Keywords: genetics, integrative taxonomy, marine parasitology, morphology, South Africa, Trematoda
Abstract
Larval stages of the widely distributed digenean species Proctoeces maculatus (Looss, 1901) were reported 40 years ago from South Africa in the common octopus, Octopus vulgaris Cuvier (Octopodidae). However, the absence of adult specimens and molecular data from this region has hindered a comprehensive understanding of its distribution. In this study, we collected three species of intertidal and near-shore marine fishes [Clinus superciliosus (L.) (Clinidae), Diplodus capensis (Smith) (Sparidae) and Sparodon durbanensis (Castelnau) (Sparidae)] along the South African coast and discovered adult specimens of P. maculatus at five localities. By employing a combination of morphological and molecular techniques, including 28S rDNA, 18S rDNA and COI mtDNA analyses, the first report of adult P. maculatus from South Africa is presented. The findings encompass a comprehensive morphological description and molecular data, illuminating the true distribution of this species in the region.
Introduction
Proctoeces maculatus (Looss, 1901) Odhner, 1911 (Digenea: Fellodistomidae) is a widespread trematode species that parasitizes the gut of a wide range of marine fishes. It was originally described as Distomum maculatum Looss, 1901 from the Brown wrasse (Labrus merula L.) in Trieste, Italy (Looss, 1901; Odhner, 1911). Over the years, adults of P. maculatus have been reported from 65 species of fish; additionally, 26 invertebrate species have been recorded as intermediate hosts for P. maculatus (WoRMS, 2023). Additionally, numerous species exhibiting morphological similarities to P. maculatus have been described, and a significant proportion of them have subsequently been synonymized with P. maculatus. This outcome stems from the conserved morphology observed among isolates, which presents a challenge in discerning clear-cut morphological characteristics to differentiate P. maculatus from other species (Freeman and Llewellyn, 1958; Bray and Gibson, 1980).
An interesting trait of Proctoeces species is the incorporation of progenetic metacercariae in their life cycles – the larval stages can attain sexual maturity while infecting an intermediate host (Freeman and Llewellyn, 1958; Bray and Gibson, 1980; Oliva and Huaquin, 2000). These trematodes have a near-cosmopolitan distribution and are known to infect a variety of hosts, mainly fishes and molluscs that mostly occur in shallow water (Bray and Gibson, 1980). Proctoeces maculatus has been reported only once, 40 years ago, in South Africa, when immature specimens were found in the common octopus Octopus vulgaris Cuvier (Bray, 1983).
While exploring the trematode biodiversity of fishes along the South African coast, adult specimens of P. maculatus were found in three intertidal and near-shore fishes: Clinus superciliosus (L.) (Clinidae), Diplodus capensis (Smith) (Sparidae) and Sparodon durbanensis (Castelnau) (Sparidae). This is the first report of adult P. maculatus from marine fishes in South Africa, along with the first molecular characterization of this species from this biodiversity-rich marine environment.
Materials and methods
Sample collection
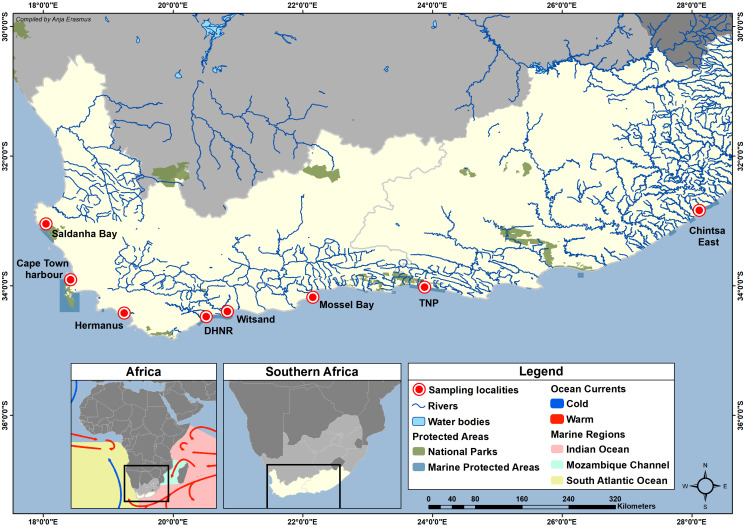
Specimens of C. superciliosus, D. capensis and S. durbanensis were collected from rocky intertidal and near-shore areas along the South African coast (Fig. 1). The collection sites of each species, along with the infection rates, can be seen in Table 1.
Figure 1.
Map of sampling localities along the South African coast. DHNR - De Hoop Nature Reserve; TNP - Tsitsikamma section of the Garden Route National Park.
Table 1.
Data on fishes collected, localities within South Africa, intensity of infection and prevalence of infection with P. maculatus.
| Host species | Locality | No. fish | Infection intensity | Prevalence, % |
|---|---|---|---|---|
| Clinus superciliosus | Cape Town harbour | 16 | 0 | 0 |
| Chintsa East | 11 | 3–4 | 27 | |
| Hermanus | 8 | 1 | 13 | |
| Saldanha Bay | 19 | 0 | 0 | |
| Tsitsikamma NP | 17 | 1 | 30 | |
| Diplodus capensis | Chintsa East | 16 | 1–4 | 25 |
| De Hoop NR | 12 | 1–22 | 67 | |
| Mossel Bay | 5 | 0 | 0 | |
| Tsitsikamma NP | 28 | 1–3 | 39 | |
| Witsand | 3 | 1–2 | 67 | |
| Sparodon durbanensis | Tsitsikamma NP | 12 | 1–13 | 42 |
NP, National Park; NR, Nature Reserve.
Sampling was carried out under the permits MALH-K2016-005a and SMIT-NJ/2020-004 for the Tsitsikamma section of the Garden Route National Park (TNP); RES2018/35 for Hermanus; RES2020/29, RES2021/49 and RES2022/44 for Cape Town harbour, Chintsa East, Langebaan marina in Saldanha Bay (henceforth called Saldanha Bay), Mossel Bay and Witsand; and CN44-87-18289 for De Hoop Nature Reserve. Fishes were collected with baited traps and hand lines and humanely killed using standard methods. Following euthanasia, fishes were subjected to a full helminthological examination by inspecting every organ. Digenean trematodes were removed, relaxed in hot saline and fixed in 80% ethanol for further analyses. The prevalence and intensity of each species was calculated according to Bush et al. (1997). Fish names and authorities follow FishBase (Froese and Pauly, 2023).
Morphological analyses
Hologenophores were selected following the concept of Pleijel et al. (2008). These, along with additional whole specimens, were rehydrated in distilled water, stained with Mayer's haematoxylin, destained with 1% hydrochloric acid, neutralized with 1% ammonia, gradually dehydrated in an ethanol series (70, 80, 90, 96, 100%), cleared in methyl salicylate and permanently mounted on slides with dammar gum. These specimens were measured, photographed and used to make detailed drawings for each species. Measurements were obtained using NIS-Elements BR Cameral Analysis software and a Nikon Ni microscope (Nikon Instruments, Tokyo, Japan), and are given as a range followed by a mean in parentheses. All measurements, unless otherwise stated, are given in micrometres (μm). Drawings were made with the aid of a drawing tube attached to the aforementioned microscope. Digitization of the specimen drawings was done using Adobe Illustrator v. 26.4.1 and Photoshop v. 23.4.2. Voucher material is deposited in the Parasite Collection of the National Museum (NMB), Bloemfontein, South Africa.
