Abstract
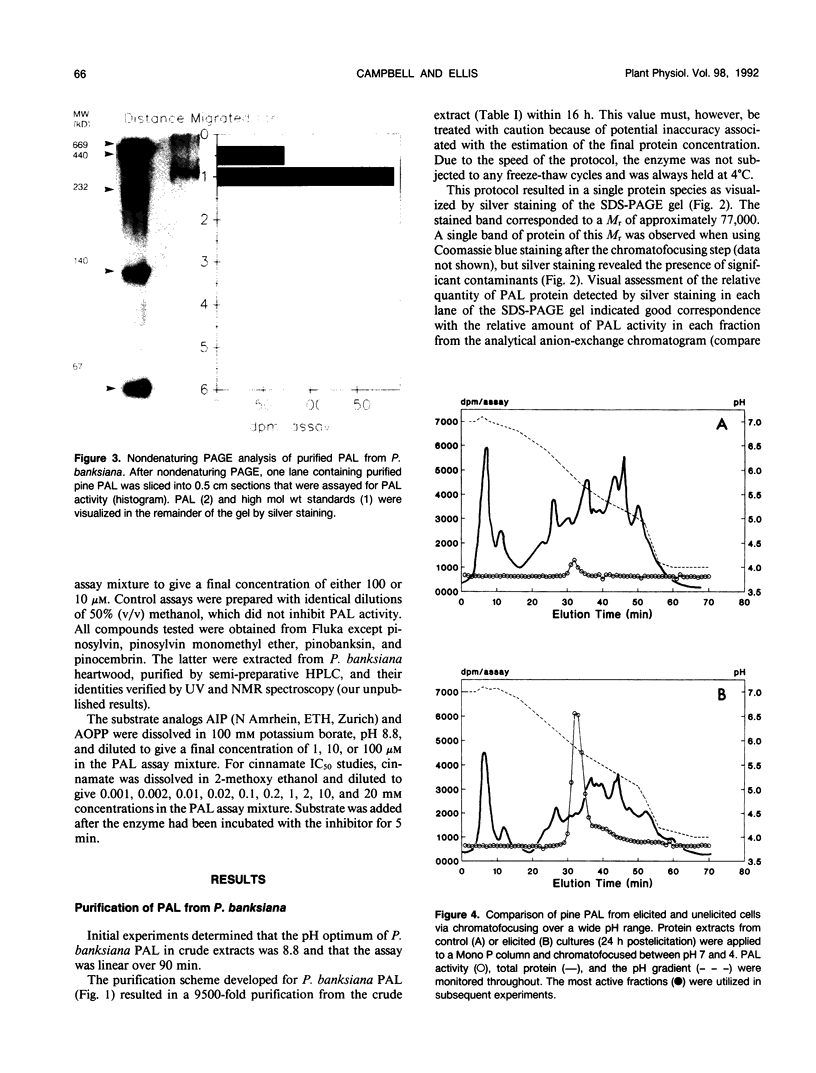
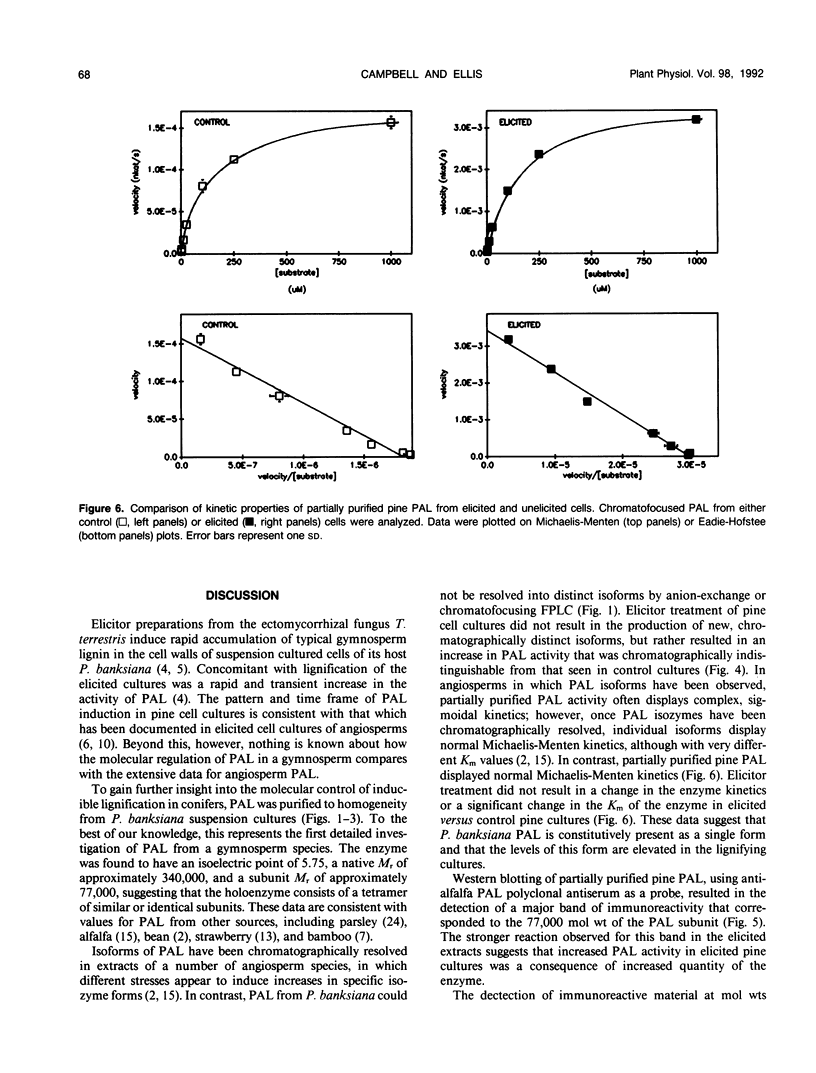
Phenylalanine ammonia-lyase (PAL, EC 4.3.1.5) is involved in the lignification of pine suspension cultures in response to an elicitor prepared from an ectomycorrhizal fungus. To elucidate the molecular basis of this response, PAL was purified to homogeneity from jack pine (Pinus banksiana) suspension cultures using anion-exchange and chromatofocussing fast protein liquid chromatography. Physical characterization of the enzyme revealed that pine PAL was similar to PAL from other plant sources. Pine PAL had a pH optimum of 8.8, an isoelectric point of 5.75, and a native molecular mass of 340 kilodaltons. The enzyme appears to be a tetramer composed of 77 kilodalton subunits. Chromatographic and western blot analyses were used to identify possible isoenzymic changes in pine PAL in response to elicitation and to determine the nature of the increase in PAL activity associated with inducible lignification in these cultures. Only one species of PAL was detected in P. banksiana cell cultures and increased quantities of this protein were correlated with the enhanced enzyme activity observed in elicited cultures. P. banksiana PAL was not feedback-inhibited by a wide range of phenolic compounds at micromolar concentrations, including the reaction product cinnamic acid. Our data suggest that a different set of metabolic and molecular controls must be in place for the regulation of PAL in pine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Dalkin K., Edwards R., Edington B., Dixon R. A. Stress Responses in Alfalfa (Medicago sativa L.): I. Induction of Phenylpropanoid Biosynthesis and Hydrolytic Enzymes in Elicitor-Treated Cell Suspension Cultures. Plant Physiol. 