Abstract
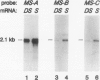
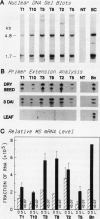
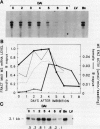
To study gene regulation during the transition from late embryogeny to germination, we have analyzed the expression of a gene encoding the glyoxylate cycle enzyme malate synthase in transgenic tomato (Lycopersicon esculentum) plants. We have shown that although there are at least four classes of malate synthase genes in Brassica napus L., one gene is expressed at a high level during both late embryogeny and postgermination. Analyses of transgenic tomato plants containing the expressed B. napus gene along with 4.7 and 1.0 kilobase pairs of 5′ and 3′ flanking sequences, respectively, confirmed that a single gene is expressed at both stages of development. Furthermore, localization studies have shown that mRNA encoded by the B. napus gene is distributed throughout the tissues of a mature embryo but is not detected in the vascular cylinder of a seedling. We conclude that the sequences required to qualitatively regulate the gene correctly over the plant life cycle are present within the transferred gene and/or flanking regions. Moreover, the malate synthase gene is regulated differently during late embryogeny and postgermination in the developing vascular cylinder.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benfey P. N., Chua N. H. The Cauliflower Mosaic Virus 35S Promoter: Combinatorial Regulation of Transcription in Plants. Science. 1990 Nov 16;250(4983):959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- Comai L., Baden C. S., Harada J. J. Deduced sequence of a malate synthase polypeptide encoded by a subclass of the gene family. J Biol Chem. 1989 Feb 15;264(5):2778–2782. [PubMed] [Google Scholar]
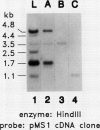
- Comai L., Dietrich R. A., Maslyar D. J., Baden C. S., Harada J. J. Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell. 1989 Mar;1(3):293–300. doi: 10.1105/tpc.1.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Harada J. J. Transcriptional activities in dry seed nuclei indicate the timing of the transition from embryogeny to germination. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2671–2674. doi: 10.1073/pnas.87.7.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch M. L. Regulation of gene expression during seed development in flowering plants. Dev Biol (N Y 1985) 1988;5:367–404. doi: 10.1007/978-1-4615-6817-9_14. [DOI] [PubMed] [Google Scholar]
- Dietrich R. A., Maslyar D. J., Heupel R. C., Harada J. J. Spatial patterns of gene expression in Brassica napus seedlings: identification of a cortex-specific gene and localization of mRNAs encoding isocitrate lyase and a polypeptide homologous to proteinases. Plant Cell. 1989 Jan;1(1):73–80. doi: 10.1105/tpc.1.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger W. F., Harada J. J. Translational or post-translational processes affect differentially the accumulation of isocitrate lyase and malate synthase proteins and enzyme activities in embryos and seedlings of Brassica napus. Arch Biochem Biophys. 1990 Aug 15;281(1):139–143. doi: 10.1016/0003-9861(90)90423-v. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Legocki A. B., Greenway S. C., Dure L. S., 3rd Cotton messenger RNA sequences exist in both polyadenylated and nonpolyadenylated forms. J Biol Chem. 1981 Mar 10;256(5):2551–2560. [PubMed] [Google Scholar]
- Goldberg R. B., Barker S. J., Perez-Grau L. Regulation of gene expression during plant embryogenesis. Cell. 1989 Jan 27;56(2):149–160. doi: 10.1016/0092-8674(89)90888-x. [DOI] [PubMed] [Google Scholar]
- Graham I. A., Smith L. M., Brown J. W., Leaver C. J., Smith S. M. The malate synthase gene of cucumber. Plant Mol Biol. 1989 Dec;13(6):673–684. doi: 10.1007/BF00016022. [DOI] [PubMed] [Google Scholar]
- Graham I. A., Smith L. M., Leaver C. J., Smith S. M. Developmental regulation of expression of the malate synthase gene in transgenic plants. Plant Mol Biol. 1990 Oct;15(4):539–549. doi: 10.1007/BF00017829. [DOI] [PubMed] [Google Scholar]
- Hughes D. W., Galau G. A. Temporally modular gene expression during cotyledon development. Genes Dev. 1989 Mar;3(3):358–369. doi: 10.1101/gad.3.3.358. [DOI] [PubMed] [Google Scholar]
- Liang X. W., Dron M., Cramer C. L., Dixon R. A., Lamb C. J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J Biol Chem. 1989 Aug 25;264(24):14486–14492. [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- McGrath J. M., Quiros C. F., Harada J. J., Landry B. S. Identification of Brassica oleracea monosomic alien chromosome addition lines with molecular markers reveals extensive gene duplication. Mol Gen Genet. 1990 Sep;223(2):198–204. doi: 10.1007/BF00265054. [DOI] [PubMed] [Google Scholar]
- Miernyk J. A., Trelease R. N., Choinski J. S. Malate synthase activity in cotton and other ungerminated oilseeds: a survey. Plant Physiol. 1979 Jun;63(6):1068–1071. doi: 10.1104/pp.63.6.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Leaver C. J. Glyoxysomal Malate Synthase of Cucumber: Molecular Cloning of a cDNA and Regulation of Enzyme Synthesis during Germination. Plant Physiol. 1986 Jul;81(3):762–767. doi: 10.1104/pp.81.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley R. B., Trelease R. N. Development and regulation of three glyoxysomal enzymes during cotton seed maturation and growth. Plant Mol Biol. 1990 Feb;14(2):137–146. doi: 10.1007/BF00018555. [DOI] [PubMed] [Google Scholar]
- Weir E. M., Riezman H., Grienenberger J. M., Becker W. M., Leaver C. J. Regulation of glyoxysomal enzymes during germination of cucumber. Temporal changes in translatable mRNAs for isocitrate lyase and malate synthase. Eur J Biochem. 1980 Dec;112(3):469–477. [PubMed] [Google Scholar]