Generation of molecular data
Total genomic DNA was extracted with the KAPA Express Extract Kit (Kapa Biosystems, Cape Town, South Africa) and the PCRBiosystems Rapid DNA Extraction Kit (PCRBiosystems available from Analytical Solutions, Randburg, South Africa), following the manufacturers’ protocols. However, the following adaptations were made to the protocol of the PCRBiosystems Rapid DNA Extraction Kit to obtain quality DNA: only 10 μL lysis buffer was used, 5 μL proteinase K-containing buffer was used and the final reaction was diluted with 450 μL water. The D1–D3 fragment of the 28S nuclear ribosomal RNA gene was amplified using the primers Digl2 (5′-AAG CAT ATC ACT AAG CGG-3′) (Tkach et al., 2001) and 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) (Snyder and Tkach, 2001), following the protocol of Tkach et al. (2003). Two internal primers were used for sequencing of 28S rDNA: ECD2 (5’-CTT GGT CCG TGT TTC AAG ACG GG-3’) (Tkach et al., 2003) and 300F (5’-CAA GTA CCG TGA GGG AAA GTT G-3’) (Littlewood et al., 2000). For the amplification of the 18S rRNA fragment, the universal forward and reverse primers 18SU467F (5’-ATC CAA GGA AGG CAG CAG GC-3’) and 18SL1310R (5’-CTC CAC CAA CTA AGA ACG GC-3’) (Suzuki et al., 2006) were used; polymerase chain reaction (PCR) conditions were set to 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 55°C for 1 min, 72°C for 2 min and final extension at 72°C for 7 min. The cytochrome c oxidase subunit I (COI) genes were amplified using the forward primer Dice1F (5’-ATT AAC CCT CAC TAA ATT WCN TTR GAT CAT AAG-3’) (Moszczynska et al., 2009) and the reverse primer Dice 14R (5’-TAA TAC GAC TCA CTA TAC CHA CMR TAA ACA TAT GATG-3’) (Van Steenkiste et al., 2015); PCR conditions were set to 94°C for 4 min, followed by 40 cycles of 94°C for 40 s, 51°C for 40 s, 72°C for 1 min and final extension at 72°C for 10 min. The PCR products were visualized with 1% agarose gel electrophoresis and sent to a commercial sequencing company in Pretoria, South Africa for purification and sequencing (Inqaba Biotechnical Industries [Pty] Ltd.). The resulting sequences were assembled and edited using Geneious v. 11.1.4 bioinformatics software (Biomatters, Auckland, New Zealand). Novel sequence data have been deposited in GenBank (see Table 2).
Table 2.
Sequences used for phylogenetic analyses of the 18S, 28S and COI gene/regions
| Species | Host | Locality | GenBank accession numbers | Reference | ||
|---|---|---|---|---|---|---|
| 18S | 28S | COI | ||||
| Proctoeces choerodoni | Choerodon cyanodus | Heron Island, AUS | KX671310 | KX671299 | KY073877 | Wee et al. (2017) |
| Proctoeces humboldti | Semicossyphus darwini | Chile | MF414438 | – | – | Ñacari et al. (2018) |
| Sicyases sanguineus | Chile | – | KY432601 | KY432628 | Oliva et al. (2018) | |
| S. sanguineus | Chile | – | – | KU236023a | Oliva et al. (2018) | |
| Proctoeces insolitus | Acanthopagrus australis | Queensland, AUS | KX671312 | KX671300 | KY073873 | Wee et al. (2017) |
| Proctoeces cf. lintoni | Fissurella costatab | Chile | EU423050c | – | – | Wee et al. (2017) |
| Proctoeces maculatus | Sparodon durbanensis | TNP, SA | – | – | OR723765 | Present study |
| S. durbanensis | TNP, SA | – | – | OR723766 | Present study | |
| S. durbanensis | TNP, SA | – | OR724714 | – | Present study | |
| Clinus superciliosus | TNP, SA | – | OR724715 | – | Present study | |
| C. superciliosus | Chintsa East, SA | – | OR724716 | – | Present study | |
| Diplodus capensis | TNP, SA | – | OR724713 | – | Present study | |
| D. capensis | TNP, SA | OR724708 | – | OR723768 | Present study | |
| D. capensis | TNP, SA | – | – | OR723769 | Present study | |
| D. capensis | DHNR, SA | – | OR724718 | – | Present study | |
| D. capensis | TNP, SA | – | – | OR723767 | Present study | |
| D. capensis | Chintsa East, SA | – | OR724717 | OR723770 | Present study | |
| Archosargus probatocephalus | Mississippi, USA | AY222161 | AY222284 | – | Olson et al. (2003) | |
| Sabella pavoninab | Tunisia | KX671315 | – | – | Wee et al. (2017) | |
| Sparus aurata | Tunisia | – | KX671302 | – | Wee et al. (2017) | |
| Lithognathus mormyrus | Tunisia | – | KU052937 | – | Antar and Gargouri (2016) | |
| S. pavoninab | Tunisia | – | KU052941 | – | Antar and Gargouri (2016) | |
| Thalassoma jansenii | Queensland, AUS | KX671325 | – | – | Wee et al. (2017) | |
| Monodactylus argenteus | Queensland, AUS | – | KX671309 | – | Wee et al. (2017) | |
| Chrysophrys auratus | Queensland, AUS | – | – | KY073875 | Wee et al. (2017) | |
| Octopus sinensisb | Japan | – | LC618023 | – | Izumi et al. (2021) | |
| S. sanguineus | Chile | – | KT865207d | – | Oliva et al. (2018) | |
| Proctoeces major | S. sanguineus | Chile | KY432595d | KY432618d | – | Oliva et al. (2018) |
| S. sanguineus | Chile | JX306110d | – | – | Oliva et al. (2018) | |
| Perumytilus purpuratusb | Chile | JQ782525 | – | – | Muñoz et al. (2013) | |
| Proctoeces cf. major | Octopus sinensisb | Japan | – | LC618023 | – | Izumi et al. (2021) |
| Proctoeces sicyases | M. argenteus | Hope Island, AUS | AJ224469 | – | – | Hall et al. (1999) |
| M. argenteus | Moreton Bay, AUS | – | MZ687078 | – | Cribb et al. (2021) | |
| Anarhichas lupus | North Sea, UK | Z12601 | AY222282 | – | Olson et al. (2003) | |
| Cerastoderma eduleb | Wadden Sea, The Netherlands | – | – | KF880498 | Feis et al. (2015) | |
| Outgroup | ||||||
| Coomera brayi | M. argenteus | Hope Island, AUS | AJ224469 | – | – | Hall et al. (1999) |
| M. argenteus | Moreton Bay, AUS | – | MZ687078 | – | Cribb et al. (2021) | |
| Fellodistomum fellis | Anarhichas lupus | North Sea, UK | Z12601 | AY222282 | – | Olson et al. (2003) |
| Gymnophallus choledochus | Cerastoderma eduleb | Unspecified | – | – | KF880498 | Feis et al. (2015) |
AUS, Australia; TNP, Tsitsikamma section of the Garden Route National Park; SA, South Africa; UK, United Kingdom; USA, United States of America.
Listed on GenBank as Proctoeces cf. lintoni.
Host is not a fish.
Listed on GenBank as Proctoeces lintoni.
Listed on GenBank as Proctoeces sp.
Phylogenetic analyses
Sequences included in the phylogenetic analyses were selected based on the results of Wee et al. (2017) and Oliva et al. (2018). Sequences available for this genus as well as the outgroup sequences were retrieved from GenBank (Table 2).
