tRNA genes. To test this hypothesis, we examined the physical localization of Isw2p in the bdp1-Δ240 mutant by chromatin immunoprecipitation (ChIP). We recently found that the cross-linking of Isw2p-K215R, but not wild-type Isw2p, is enriched at Isw2 targets in vivo in a genome-wide analysis (Gelbart et al. 2005). Robust cross-linking of Isw2p-K215R to the tDNA upstream region is observed in the presence of both full-length Bdp1p and Bdp1p 1-509 (Fig. 6). Cross-linking is reduced by approximately fourfold when the N-terminal 240 residues of Bdp1p are absent. The N terminus of Bdp1p is not generally required for Isw2p-K215R association with chromatin as Isw2p-K215R cross-linking is not affected at the control PPA1 locus in the bdp1 mutants. The level of expression of Isw2p-K215R is the same in each of the bdp1 mutant strains (Supplementary Fig. 1). These results indicate that Isw2p recruitment to the tDNA upstream region is dependent on the N-terminal 240 residues of Bdp1p.
Figure 6.
ChIP of Isw2p-K215R upstream of three tDNAs in the bdp1 mutant strains. ChIPs were performed in each strain using a 3× Flag-tagged Isw2p-K215R. For each sample, ChIP of the tDNA and PPA1 (loading control) is calculated as a percentage of the input.
Discussion
Previous analysis of populations of Ty1 insertions upstream of two families of tDNAs, as well as single copies in the yeast genome, showed a periodic pattern of insertion within the integration windows (Bachman et al. 2004). The accompanying paper (Gelbart et al. 2005) shows that the chromatin remodeling complex Isw2 contributes to nucleosome positioning upstream of tDNAs and loss of Isw2p disrupts periodic Ty1 integration. Here we demonstrate a similar disruption of periodic Ty1 integration upon deletion of the first 240 residues of Bdp1p. tDNA-adjacent chromatin is similarly altered in both the isw2 mutants and the bdp1-Δ240 mutants compared with wild type and targeting of Isw2p upstream of tDNAs tested is dependent on the N terminus of Bdp1p.
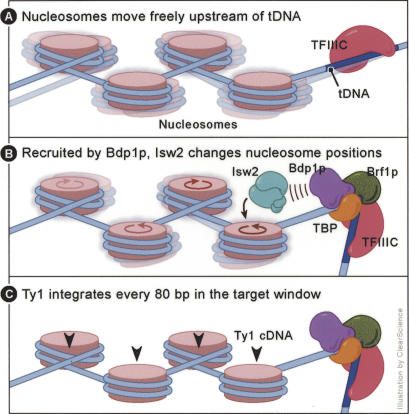
Together, our results suggest a model in which Isw2p and its nucleosome remodeling activity are recruited upstream of tDNAs in yeast by the N terminus of Bdp1p. Prior to Bdp1p entry into the pol III preinitiation complex, TFIIIC is bound to the internal promoter of the tDNA (Fig. 7A). TFIIIB binds and generates a sharp bend in the DNA. The Isw2 complex is recruited by Bdp1p and orders the nucleosomes upstream of the tDNA (Fig. 7B). The chromatin structure established by Isw2 upstream of tDNAs consequently affects integration site selection by Ty1 integrase in this permissive window, resulting in the observed periodicity of ∼80 bp (Fig. 7C). The insertion events are shown occurring into the DNA at the dyad axis; however, there is no explicit evidence for or against this positioning. The hotspots might also be located, for example, on the opposite face of the nucleosome, which would result in similar spacing of the integration events. Isw2-dependent chromatin structure upstream of tDNAs, and not simply the presence of Isw2p, is necessary for periodic integration by Ty1 integrase, as evidenced by the fact that inactivation of the catalytic activity of Isw2p, rather than deletion of the protein, is sufficient to disrupt periodic Ty1 integration. These results support the hypothesis that periodicity of Ty1 integration occurs based on the physical characteristics of the target chromatin structure.
Figure 7.
