Abstract
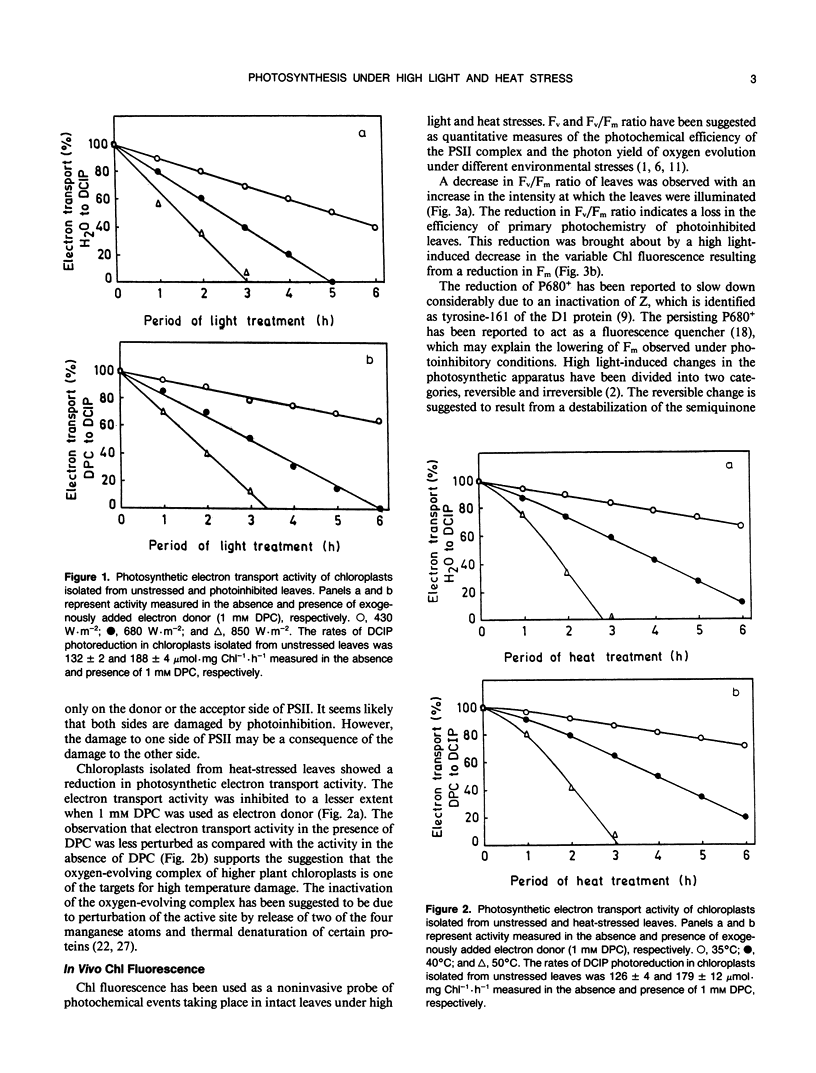
Effects of high light and temperature stress on the structure and function of the photosynthetic apparatus of wheat (Triticum aestivum) were studied. There was a decrease in the electron transport activity of chloroplasts isolated from photoinhibited and heat-stressed leaves. Chlorophyll fluorescence was measured in photoinhibited and heat-stressed leaves and the decrease in variable fluorescence and variable to maximum fluorescence ratio of the stressed leaves indicated a loss in the quantum yield of photosynthesis. The decrease in electron transport activity was accompanied by an increase in peroxidation of thylakoid lipids. Lipid peroxidation indicated the oxidative degradation of polyunsaturated fatty acyl residues of the thylakoid lipids. A negative correlation was observed between electron transport activity and lipid peroxidation. The electron transport activity was completely lost as the peroxidation level reached a threshold equivalent to 0.6 micromoles malondialdehyde. The threshold of lipid peroxidation for complete loss of activity was the same for both photoinhibition and heat treatment, suggesting that the nature of the environmental stress may be less important with respect to the relationship between electron transport and lipid peroxidation. Thus, it seems likely that lipids are required for sustaining the photosynthetic activity under environmental stress, and a loss in activity is observed as the lipids are degraded either by high light or high temperature stress.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Sithole I., Babcock G. T., McIntosh L. Directed mutagenesis indicates that the donor to P+680 in photosystem II is tyrosine-161 of the D1 polypeptide. Biochemistry. 1988 Dec 27;27(26):9071–9074. doi: 10.1021/bi00426a001. [DOI] [PubMed] [Google Scholar]
- Girotti A. W. Photodynamic lipid peroxidation in biological systems. Photochem Photobiol. 1990 Apr;51(4):497–509. doi: 10.1111/j.1751-1097.1990.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Heath R. L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968 Apr;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. K., Blaylock R., Sturtevant J. M., Brudvig G. W. Molecular basis of the heat denaturation of photosystem II. Biochemistry. 1989 Aug 8;28(16):6686–6695. doi: 10.1021/bi00442a023. [DOI] [PubMed] [Google Scholar]