An alignment was built for each gene, using MUSCLE (Edgar, 2004) as implemented in Geneious v. 11.1.4. The best nucleotide substitution model was predicted using jModelTest 2.1 (Posada, 2008), based on the Akaike information criterion. The general time-reversible model with gamma distribution rate variation among sites (GTR + G) was used to construct both phylogenetic trees. The COI alignment was only used to calculate genetic distance matrices. Both phylogenies are based on Bayesian inference (BI) and maximum likelihood (ML) estimate analyses. BI analyses were performed with MrBayes software and ML analyses were performed with PhyML v. 3.0 (available at http://www.atgc-montpellier.fr/phyml/). For the BI analyses of both alignments, the following parameters were set: Markov chain Monte Carlo chains were run for 3 000 000 generations; the ‘burn-in’ parameter was set for the first 25% of the sampled trees. A hundred bootstrap pseudo replicates were run to determine the nodal support for ML analyses. Phylogenetic trees were visualized using FigTree v. 1.4.4 (Rambaut 2012) and combined and edited using Adobe Illustrator v. 26.4.1. Pairwise genetic distance matrices were calculated in MEGA v. X using the parameters ‘model/method = No. of differences’, ‘variance estimation method = none’, ‘substitutions to include = d: transitions + transversions’ and ‘gaps/missing data treatment = pairwise deletion’.
Results
General results
Among all the localities sampled, De Hoop Nature Reserve exhibited the highest prevalence and intensity of infection with P. maculatus in D. capensis (see Table 1). Proctoeces maculatus was most prevalent in C. superciliosus from TNP, but had a higher intensity of infection at Chintsa East. However, this species was absent from C. superciliosus collected in Cape Town harbour and Saldanha Bay, as well as from D. capensis collected in Mossel Bay. Nearly half of the S. durbanensis collected from TNP were infected with P. maculatus. Having considered the lines of evidence provided by molecular, morphological and ecological (i.e. host) data, we are confident that these collected specimens belong to P. maculatus.
Morphological characterization
Family Fellodistomidae Nicoll, 1909
Subfamily Fellodistominae Nicoll, 1909
Genus Proctoeces Odhner, 1911
Proctoeces maculatus (Looss, 1902) Odhner, 1911
Type-host: Labrus merula L.
Type-locality: Trieste, Italy
New hosts: Clinus superciliosus (L.) (Clinidae); Diplodus capensis (Smith) (Sparidae); Sparodon durbanensis (Castelnau) (Sparidae).
New localities: Chintsa East, De Hoop Nature Reserve, Hermanus, Tsitsikamma section of the Garden Route National Park, and Witsand, South Africa.
Site of infection: Intestine.
Representative DNA sequences: OR724708 (18S); OR724713–OR724718 (28S); OR723765–OR723770 (COI).
Voucher material: A total of 58 voucher specimens deposited in NMB ‒ 22 stained and permanently mounted specimens (accession no. NMB P 999–1020) and 36 specimens in ethanol (accession no. NMB P 991–998).
Description (based on 22 whole mounts; Fig. 2; Table 3). Body subcylindrical, robust, tapering at both ends; widest at level of ventral sucker, occasionally at level of testes; forebody occupying about 26.1% of total body length. Tegument unarmed.
Figure 2.
Proctoeces maculatus whole mount. Ventral view (A), terminal genitalia (B), lateral view (C). Abbreviations: E, egg; CS, cirrus sac; GA, genital atrium; GP, genital pore; IC, intestinal caeca; M, metraterm; OV, ovary; P, pharynx; PP, pars prostatica; SV, seminal vesicle; T, testis; U, uterus; VF, vitelline follicles. Scale bars: 500 μm (A, C); 100 μm (B).
Table 3.
Morphometrics of newly collected specimens of Proctoeces maculatus, compared to examples of published measurements in literature for adult P. maculatus
| Host(s) | Diplodus capensis, Clinus superciliosus | Labrus merula | Blennius ocellaris | Crenilabrus sp. | Acanthopagrus schlegelii, Epinephelus akaara, Pagrus auratus, Rhabdosargus sarba | Halichoeres bivittatus | Parapercis colias | Lithognathus mormyrus, Sparus aurata, Trachinotus ovatus | Myoxocephalus stelleri, Platichthys stellatus, Pseudopleuronectes schrenki | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | Various, South Africa | Trieste, Italy | Naples, Italy | Black Sea, Russia | Seto Inland Sea, Japan | Bermuda | New Zealand | Bizerte Lagoon, Tunisia | Wakanai, Hokkaido, Japan | |||
| Reference | Present study | Looss (1901) | Odhner (1911) | Vlasenko (1931) | Yamaguti (1934) | Bray and Gibson (1980) | Bray (1983) | Antar and Gargouri (2016) | Shimazu (1984) | |||
| Range (n = 19) | Mean | Range (n = unkown) | Max. | Range (n = unknown) | Max. | Range (n = unknown) | Range (n = 9) | Range (n = unknown) | Range (n = 1) | Range (n = 4) | Range (n = 39) | |
| Body length | 1151‒2870 | 1811 | ‒ | 3200 | ‒ | 2500 | ~3000 | 1730–4460 | 1460 | 2450 | 1277‒1506 | 2370–6170 |
| Body width | 322‒695 | 484 | ‒ | 800 | 300‒450 | ‒ | 700 | 340–1100 | 650 | 950 | 449‒582 | 700–1420 |
| Forebody length | 283‒546 | 428 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 420‒587 | ‒ |
| Hindbody length | 839‒2133 | 1325 | ‒ | ‒ | 500‒700 | ‒ | ‒ | ‒ | ‒ | ‒ | 612‒936 | ‒ |
| Body width:length ratio | 1:2.41‒5.98 | 3.73 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Forebody length as % body length | 18.4‒35.2 | 26.1 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Oral sucker length | 167‒317 | 217 | ‒ | ‒ | ‒ | ‒ | ‒ | 200–560 | 200 | 360 | 146‒171 | 350–700 |
| Oral sucker width | 148‒270 | 213 | 370 | ‒ | 200‒300 | ‒ | ~250 | 230–570 | 280 | 370 | 150‒191 | 350–750 |
| Pharynx length | 143‒278 | 193 | ‒ | ‒ | ‒ | ‒ | ‒ | 190–400 | 230 | 350 | 100‒137 | 250–450 |
| Pharynx width | 88‒244 | 171 | 280 | ‒ | 150 ‒230 | ‒ | ~200 | 190–360 | 170 | 280 | 100‒142 | 250–450 |
| Oesophagus length | 9‒52 | 28 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 50 | ‒ | 20‒54 | ‒ |
| Ventral sucker length | 223‒324 | 265 | ‒ | ‒ | 280‒420 | ‒ | 390 | 230–640 | 330 | 430 | 166‒246 | 470–970 |
| Ventral sucker width | 293‒465 | 370 | 630 | ‒ | 420‒700 | ‒ | 610 | 290–840 | 400 | 670 | 237‒287 | 570–1000 |
| Oral sucker length:ventral sucker length | 1:0.95‒1.48 | 1:1.23 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1:1.54‒1.14 | ‒ |
| Oral sucker width:ventral sucker width | 1:1.49‒1.98 | 1:1.74 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1:1.4 | 1:1.80 | 1:1.59‒1.41 | 1:1.13‒1.66 |
| Oral sucker length:pharynx length | 1:0.71‒1.08 | 1:0.90 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Oral sucker width:pharynx width | 1:0.57‒0.95 | 1:0.80 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1:0.79‒0.67 | ‒ |
| Ovary length | 128‒244 | 200 | ‒ | ‒ | ‒ | ‒ | ‒ | 170–400 | ‒ | 220 | 87‒129 | 250–500 |
| Ovary width | 112‒235 | 170 | ‒ | ‒ | ‒ | ‒ | ~220 | 140–410 | ‒ | 280 | 75‒87 | 220–420 |
| Egg length | 23‒49 | 41 | 70 | ‒ | 72‒79 | ‒ | 74 | 66–76 | ‒ | 40‒62 | ‒ | 50–65 |
| Anterior testis length | 110‒255 | 186 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 83‒141 | ‒ |
| Anterior testis width | 143‒268 | 204 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 71‒121 | ‒ |
| Posterior testis length | 121‒284 | 197 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 87‒158 | ‒ |
| Posterior testis width | 138‒323 | 220 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 79‒116 | ‒ |
| Average testis length | 116‒270 | 192 | ‒ | ‒ | ‒ | ‒ | ~220 | 190–420 | 160‒230 | 150‒160 | ‒ | 250–750 |
| Average testis width | 141‒281 | 121 | ‒ | ‒ | ‒ | ‒ | ~220 | 190–570 | 130‒140 | 250‒260 | ‒ | 270–620 |
| Cirrus sac length | 314‒633 | 471 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 130‒340 | 600 | 250‒374 | 500–1050 |
| Cirrus sac width | 80‒148 | 124 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 160 | 58‒79 | 120–300 |
| Post-testicular region | 223‒1217 | 576 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 162‒337 | ‒ |
| Post-testicular region as % body length | 17‒42 | 26 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
Oral sucker subterminal, spherical to subspherical. Prepharynx absent. Pharynx well developed, globular, muscular. Oral sucker to pharynx length ratio 1:0.7–1.1 (1:0.9). Oesophagus short, often indistinct. Intestine thick-walled. Intestinal bifurcation in mid forebody, often overlaps pharynx dorsally. Caeca end blindly in hindbody between testes and posterior extremity; ends often covered by uterus, thus indistinguishable. Ventral sucker pre-equatorial, transversely oval when viewed ventrally, muscular, larger than oral sucker. Oral sucker to ventral sucker length ratio 1:0.9–1.5 (1:1.2); width ratio 1:1.5–2.0 (1:1.7).