A model for Isw2 recruitment by the Bdp1p N terminus and its effect on Ty1 integration pattern. (A) The pol III preinitiation factor TFIIIC is constitutively bound to the internal promoter of tDNAs, and nucleosomes in unstable positions may be present in the surrounding area. (B) TFIIIB binding results in a distortion of the DNA by virtue of the DNA-bending activities of TBP and Bdp1p. Isw2 is recruited to the tDNA upstream region by the Bdp1p N terminus, and acts on the nucleosomes there, sliding them into a regular array. (C) Ty1 integration occurs into the nucleosomal DNA at regular intervals of ∼80 bp, based on the spacing of the nucleosomes. The position of integration on the nucleosome is not known. Insertion events are shown here occurring into DNA at the dyad axis.
Ty1 integration continues to occur upstream of tDNAs in both the isw2 mutants and the bdp1-Δ240 mutants, indicating that neither Isw2p nor the first 240 residues of Bdp1p are required for recruitment of the preintegration complex to the tDNA upstream region. Other proteins, or domains, of Bdp1p other than those tested in this work are required for recruitment of the preintegration complex to the tDNA upstream region. The requirements for tDNA-targeted integration and periodic integration therefore appear to be distinct.
The results described here are the first to ascribe an in vivo phenotype to the N-terminal half of Bdp1p. This domain contains features that could mediate an interaction between Bdp1p and the Isw2 complex or intermediary proteins. The Bdp1 protein undergoes a major conformational change upon binding to the TBP/Brf1p/DNA complex (Kumar et al. 1997). The region between residues 190-210 is the only region to become exposed to surrounding solution upon binding, and this conformational change could mediate a DNA-binding-specific recruitment of Isw2 to the tDNA upstream region. In addition, the serine at position 164 is the only cAMP-dependent kinase phosphorylation site in Bdp1p (Shah et al. 1999); phosphorylation of this residue could potentially regulate protein-protein interactions.
Interestingly, deletion of the N terminus does not affect the tDNA-proximal position of the Ty1 integration window. Integration events occurred on average 80-90 bp upstream of the tRNA transcription start site, and never occurred closer than -65 bp upstream (Fig. 3; Bachman et al. 2004), though the boundary of the TFIIIB complex footprint on DNA is ∼42 bp upstream of the tRNA transcription start site (White 1998). Because the DNA-binding domain of Bdp1p encompasses only a small fraction of the protein, amino acids 299-315, and the rest of the protein is thought to be positioned away from the DNA (Kumar et al. 1997; Shah et al. 1999), the loss of the Bdp1p N terminus does not affect its DNA-binding footprint. The question of what protein or activity prevents integration events from occurring closer to the tDNA remains open; one possible candidate is the preintegration complex itself.
The requirement for the first 240 residues of Bdp1p in targeting Isw2p to the tDNA upstream region is the first example of recruitment of an ATP-dependent chromatin-remodeling factor by a general transcription factor. Previous studies have shown that ATP-dependent chromatin-remodeling factors are delivered to promoters by gene-specific transcription factors; the recruitment of Isw2p to specific promoters by Ume6p and α2p is an example. While much work has focused on the effects of chromatin structure on the regulation of pol II-transcribed genes, relatively little is known about the role of ATP-dependent chromatin remodeling at pol I- and pol III-transcribed genes. The NoRC complex in mammalian cells is associated with pol I transcription and epigenetic silencing of specific rDNA copies (Li et al. 2005). NoRC also plays a role in DNA replication timing, as rDNA copies associated with NoRC are late-replicating, while others replicate early in S phase (Li et al. 2005). In a genome-wide location analysis, the RSC ATP-dependent remodeling complex was found to be associated with pol III-transcribed genes (Ng et al. 2002); however, the mechanism for targeting RSC and the role of RSC at these loci remain unknown. Our results demonstrate that the pol III general transcription factor, Bdp1p, recruits Isw2 to tDNA upstream regions, and suggest intriguing possibilities about the function of Isw2p at tDNA targets. There is mounting evidence that tDNAs are associated with global chromatin structuring activities and that they have genomic functions beyond acting as simple sources of tRNAs. In addition to their ability to direct retrotransposon integration, active tDNAs act as boundary elements limiting the spread of silent chromatin (Donze et al. 1999; Simms et al. 2004), repress transcription from nearby weak pol II promoters (Hull et al. 1994; Kendall et al. 2000; Bolton and Boeke 2003), function as DNA replication pause sites (Deshpande and Newlon 1996), and are associated with late-replicating origins of replication (Wyrick et al. 2001). The association between Isw2p and Bdp1p raises the possibility that Isw2p could contribute to the global chromatin marking activity of tDNAs in the yeast genome.