1990 Feb;92(2):440–446. doi: 10.1104/pp.92.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Mindrinos M., Davis K. R., Ausubel F. M. Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell. 1991 Jan;3(1):61–72. doi: 10.1105/tpc.3.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorrin J., Dixon R. A. Stress Responses in Alfalfa (Medicago sativa L.): II. Purification, Characterization, and Induction of Phenylalanine Ammonia-Lyase Isoforms from Elicitor-Treated Cell Suspension Cultures. Plant Physiol. 1990 Feb;92(2):447–455. doi: 10.1104/pp.92.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Shaw N. M., Bolwell G. P., Smith C. Wound-induced phenylalanine ammonia-lyase in potato (Solanum tuberosum) tuber discs. Significance of glycosylation and immunolocalization of enzyme subunits. Biochem J. 1990 Apr 1;267(1):163–170. doi: 10.1042/bj2670163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields S. E., Wingate V. P., Lamb C. J. Dual control of phenylalanine ammonia-lyase production and removal by its product cinnamic acid. Eur J Biochem. 1982 Apr 1;123(2):389–395. doi: 10.1111/j.1432-1033.1982.tb19781.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann S., Hahlbrock K. Light-induced changes of enzyme activities in parsley cell suspension cultures. Purification and some properties of phenylalanine ammonia-lyase (E.C.4.3.1.5). Arch Biochem Biophys. 1975 Jan;166(1):54–62. doi: 10.1016/0003-9861(75)90364-1. [DOI] [PubMed] [Google Scholar]