Testes two, intercaecal, obliquely tandem, occasionally tandem, margins entire; anterior testis triangular to elongate, often contiguous with ovary; posterior testis triangular to elongate, contiguous with anterior testis. Post-testicular field represents 17–42% (26%) of body length. Cirrus sac situated between posterior end of ventral sucker and mid-level of anterior testis, encloses seminal vesicle and pars prostatica, ejaculatory duct not observed. Seminal vesicle in posterior part of cirrus sac, tubular, highly convoluted. Pars prostatica fills most of anterior cirrus sac, well developed, straight or slightly curved, covered by dense gland cells. Prominent muscular papilla at distal end of cirrus sac. Genital atrium thin-walled, extends from about mid or anterior level of ventral sucker to meet genital pore. Genital pore at about level of intestinal bifurcation, slightly sinistral.
Ovary median to slightly dextral, often contiguous to anterior testis, subspherical to elongate oval but occasionally slightly lobed. Mehlis’ gland not observed. Uterus highly convoluted; uterine coils restricted to between mid-level of ventral sucker and posterior extremity, filling most of ventral hindbody, filled with eggs in all specimens. Metraterm at distal end of uterus, enters genital atrium, faint. Eggs oval, operculate, yellow, without filaments.
Vitellarium follicular; follicles vary greatly in size, situated in two lateral fields, extend from slightly anterior to ovary to posterior limit of posterior testis, occasionally overreaching these limits, sometimes difficult to distinguish.
Excretory pore terminal, forming slight concavity at posterior body extremity. Excretory vesicle Y-shaped; site of bifurcation of vesicle not observed due to large number of eggs present in uterus; arms of vesicle terminate in two blind ends near posterior limit of pharynx, often difficult to distinguish.
Remarks
The specimens of P. maculatus in the present study agree well with the original description of the species by Looss (1901) from brown wrasse, L. merula (Labridae) collected off Trieste, Italy, and the redescription by Odhner (1911) based on specimens collected from the butterfly blenny, Blennius ocellaris L., (Blenniidae) collected off Naples, Italy (Table 3), except that the specimens in the present study are smaller, having lower maxima for body length and width, slightly smaller suckers, pharynx and eggs. Those specimens of Odhner (1911) also have a notably shorter hindbody and higher maxima for ventral sucker length and width. A faint metraterm has been noted in some specimens, including our own; Looss (1901) also noted a metraterm, however Bray and Gibson (1980) described the metraterm as being muscular.
More recent descriptions of P. maculatus by Bray and Gibson (1980) and Antar and Gargouri (2016) are also considered. Bray & Gibson (1980) also note the bifurcation site of the y-shaped excretory vesicle, but this was not observed in any of the specimens of the present study, due to the large number of eggs present in the uterus that fills the hindbody. However, it was observed that the excretory vesicle terminates blindly in the anterior forebody, suggesting that the vesicle might be y-shaped. Otherwise, the morphometrics of these specimens generally agree well with the specimens in the present study.
The specimens of Antar and Gargouri (2016) from Tunisia are also similar to those of the present study, with the exception of having lower maxima for body length, body width, hindbody length, as well as smaller suckers, ovary, testes and post-testicular field. Specimens collected in the present study are overall slightly smaller than those collected in the Black Sea (Vlasenko, 1931), but contain eggs that are nearly half the length of those observed by Vlasenko (1931). The upper limits of all structures of the specimens collected in Japan are much higher than that noted in the present study, although there is some overlap in the lower limits (Yamaguti, 1934; Shimazu, 1984).
Molecular characterization
The alignment of the 28S rDNA dataset generated 729 characters for analyses. Newly sequenced isolates formed a highly supported clade within the 28S analyses (Fig. 3A), together with the P. maculatus isolates found from the sparid fish hosts Lithognathus mormyrus (L.) (KU052937: juvenile) and Sparus aurata L. (KX671302), as well as the polychaete Sabella pavonina (KU052941: metacercariae) all collected in the Bizerte Lagoon in Tunisia (Antar and Gargouri, 2016; Wee et al., 2017). An isolate collected from the sheepshead, Archosargus probatocephalus (Walbaum) (Sparidae), in the Gulf of Mexico, Mississippi, USA, was identified as P. maculatus (AY222284) (Olson et al., 2003), but did not cluster with the abovementioned isolates of P. maculatus; our analyses instead recover it in a clade with Proctoeces sicyases Oliva, Valdivia, Cárdena, Muñoz, Escribano and George-Nascimento, 2018, P. choerodoni Wee, Cribb, Bray and Cutmore, 2017 and P. insolitus (Nicoll, 1915). Newly generated sequences differed from each other by 0–0.14% (0–1 nt) and from other isolates of P. maculatus (KU052937, KU052941, KX671302) by 0–0.41% (0–3 nt). The isolate identified as P. maculatus (AY222284) differed from sequences generated in the present study by 4.40–4.53% (32–33 nt), and from the abovementioned three isolates of P. maculatus by 4.53–4.67% (33–34 nt). The overall interspecific variation for the Proctoeces isolates in this dataset is 0.14–7.43% (1–54 nt).
Figure 3.
Bayesian inference (BI) trees based on the 28S rDNA (A) and 18S rDNA (B) datasets of the genus Proctoeces. Nodal support given as BI/ML (maximum likelihood). Support values lower than 0.90 (BI) and 70 (ML) are not shown. The scale bar indicates the expected number of substitutions per site.