Materials and methods
Construction of plasmids
pVIT41 was previously described (Lauermann et al. 1997).
Most of the bdp1 truncation alleles were constructed by PCR and inserted into pRS315 as described (Ishiguro et al. 2002) and kindly provided by G. Kassavetis and E.P. Geiduschek (Department of Biology and Center for Molecular Genetics, University of California, San Diego, La Jolla, CA). DNA sequencing revealed that these constructs contain 500 bases of genomic sequence upstream of the BDP1 ATG codon and 500 bp downstream of the stop codon. The Bdp1p 1-594 (pLH19), Bdp1p 241-594 (pLH 20), Bdp1p -509 (pLH21), and Bdp1p 241-521 (pLH22) were a gift of S. Hahn (The Fred Hutchinson Cancer Research Center, Seattle, WA), and were made by adding a single HA tag to the C terminus of the protein and inserting each BamHI-XhoI PCR fragment into pRS315. These constructs contain 481 bases of genomic sequence upstream of the ATG start codon and lack genomic flanking sequence downstream of the stop codon or HA tag.
Yeast strains and media
Media were prepared by standard methods as described (Burke et al. 2000).
The Δbdp1 strain (Roberts et al. 1996), BY5025 (ura3 lys2 ade2 trp1 his3 leu2Δbdp1::TRP1/[URA3 BDP1]), was obtained from S. Hahn and was made by replacing the genomic copy of BDP1+ with the TRP1 gene and complementing with BDP1 on a URA3-expressing plasmid, to allow growth. Cells were transformed with each bdp1 mutant plasmid (see above), and the BDP1+ plasmid was shuffled out by selection on 5-flouroorotic acid. Strains and genotypes are listed in Supplementary Table 1.
PCR assay for Ty1-targeted integration
In order to test periodic integration pattern, the strains YNB143, YNB145, YNB147, YNB149, YNB352-YNB356, YNB358-YNB360, and YNB362 were transformed with pVIT41 for bdp1 mutant analysis, as were strains YTT3078, YTT3079, YTT3141, YTT3112, YTT3113, and YTT3116 for isw2/bdp1 mutant analysis (see Supplementary Table 1). Transposition induction, genomic DNA preparation, and PCR were all performed as described previously (Bachman et al. 2004). PCR reactions shown in Figures 1 and 2 were run on 1.5% agarose gels, while PCRs shown in Figure 4 were run on 1.7% agarose gels. The tG(GCC)G2-specific primer used for PCR shown in Figure 4 was 5′-TGAGAGGTGATTTTCTAGAGC-3′.
PCR assay with end-labeled primer
One-hundred-fifty nanograms genomic DNA was used for the PCR. Oligo JB3361 was end-labeled with fresh [γ 32P]-ATP by mixing 30 pmol of oligo, 1× kinase buffer (New England Biolabs), 20 U T4 polynucleotide kinase (New England Biolabs), and 2 μL of [γ 32P]-ATP and incubating at 37°C for 1-2 h. Unincorporated counts were removed using a Probe Quant G-50 microcolumn (Amersham Biosciences) per manufacturer's instructions. The labeled oligo was diluted with cold oligo to a final concentration of 2.5 μM at 200,000 counts/μL. PCR reaction conditions were otherwise identical to those described above.
Four-percent polyacrylamide gels were poured as described (Slatko and Albright 1998). Three microliters of the 25-μL PCR reaction was loaded in each lane. Gels were run at 70 W (constant power) for ∼3.5 h. Dried gels were placed on a PhosphorImager plate (Molecular Dynamics, Amersham Bioscience), scanned, and analyzed using ImageQuant software (Molecular Dynamics).
Nucleosome mapping in bdp1-Δ240 and Δisw2 mutants
MNase digestions and indirect end-labeling were performed as described (Fazzio and Tsukiyama 2003), except that cells were grown in YEPD and were harvested at OD660 = 0.7. Zymolyase was used at 10 mg/mL, and each 200μL aliquot of spheroplasts was digested with 25-75 U MNase.
ChIP assay
ChIP was performed as described (McConnell et al. 2004) except that the fixation time was decreased to 5 min. After reversal of cross-linking, the DNA was subjected to phenol and phenolchloroform extractions, followed by ethanol precipitation. Locus-specific PCR reactions were performed in the presence of α32P-dCTP, and each experiment was repeated in triplicate with three immunoprecipitations from two independent chromatin samples. PPA1 was used as a loading control. Primer sequences are available upon request.
Acknowledgments
We thank S. Hahn for bdp1 mutant strains and antibodies to Bdp1p. We thank G. Kassavetis and P. Geiduschek for bdp1 mutant strains. We thank David Clark and members of the Boeke and Tsukiyama laboratories for helpful discussions and Bang Wong for assistance with figures. This work was supported by NIH grant GM36481 to J.D.B. N.B. was supported in part by training grant 5T32CA09139. This work was supported by NIH grant GM58465 to T.T. M.E.G. was supported by a Predoctoral Fellowship from HHMI. T.T. is a Leukemia and Lymphoma Society Scholar.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1299105.
References
- Alen C., Kent, N.A., Jones, H.S., O'Sullivan, J., Aranda, A., and Proudfoot, N.J. 2002. A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol. Cell 10: 1441-1452. [DOI] [PubMed] [Google Scholar]
- Bachman N., By, Y., and Boeke, J.D. 2004. Local definition of Ty1 target preference by long terminal repeats and clustered tRNA genes. Genome Res. 14: 1232-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P., Dingermann, T., and Winckler, T. 2002. Transfer RNA gene-targeted retrotransposition of Dictyostelium TRE5-A into a chromosomal UMP synthase gene trap. J. Mol. Biol. 318: 273-285. [DOI] [PubMed] [Google Scholar]
- Boeke J.D. and Devine, S.E. 1998. Yeast retrotransposons: Finding a nice quiet neighborhood. Cell 93: 1087-1089. [DOI] [PubMed] [Google Scholar]
- Bolton E.C. and Boeke, J.D. 2003. Transcriptional interactions between yeast tRNA genes, flanking genes and Ty elements: A genomic point of view. Genome Res. 13: 254-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Dawson, D., and Stearns, T. 2000. Methods in yeast genetics: A Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Deshpande A.M. and Newlon, C.S. 1996. DNA replication fork pause sites dependent on transcription. Science 272: 1030-1033. [DOI] [PubMed] [Google Scholar]
- Devine S.E. and Boeke, J.D. 1996. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA polymerase III. Genes & Dev. 10: 620-633. [DOI] [PubMed] [Google Scholar]
- Donze D., Adams, C.R., Rine, J., and Kamakaka, R.T. 1999. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes & Dev. 13: 698-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzio T.G. and Tsukiyama, T. 2003. Chromatin remodeling in vivo: Evidence for a nucleosome sliding mechanism. Mol. Cell 12: 1333-1340. [DOI] [PubMed] [Google Scholar]