The alignment of the 18S rDNA dataset generated 308 characters for analyses. A similar topology was observed for the 18S dataset (Fig. 3B), where the isolate from the present study formed a highly supported clade with P. maculatus infecting S. pavonina from the Bizerte Lagoon, Tunisia (KX671315) (Wee et al., 2017). These two sequences are identical. Again, the new sequence did not cluster with the isolate identified as P. maculatus by Olson et al. (2003) (AY222161); the latter was instead recovered in a clade with Proctoeces major Yamaguti, 1934 + [P. humboldti George-Nascimento and Quiroga, 1983 + P. lintoni Siddiqi and Cable, 1960], sister to the clade formed by sequences of P. maculatus. This isolate differed from newly generated sequences and an isolate of P. maculatus (KX671315) by 3.58% (11 nt). The overall interspecific variation for the Proctoeces isolates in this dataset is 2.61–10.46% (8–32 nt).
The COI dataset was only used to calculate genetic difference matrices, as there are no COI sequences of P. maculatus available in GenBank with which to compare our data. Newly generated sequences of P. maculatus differed from each other by 0–0.9% (0–3 nt). The interspecific variation between other species of Proctoeces and newly sequenced isolates is 2.4–23.8% (8–79 nt). This study provides the first COI sequences for this species, which can be used in future phylogenies to study the true diversity of this genus.
Discussion
Due to a lack of reliable characteristics on which the species of Proctoeces can be distinguished and the great morphological variation exhibited within this genus, the species of Proctoeces are notoriously difficult to identify (Freeman and Llewellyn, 1958; Bray and Gibson, 1980). Proctoeces maculatus has not been re-collected or genotyped from its type-host at its type-locality (Trieste, Italy). Specimens identified as P. maculatus have been recorded and sequenced from Bizerte, Tunisia, which is also in the Mediterranean Basin but somewhat distant from Trieste, being about 950 km straight-distance away in the Tyrrhenian rather than Adriatic Sea and largely separated by the Italian mainland (Antar and Gargouri, 2013, 2016). This geographical distance, and the difference in fish hosts (the type-host being a labrid and those of Antar and Gargouri being from a sparid) further enhance the possibility that the specimens of Antar and Gargouri might not represent P. maculatus sensu stricto. This uncertainty can only be resolved with the molecular characterization of specimens collected from the type-host and the type-locality.
In weighing the merits of considering the P. maculatus of Looss from the type-locality conspecific with those of Antar and Gargouri from Tunisia, we need to consider two factors: the connectivity of populations and the propensity of P. maculatus to both switch fish hosts and use invertebrate (molluscan, annelid and echinoderm) hosts. Looss (1901), in describing P. maculatus, reported it from L. merula and two other labrid species, Symphodus cinereus (Bonnaterre) and S. tinca (L.). Linton (1907) subsequently described Proctoeces subtenuis (Linton, 1907) (as Distomum subtenue) from Bermuda, recording it from three labrid species and the sparid Calamus calamus (Valenciennes). Odhner (1911), while proposing the genus Proctoeces and redescribing P. maculatus, also described a second species, Proctoeces erythraeus Odhner, 1911 from Acanthopagrus bifasciatus (Forsskål) (Sparidae) and Thalassoma lunare (L.) (Labridae) from the Red Sea. Bray and Gibson (1980) reviewed the case for both species being synonymous with P. maculatus; P. subtenuis remains as such, although Wee et al. (2017) argued that P. erythraeus should best be treated as species inquirenda. Nevertheless, it had become established relatively early on that species of Proctoeces readily infected both labrids and sparids in sympatry. Wee et al. (2017) demonstrated that P. major Yamaguti, 1934 infected sympatric sparids and labrids (as well as lethrinids, monacanthids, monodactylids and pomacentrids) in Moreton Bay, Australia, but also found that Proctoeces choerodoni Wee, Cribb, Bray and Cutmore, 2017, exclusively infected labrids of the genus Choerodon Bleeker, showing that the species of Proctoeces could (but not always) have wide host ranges incorporating both sparids and labrids. Demarcating the true host-specificity of P. maculatus is particularly fraught due to the fact that the majority of records putatively assigned to this species have never been tested with molecular sequence methods, nor accompanied by morphological vouchers or depictions. It is hence highly likely that the host range of P. maculatus has, to some extent, been wrongly estimated. That this might be true, however, does not preclude the fact that its host range is wide, nor does a wide host and geographic range mean divergence and speciation cannot occur in certain circumstances.
It is well understood that connectivity in the marine environment is a significant function of population spatial structure, genetic variability and, ultimately, speciation (see Hodge and Bellwood, 2016, for example). Marine taxa in the Mediterranean Basin show varying levels of population connectivity, with even single or two ecologically similar species showing differing levels of genetic variation and connectivity between different Mediterranean regions (Sahyoun et al., 2016; Exadactylos et al., 2019; Falcini et al., 2020; López-Márquez et al., 2021). However, it is clear from many studies that high connectivity and therefore high gene flow is a feature of many Mediterranean marine species at least some of the time (González-Wangüemert et al., 2010; Exadactylos et al., 2019; López-Márquez et al., 2021), effectively reducing the chances that the P. maculatus on the north coast of Africa might have speciated from those on the south coast of Europe. The ability of the species of Proctoeces to infect and even reproduce within a wide range of sessile invertebrate hosts as progenetic metacercariae compounds their ability to reduce impediments to connectivity and link populations (Valdivia et al., 2014). Although the lack of molecular sequence data from the type-locality of P. maculatus again poses problems, sequence matching of species of Proctoeces from sympatric invertebrate and fish hosts has already been achieved (Valdivia et al., 2010; Antar and Gargouri, 2016; Wee et al., 2017). From all this information, we can (with the significant caveat that the status of P. maculatus from its type-locality and that of many putative records of this species from around the world is currently unknowable) infer that P. maculatus from labrids in the northern Mediterranean being a different species to those from sparids in the south is less likely than them being the same species, and, until the precise molecular nature of P. maculatus from its type-locality is known, it is safe and pragmatic to consider those specimens from Tunisia to be the same species.
Since its original description, P. maculatus has been reported from 65 fish species (including our three new host records) and 26 invertebrate species globally (WoRMS, 2023). The species is therefore rare among marine trematodes in that it appears to be truly euryxenous, i.e. infecting a wide range of hosts. Only a minority of marine fish-infecting trematodes are euryxenous, with the tendency being firmly towards higher, rather than lower, host-specificity (Miller et al., 2011). The phenomenon is most often observed among species of Hemiuroidea, including Aponurus laguncula Looss, 1907 (Lecithasteridae), reported from 95 fish species; Thulinia microrchis (Yamaguti, 1934) (Lecithasteridae), reported from 34 fish species; and most dramatically in Derogenes varicus (Müller, 1784) (Derogenidae), which has been reported from 317 fish species and habitats ranging from tropical coral reefs to abyssobenthic Antarctic waters (WoRMS, 2023). Such vast host ranges intuitively feel over-estimated; in cases of such disparate host and geographical range, they almost certainly are. However, judging their validity is complicated by the dubious reliability of many records, which were often made before the advent of modern molecular sequencing and provided only perfunctory morphological information (Bray et al., 2016). Further complicating the matter is the issue of morphological ‘variation along a theme’, with individuals from disparate localities and hosts showing a degree of variation in size or anatomy, but sufficiently conserved morphology that distinguishing them from one another is difficult or even impossible, and easily confounded by poor specimen condition or preparation practises such as flattening. Renewed scrutiny of such taxa has, in some instances, supported the notion that they actually represent complexes of multiple, often cryptic species (for example Carreras-Aubets et al., 2011), although the converse has also been demonstrated – specimens sampled across a wide host range are shown to be conspecific and thus reinforcing the breadth of the host range [as has happened in the case of T. microrchis (Miller et al., 2011)]. It is likely that P. maculatus represents both a truly euryxenous and widespread species, and also a complex of multiple species. However, without the ability to access more specimens and generate more molecular sequence data from localities throughout its range, however, no firm conclusions can be drawn.