- Fazzio T.G., Kooperberg, C., Goldmark, J.P., Neal, C., Basom, R., Delrow, J., and Tsukiyama, T. 2001. Widespread collaboration of Isw2 and Sin3-Rpd3 chromatin remodeling complexes in transcriptional repression. Mol. Cell. Biol. 21: 6450-6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D.J., DeLuca, C., Berger, I., Gaillard, H., Sigrist, R., Schimmele, K., and Richmond, T.J. 2004. Reaction cycle of the yeast Isw2 chromatin remodeling complex. EMBO J. 23: 3836-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard H., Fitzgerald, D.J., Smith, C.L., Peterson, C.L., Richmond, T.J., and Thoma, F. 2003. Chromatin remodeling activities act on UV-damaged nucleosomes and modulate DNA damage accessibility to photolyase. J. Biol. Chem. 278: 17655-17663. [DOI] [PubMed] [Google Scholar]
- Geiduschek E.P. and Kassavetis, G.A. 2001. The RNA polymerase III transcription apparatus. J. Mol. Biol. 310: 1-26. [DOI] [PubMed] [Google Scholar]
- Gelbart M.E., Bachman, N., Delrow, J., Boeke, J.D., and Tsukiyama, T. 2005. Genome-wide identification of Isw2 chromatin-remodeling targets by localization of a catalytically inactive mutant. Genes & Dev. (this issue). [DOI] [PMC free article] [PubMed]
- Goldmark J.P., Fazzio, T.G., Estep, P.W., Church, G.M., and Tsukiyama, T. 2000. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell 103: 423-433. [DOI] [PubMed] [Google Scholar]
- Grove A., Kassavetis, G.A., Johnson, T.E., and Geiduschek, E.P. 1999. The RNA polymerase III-recruiting factor TFIIIB induces a DNA bend between the TATA box and the transcriptional start site. J. Mol. Biol. 285: 1429-1440. [DOI] [PubMed] [Google Scholar]
- Hull M.W., Erickson, J., Johnston, M., and Engelke, D.R. 1994. tRNA genes as transcriptional repressor elements. Mol. Cell. Biol. 14: 1266-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T. and Araki, H. 2004. Noncompetitive counteractions of DNA polymerase ε and ISW2/yCHRAC for epigenetic inheritance of telomere position effect in Saccharomyces cerevisiae. Mol. Cell. Biol. 24: 217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro A., Kassavetis, G.A., and Geiduschek, E.P. 2002. Essential roles of Bdp1, a subunit of RNA polymerase III initiation factor TFIIIB, in transcription and tRNA processing. Mol. Cell. Biol. 22: 3264-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Moore, D.P., Blomberg, M.A., Braiterman, L.T., Voytas, D.F., Natsoulis, G., and Boeke, J.D. 1993. Hotspots for unselected Ty1 transposition events on yeast chromosome III are near tRNA genes and LTR sequences. Cell 73: 1007-1018. [DOI] [PubMed] [Google Scholar]
- Kassabov S.R., Henry, N.M., Zofall, M., Tsukiyama, T., and Bartholomew, B. 2002. High-resolution mapping of changes in histone-DNA contacts of nucleosomes remodeled by ISW2. Mol. Cell. Biol. 22: 7524-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G.A., Braun, B.R., Nguyen, L.H., and Geiduschek, E.P. 1990. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell 60: 235-245. [DOI] [PubMed] [Google Scholar]
- Kassavetis G.A., Kumar, A., Letts, G.A., and Geiduschek, E.P. 1998. A post-recruitment function for the RNA polymerase III transcription-initiation factor IIIB. Proc. Natl. Acad. Sci. 95: 9196-9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall A., Hull, M.W., Bertrand, E., Good, P.D., Singer, R.H., and Engelke, D.R. 2000. A CBF5 mutation that disrupts nucleolar localization of early tRNA biosynthesis in yeast also suppresses tRNA gene-mediated transcriptional silencing. Proc. Natl. Acad. Sci. 97: 13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey P.T. and Sandmeyer, S.B. 1991. Adjacent pol II and pol III promoters: Transcription of the yeast retrotransposon Ty3 and a target tRNA gene. Nucleic Acids Res. 19: 1317-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kassavetis, G.A., Geiduschek, E.P., Hambalko, M., and Brent, C.J. 1997. Functional dissection of the B″ component of RNA polymerase III transcription factor IIIB: A scaffolding protein with multiple roles in assembly and initiation of transcription. Mol. Cell. Biol. 17: 1868-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauermann V., Hermankova, M., and Boeke, J.D. 1997. Increased length of long terminal repeats inhibits Ty1 transposition and leads to the formation of tandem multimers. Genetics 145: 911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Santoro, R., Koberna, K., and Grummt, I. 2005. The chromatin remodeling complex NoRC controls replication timing of rRNA genes. EMBO J. 24: 120-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell A.D., Gelbart, M.E., and Tsukiyama, T. 2004. Histone fold protein Dls1p is required for Isw2-dependent chromatin remodeling in vivo. Mol. Cell. Biol. 24: 2605-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H.H., Robert, F., Young, R.A., and Struhl, K. 2002. Genome-wide location and regulated recruitment of the RSC nucleosome-remodeling complex. Genes & Dev. 16: 806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S., Miller, S.J., Lane, W.S., Lee, S., and Hahn, S. 1996. Cloning and functional characterization of the gene encoding the TFIIIB90 subunit of RNA polymerase III transcription factor TFIIIB. J. Biol. Chem. 271: 14903-14909. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S. 1998. Targeting transposition: At home in the genome. Genome Res. 8: 416-418. [DOI] [PubMed] [Google Scholar]
- Schramm L. and Hernandez, N. 2002. Recruitment of RNA polymerase III to its target promoters. Genes & Dev. 16: 2593-2620. [DOI] [PubMed] [Google Scholar]
- Shah S.M., Kumar, A., Geiduschek, E.P., and Kassavetis, G.A. 1999. Alignment of the B″ subunit of RNA polymerase III transcription factor IIIB in its promoter complex. J. Biol. Chem. 274: 28736-28744. [DOI] [PubMed] [Google Scholar]
- Simms T.A., Miller, E.C., Buisson, N.P., Jambunathan, N., and Donze, D. 2004. The Saccharomyces cerevisiae TRT2 tRNAThr gene upstream of STE6 is a barrier to repression in MATα cells and exerts a potential tRNA position effect in MATa cells. Nucleic Acids Res. 32: 5206-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatko B.E. and Albright, L.M. 1998. Denaturing gel electrophoresis for sequencing. In Current protocols in molecular biology (ed. F.M. Ausubel), pp. 7.6.1-7.6.11. Wiley, Hoboken, NJ. [DOI] [PubMed]
- Tsukiyama T., Palmer, J., Landel, C.C., Shiloach, J., and Wu, C. 1999. Characterization of the imitation switch subfamily of ATP-dependent chromatin-remodeling factors in Saccharomyces cerevisiae. Genes & Dev. 13: 686-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.J. 1998. RNA polymerase III transcription. Landes Bioscience, Austin, TX.
- Winckler T., Dingermann, T., and Glockner, G. 2002. Dictyostelium mobile elements: Strategies to amplify in a compact genome. Cell. Mol. Life Sci. 59: 2097-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick J.J., Aparicio, J.G., Chen, T., Barnett, J.D., Jennings, E.G., Young, R.A., Bell, S.P., and Aparicio, O.M. 2001. Genome-wide distribution of ORC and MCM proteins in S. cerevisiae: High-resolution mapping of replication origins. Science 294: 2357-2360. [DOI] [PubMed] [Google Scholar]
- Yieh L., Kassavetis, G., Geiduschek, E.P., and Sandmeyer, S.B. 2000. The Brf and TATA-binding protein subunits of the RNA polymerase III transcription factor IIIB mediate position-specific integration of the gypsy-like element, Ty3. J. Biol. Chem. 275: 29800-29807. [DOI] [PubMed] [Google Scholar]
- Yieh L., Hatzis, H., Kassavetis, G., and Sandmeyer, S.B. 2002. Mutational analysis of the transcription factor IIIB-DNA target of Ty3 retroelement integration. J. Biol. Chem. 277: 25920-25928. [DOI] [PubMed] [Google Scholar]