Using an integrated taxonomic approach (based on a combination of molecular and morphological characteristics), we have identified the specimens in the present study as P. maculatus. This study provides the first molecular characterization of P. maculatus from South Africa, in combination with morphological characterization. This is also the first report of adult P. maculatus from South Africa, as well as the first report of this species from a fish host in the southern African region. Antar and Gargouri (2016) observed intraspecific variation in their sequences of the partial 28S gene of P. maculatus of 0‒0.42% (0‒5 nt); we consider the 0–0.41% (0–3 nt) difference between the newly generated sequences and the P. maculatus sequences available on GenBank as also consistent with intraspecific variation. Newly sequenced isolates are highly similar to isolates collected in the Mediterranean (Antar and Gargouri, 2016; Wee et al., 2017), differing by a maximum of 3 base-pairs. However, the isolate identified as P. maculatus collected in the Gulf of Mexico (Olson et al., 2003) did not cluster among other isolates of P. maculatus, thus it likely represents another species of Proctoeces. This was also noted by Antar and Gargouri (2016). Similar results were seen within the 18S dataset analysed. Bray (1984) reported P. maculatus as progenetic metacercariae from the common octopus O. vulgaris off Durban, South Africa; it is very likely that the specimens found during the present study represent this species, especially given that these host species share a habitat and similar food sources and are thereby exposed to larval stages of the same parasitic species (Smale and Buchan, 1981; Bennett et al., 1983).
The sequence data generated by Antar and Gargouri (2016) and Wee et al. (2017) from sparids and carangids from off Tunisia are the closest available to the type-locality, being also from the Mediterranean Basin. Our P. maculatus sequences from sparids and clinids differ from those of Antar and Gargouri (2016) and Wee et al. (2017) by a maximum of 3 bp in the partial 28S rDNA region and are identical in the 18S rDNA region, supporting the notion that P. maculatus is not only euryxenous, but also has a wide geographical range. This ability to spread across such a wide area is likely facilitated by the versatility of P. maculatus, exploiting multiple hosts which are similarly wide-ranging and highly vagile (Feis et al., 2015). South Africa shares several known hosts of P. maculatus with the Mediterranean, e.g. the sparids L. mormyrus and Diplodus vulgaris (Geoffroy Saint-Hilaire), the common octopus, O. vulgaris and the Mediterranean mussel, Mytilus galloprovincialis Lamarck (Mytilidae), the latter having been introduced to South Africa in the 1970s (Branch and Steffani, 2004). Another known host in the Mediterranean, Diplodus sargus, is also found along most of the West African coast and, until recently, was considered conspecific with our new host, D. capensis. As discussed above, the ability of P. maculatus to incorporate a progenetic stage in its life cycle, thereby thriving even when suitable definitive fish hosts are not present, likely further contributes to the wide distribution and ability of this species to exploit a wide range of hosts.
Interestingly, our results showed that fish sampled from sites within marine protected areas (MPAs) had the highest prevalence of P. maculatus (TNP, 30.0% from C. superciliosus, 39.0% from D. capensis and 42% from S. durbanensis; DHNR, 67.0% from D. capensis), compared with sites not within MPAs and adjacent to highly urbanized areas (0% in Cape Town harbour, Saldanha Bay and Mossel Bay) (see Table 1). This suggests that these parasites might be sensitive to pollution or other anthropogenic effects and therefore could be good indicators of ecosystem health. Similar results were noted by Erasmus et al. (2022), where the parasite diversity of C. superciliosus was lower in areas with a higher anthropogenic influence. Such findings are consistent with what we know regarding the deleterious effects that anthropogenic environmental changes have on both the richness and abundance of aquatic parasites (Sures et al., 2023). One possible explanation of this phenomenon could be the absence or reduced presence of suitable intermediate or definitive hosts, which might be more susceptible to the effects of anthropogenic activities in non-MPA areas (Erasmus et al., 2022). Apart from the record of metacercariae by Bray (1983) from O. vulgaris, intermediate hosts of P. maculatus are unknown in South Africa. Elsewhere, first intermediate stages of the species have been observed from mytilid bivalves (Stunkard, 1970; Wardle, 1980), while both progenetic and non-progenetic metacercariae have been found in a wide range of invertebrates, including buccinid (Shimazu, 1984), haliotid (Shimazu, 1972), hydrobiid (Belousova, 2022), patellid (Prevot, 1965) and rissoid (Machkevsky and Parukhin, 1981) gastropods; acanthochitonid chitons (Polyplacophora) (Prevot, 1965); pectinid bivalves (Bray, 1983); nereid (Machkevsky, 1985), sabellid (Antar and Gargouri, 2016) and serpulid (Prevot, 1965) polychaetes (Annelida); and strongylocentrotid echinoids (Echinodermata) (Shimazu, 1979). Most of these host groups, and the definitive fish hosts in which we found P. maculatus, are well represented along the South African coast, which means that, in theory, P. maculatus is well provisioned with intermediate and definitive hosts. However, the shallow-water marine communities, both in South Africa and elsewhere, are known to be vulnerable to anthropogenic disturbance, such as that caused by excessive harvesting (Crowe et al., 2000; Cole et al., 2011) and urbanization (Celliers and Ntombela, 2015; Momota and Hosokawa, 2021). Further marine environmental parasitological studies, focussing on digeneans and their intermediate and definitive hosts, will be needed to determine the extent to which anthropogenic environmental disturbance could compromise host population/community structure and, by extension, the parasite community.
Acknowledgements
We thank the staff of Two Oceans Aquarium for collecting C. superciliosus from Cape Town harbour; members of the North-West University (NWU) Water Research Group (WRG) for their assistance with fish collection and fieldwork; and Dr Anja Erasmus for constructing the map of the sampling localities. This is contribution number 827 from the NWU-WRG.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Author contributions
Conceptualization, N. J. S. and O. K.; methodology, A. V. and O. K.; validation, N. J. S., O. K. and A. V.; formal analysis, A. V. and O. K.; investigation, A. V. and O. K.; resources, N. J. S. and O. K.; data curation, A. V. and O. K.; writing – original draft preparation, A. V. and R. Q-Y. Y.; writing – review and editing, N. J. S., R. Q-Y. Y., A.V. and O. K.; visualization, A. V.; supervision, N. J. S. and O. K.; project administration, N. J. S. and O. K.; funding acquisition, N. J. S. All authors have read and agreed to the published version of the manuscript.
Financial support
This study was supported by a Postdoctoral Fellowship from the NWU, South Africa and a Claude Leon Foundation Postdoctoral Fellowship (2017–2018) to O. K. A. V. was partially funded by a South African National Research Foundation (NRF) scholarship (grant number: 122640 and MND200420515000). Opinions, findings, conclusions and recommendations expressed in this publication are that of the authors, and the NRF accepts no liability whatsoever in this regard.
Competing interests
None.
Ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Ethical approval for this study was provided by the North-West University's AnimCare Ethics committee (NWU-00565-19-A5 and NWU-00759-22-A5).
References
- Antar R and Gargouri L (2013) Trematodes in fishes of the genus Diplodus (Teleostei, Sparidae) from Bizerte Lagoon (Northern coast of Tunisia). Bulletin of the European Association of Fish Pathologists 33, 44–52. [Google Scholar]
- Antar R and Gargouri L (2016) Morphology and molecular analysis of life-cycle stages of Proctoeces maculatus (Looss, 1901) (Digenea: Fellodistomidae) in the Bizerte Lagoon, Tunisia. Journal of Helminthology 90, 726–736. [DOI] [PubMed] [Google Scholar]
- Belousova YV (2022) The first data on larvae of trematodes from the gastropod Hydrobia acuta in the Black Sea. Biological Bulletin 49, 21–28. [Google Scholar]
- Bennett B, Griffiths CL and Penrith M (1983) The diets of littoral fish from the Cape Peninsula. South African Journal of Zoology 18, 343–352. [Google Scholar]
- Branch GM and Steffani CN (2004) Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). Journal of Experimental Marine Biology and Ecology 300, 189–215. [Google Scholar]
- Bray RA (1983) On the fellodistomid genus Proctoeces Odhner, 1911 (Digenea), with brief comments on two other fellodistomid genera. Journal of Natural History 17, 321–339. [Google Scholar]
- Bray RA (1984) Some helminth parasites of marine fishes and cephalopods of South Africa: Aspidogastrea and the digenean families Bucephalidae, Haplosplanchnidae, Mesometridae and Fellodistomidae. Journal of Natural History 18, 271–292. [Google Scholar]
- Bray RA and Gibson DI (1980) The Fellodistomidae (Digenea) of fishes from the northeast Atlantic. Bulletin of the British Museum of Natural History 37, 199–293. [Google Scholar]
- Bray RA, Diaz PE and Cribb TH (2016) Knowledge of marine fish trematodes of Atlantic and Eastern Pacific Oceans. Systematic Parasitology 93, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM and Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis, et al. revisited. Journal of Parasitology 83, 575–583. [PubMed] [Google Scholar]
- Carreras-Aubets M, Repullés-Albelda A, Kostadinova A and Carassón M (2011) A new cryptic species of Aponurus Looss, 1907 (Digenea: Lecithasteridae) from Mediterranean goatfish (Teleostei: Mullidae). Systematic Parasitology 79, 145–159. [DOI] [PubMed] [Google Scholar]
- Celliers L and Ntombela C (2015) Urbanisation, coastal development and vulnerability, and catchments. In Paula J (ed.), Regional State of the Coast Report: Western Indian Ocean. Nairobi, Kenya: United Nations Environment Programme/Nairobi Convention Secretariat, pp. 387–406. [Google Scholar]
- Cole VJ, McQuaid CD and Nakin MDV (2011) Marine protected areas export larvae of infauna, but not of bioengineering mussels to adjacent areas. Biological Conservation 144, 2088–2096. [Google Scholar]
- Cribb TH, Martin SB, Diaz PE, Bray RA and Cutmore SC (2021) Eight species of Lintonium Stunkard & Nigrelli, 1930 (Digenea: Fellodistomidae) in Australian tetraodontiform fishes. Systematic Parasitology 98, 595–624. [DOI] [PubMed] [Google Scholar]
- Crowe TP, Thompson RC, Bray S and Hawkins SJ (2000) Impacts of anthropogenic stress on rocky intertidal communities. Journal of Aquatic Ecosystem Stress and Recovery 7, 273–297. [Google Scholar]
- Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acid Research 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus A, Wepener V, Hadfield KA, Sures B and Smit NJ (2022) Metazoan parasite diversity of the endemic South African intertidal klipfish, Clinus superciliosus: factors influencing parasite community composition. Parasitology International 90, 102611. [DOI] [PubMed] [Google Scholar]
- Exadactylos A, Vafidis D, Tsigenopoulos CS and Gkafas GA (2019) High connectivity of the White Seabream (Diplodus sargus, L. 1758) in the Aegean Sea, Eastern Mediterranean Basin. Animals 9, e979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcini F, Corrado R, Torri M, Mangano MC, Zarrad R, Di Cintio A, Palatella L, Jarboui O, Missaoui H, Cuttitta A, Patti B, Santoleri R, Sarà G and Lacorata G (2020) Seascape connectivity of European anchovy in the Central Mediterranean Sea revealed by weighted Lagrangian backtracking and bio-energetic modelling. Scientific Reports 10, e18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feis ME, Thieltges DW, Olsen JL, de Montaudouin X, Jensen KT, Bazaïri H, Culloty SC and Luttikhuizen PC (2015) The most vagile host as the main determinant of population connectivity in marine macroparasites. Marine Ecology Progress Series 520, 85–99. [Google Scholar]
- Freeman RFH and Llewellyn J (1958) An adult digenetic trematode from an invertebrate host: Proctoeces subtenuis (Linton) from the Lamellibranch Scorbicularia plana (Da Costa). Journal of the Marine Biological Association of the United Kingdom 37, 435–457. [Google Scholar]
- Froese R and Pauly D (2023) FishBase. World Wide Web electronic publication. Available at www.fishbase.org [Google Scholar]
- González-Wangüemert M, Cánovas F, Pérez-Ruzafa A, Marcos C and Alexandrino P (2010) Connectivity patterns inferred from the genetic structure of white seabream (Diplodus sargus L.). Journal of Experimental Marine Biology and Ecology 383, 23–31. [Google Scholar]
- Hall KA, Crobb TH and Barker SC (1999) V4 region of small subunit rDNA indicates polyphyly of the Fellodistomidae (Digenea) which is supported by morphology and life-cycle data. Systematic Parasitology 43, 81–92. [DOI] [PubMed] [Google Scholar]
- Hodge J and Bellwood DR (2016) The geography of speciation in coral reef fishes: the relative importance of biogeographical barriers in separating sister-species. Journal of Biogeography 43, 1324–1335. [Google Scholar]
- Izumi S, Akiyama N, Suzumura Y and Ogawa K (2021) Infection of a species of digenean in common octopus Octopus sinensis in Japan. Fish Pathology 56, 199–204. [Google Scholar]
- Linton E (1907) Notes on parasites of Bermuda fishes. Proceedings of the United States National Museum 33, 85–126. [Google Scholar]
- Littlewood DTJ, Curini-Galletti M and Herniou EA (2000) The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Molecular Phylogenetic Evolution 16, 449–466. [DOI] [PubMed] [Google Scholar]
- Looss A (1901) Ueber einige Distomen der Labriden des Triester Hafens. Centralblatt für Bakteriologie und Parasitenkunde 29, 398–405. [Google Scholar]
- López-Márquez V, Cushman SA, Templado J, Ho Y-W, Bothwell HM and Machordom A (2021) Genetic connectivity of two marine gastropods in the Mediterranean Sea: seascape genetics reveals species-specific oceanographic drivers of gene flow. Molecular Ecology 30, 4608–4629. [DOI] [PubMed] [Google Scholar]
- Machkevsky VK (1985) Some aspects of the biology of the trematode, Proctoeces maculatus, in connection with the development of mussel farms in the Black Sea. Parasitology and pathology of marine organisms of the world ocean. NOAA Technical Report NMFS 25. US Department of Commerce: Washington, United States of America, 109–110.
- Machkevsky VK and Parukhin AM (1981) On the role of trematodes from the Proctoeces Odhner, 1911 genus in certain littoral biocenoses of the Black Sea. Vestnik Zoologii 1, 59–61. [Google Scholar]
- Miller TL, Bray RA and Cribb TH (2011) Taxonomic approaches to and interpretation of host specificity of trematodes of fishes: lessons from the Great Barrier Reef. Parasitology 138, 1710–1722. [DOI] [PubMed] [Google Scholar]
- Momota K and Hosokawa S (2021) Potential impacts of marine urbanization on benthic macrofaunal diversity. Scientific Reports 11, 4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszczynska A, Locke SA, McLaughlin D, Marcogliese DJ and Crease TJ (2009) Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Molecular Ecology Resources 9, 75–82. [DOI] [PubMed] [Google Scholar]
- Muñoz G, López Z and Cárdenas L (2013) Morphological and molecular analyses of larval trematodes in the intertidal bivalve Perumytilus purpuratus from central Chile. Journal of Helminthology 87, 356–363. [DOI] [PubMed] [Google Scholar]
- Ñacari LA, Sepulveda FA, Escribano R, Bray RA and Oliva ME (2018) Morphological and molecular characterisation of digenean parasites of the Galápagos sheephead Semicossyphus darwini (Jenyns) with the re-description of Labifer secundus Manter, 1940 (Lepidapedidae) from the Humboldt Current Large Marine Ecosystem. Systematic Parasitology 95, 391–401. [DOI] [PubMed] [Google Scholar]
- Odhner T (1911) Zum naturlichen System der Trematoden. III. (Ein weiterer Fall von sekundarum Anus). Zoologischer Anzeiger 38, 97–117. [Google Scholar]
- Oliva ME and Huaquin LG (2000) Progenesis in Proctoeces lintoni (Fellodistomidae), a parasite of Fissurella crasse (Archaeogastropoda) in a latitudinal gradient in the Pacific coast of south America. Journal of Parasitology 86, 768–772. [DOI] [PubMed] [Google Scholar]
- Oliva ME, Valdivia IM, Cárdenas L, Muñoz G, Escribano R and George-Nascimento MA (2018) New species of Proctoeces and reinstatement of Proctoeces humboldti George-Nascimento and Quiroga 1983 (Digenea: Fellodistomidae) based on molecular and morphological evidence. Parasitology International 67, 159–169. [DOI] [PubMed] [Google Scholar]
- Olson PD, Cribb TH, Tkach VV, Bray RA and Littlewood DTJ (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology 33, 733–755. [DOI] [PubMed] [Google Scholar]
- Pleijel F, Jondelius U, Norlinder E, Nygren A, Oxelman B, Schander C, Sundberg P and Thollesson M (2008) Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetic Evolution 48, 369–371. [DOI] [PubMed] [Google Scholar]
- Posada D (2008) jModelTest: phylogenetic model averaging. Molecular Biological Evolution 25, 1253–1256. [DOI] [PubMed] [Google Scholar]
- Prevot G (1965) Complement a la connaissance de Proctoeces maculatus (Looss, 1901) Odhner, 1911 [syn. P. erythraeus Odhner, 1911 et P. subtenuis (Linton, 1907) Hanson, 1950]. (Trematoda, Digenea, Fellodistomatidae). Bulletin de la Société Zoologique de France 90, 175–179. [Google Scholar]
- Rambaut A (2012) FigTree v1.4.3. Available at http://tree.bio.ed.ac.uk/software.figtree
- Sahyoun R, Guidetti P, Di Franco A and Planes S (2016) Patterns of fish connectivity between a Marine Protected Area and surrounding fished areas. PLoS ONE 11, e0167441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu TA (1972) Metacercaria of the genus Proctoeces (Fellodistomatidae: Trematoda) from an abalone, Haliotis discus hannai, of Rebun Island, Hokkaido. Bulletin of the Japanese Society of Scientific Fisheries 38, 813–816. [Google Scholar]
- Shimazu TA (1979) Metacercaria of a digenetic trematode of the genus Proctoeces (Fellodistomidae) parasitic to the sea urchin, Strongylocentrotus intermedius. Zoological Magazine 88, 318–320. [Google Scholar]
- Shimazu T (1984) Proctoeces maculatus from Wakkanai, northern Hokkaido, Japan, with comments on the validity of other species in the genus Proctoeces (Trematoda: Fellodistomidae). Proceedings of the Japanese Society of Systematic Zoology 29, 1–15. [Google Scholar]
- Smale MJ and Buchan PR (1981) Biology of Octopus vulgaris off the east coast of South Africa. Marine Biology 65, 1–12. [Google Scholar]
- Snyder SD and Tkach VV (2001) Phylogenetic and biogeographical relationships among some Holarctic frog lung flukes (Digenea: Haematoloechidae). Journal of Parasitology 87, 1433–1440. [DOI] [PubMed] [Google Scholar]
- Stunkard HW (1970) The marine cercariae of the Woods Hole Massachusetts Region. Biological Bulletin of the Marine Biological Laboratory 138, 66–76. [Google Scholar]
- Sures B, Nachev M, Schwelm J, Grabner D and Selbach C (2023) Environmental parasitology: stressor effects on aquatic parasites. Trends in Parasitology 39, 461–474. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Murakami K, Takeyama H and Chow S (2006) Molecular attempt to identify prey organisms of lobster phyllosoma larvae. Fisheries Science 72, 342–349. [Google Scholar]
- Tkach VV, Pawlowski J, Mariaux J and Swiderski Z (2001) Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In Littlewood DTJ and Bray RA (eds), Interrelationships of Platyhelminthes. London, United Kingdom: Taylor & Francis, pp. 186–193. [Google Scholar]
- Tkach VV, Littlewood DTJ, Olson PD, Kinsella JM and Swiderski Z (2003) Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Systematic Parasitology 56, 1–15. [DOI] [PubMed] [Google Scholar]
- Valdivia IM, Cardenas L, Gonzalez K, Jofré D, George-Nascimento M, Guiñez R and Oliva ME (2010) Molecular evidence confirms that Proctoeces humboldti and Proctoeces chilensis (Digenea: Fellodistomidae) are the same species. Journal of Helminthology 84, 341–347. [DOI] [PubMed] [Google Scholar]
- Valdivia IM, Criscione CD, Cárdenas L, Durán CP and Oliva ME (2014) Does a facultative precocious life cycle predispose the marine trematode Proctoeces cf. lintoni to inbreeding and genetic differentiation among host species? International Journal for Parasitology 44, 183–188. [DOI] [PubMed] [Google Scholar]
- Van Steenkiste N, Locke SA, Castelin M, Marcogliese DJ and Abbott CL (2015) New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes). Molecular Ecology Research 15, 945–952. [DOI] [PubMed] [Google Scholar]
- Vlasenko PV (1931) On the parasitic worm fauna of fishes of the Black Sea. Trudy Karadahs’ koyi Nauchnoyi Stantsiyi imeni TI Vyazems' koho 4, 88–136. [Google Scholar]
- Wardle WJ (1980) On the life-cycle stages of Proctoeces maculatus (Digenea: Fellodistomidae) in mussels and fishes from Galveston Bay, Texas. Bulletin of Marine Sciences 30, 737–743. [Google Scholar]
- Wee NQ-X, Cribb TH, Bray RA and Cutmore SC (2017) Two known and one new species of Proctoeces from Australian teleosts: variable host-specificity for closely related species identified through multi-locus molecular data. Parasitology International 66, 16‒26. [DOI] [PubMed] [Google Scholar]
- WoRMS (2023) World Register of Marine Species. Available at https://www.marinespecies.org/. Accessed on: 14 June 2023.
- Yamaguti S (1934) Studies on the helminth fauna of Japan: Part 2, Trematodes of fishes. I. Japanese Journal of Zoology 5, 249–541. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.