Abstract
Recently, myriad studies have defined the versatile abilities of gasotransmitters and their synthesizing enzymes to play a “Maestro” role in orchestrating several oncological and non-oncological circuits and, thus, nominated them as possible therapeutic targets. Although a significant amount of work has been conducted on the role of nitric oxide (NO) and carbon monoxide (CO) and their inter-relationship in the field of oncology, research about hydrogen sulfide (H2S) remains in its infancy. Recently, non-coding RNAs (ncRNAs) have been reported to play a dominating role in the regulation of the endogenous machinery system of H2S in several pathological contexts. A growing list of microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) are leading the way as upstream regulators for H2S biosynthesis in different mammalian cells during the development and progression of human diseases; therefore, their targeting can be of great therapeutic benefit. In the current review, the authors shed the light onto the biosynthetic pathways of H2S and their regulation by miRNAs and lncRNAs in various oncological and non-oncological disorders.
Keywords: hydrogen sulfide, non-coding RNAs, CBS, CSE, 3-MST, cancer, non-oncological disorders
1. Introduction
Historically, hydrogen sulfide (H2S) has been a gas notorious for its noxious pungent odor of rotten eggs and for being identified as a toxic pollutant gas associated with several industries [1]. The identification of endogenous H2S in tissues has been reported in 1992, when Abe and Kimura reported on the endogenous production and signaling capacity of H2S as a neuromodulator [2].
The elucidation of its cellular signaling mechanisms led to the introduction of H2S into the family of gaseous signaling molecules known as gasotransmitters [3]. Until now, there have been three discovered gasotransmitters: nitric oxide (NO), identified in 1987, followed by discovery of carbon monoxide (CO) in 1990 and then the most recent member, H2S, in 1996 [4]. All the discovered gasotransmitters share a common set of characteristics, including regulated endogenous production, free permeation of cell membranes without transporters, and unique signaling function with certain interactions with cellular and molecular targets [3]. In addition, they have anti-inflammatory, anti-oxidant, anti-apoptotic, and cardioprotective activities. Yet, their point of difference is allocated in their endogenous production and their respective types of synthesizing enzymes, half-lives, and second messenger signaling [5].
H2S is colorless, flammable, and water-soluble gas with a characteristic smell of rotten eggs; it is now widely recognized as an endogenous biological mediator [6]. Similar to CO and NO, the old conventional thinking that all these gases are vicious and exert only detrimental influence on human health has gradually lost ground [7]. Since its discovery, H2S has been found to have vital functions in various physiological and pathological conditions [8,9,10,11]. Yet, its role in the malignant transformation process is still controversial and leads to a lot of puzzling conclusions. Moreover, unraveling H2S interactions within different tissues, its precise impact in different pathological conditions, and its interplay with other biochemical molecules and various signaling mediators is becoming even more complex [8]. It is worth noting that, recently, H2S has also been reported to be involved in the SARS-CoV-2 life cycle and associated symptoms [12], and others reported the protective role of H2S-releasing compounds in ameliorating SARS-CoV-2-associated lung endothelial barrier disruption [13].
Non-coding RNAs (ncRNAs) form a relatively recent class of post-transcriptional regulators that is widely expanding [14,15,16,17,18,19]. Recent work has redefined the perception of ncRNAs from ‘junk’ non-coding transcripts into functional regulatory mediators [20,21,22,23,24,25,26,27,28]. NcRNAs can regulate several cellular processes, such as chromatin re-modeling, post-transcriptional modifications, and the regulation of signal transduction pathways, through targeting several target mRNAs simultaneously [17,19,29,30]. Recently, we and others have highlighted the potential roles of ncRNAs in regulating H2S biosynthesis in several physiological and pathological contexts [31,32,33,34,35,36,37,38]. H2S and ncRNAs interact during the development and progression of several human diseases; therefore, their targeting can be of great therapeutic benefit [17]. It is also worth mentioning that miRNAs and lncRNAs are the dominating ncRNAs for regulating H2S biosynthesis in mammalian cells. However, this does not exclude the possibility that other ncRNAs are also involved in H2S biosynthesis regulation. We aimed, in this review, to shed the light onto the biosynthetic pathways of H2S and their regulation by ncRNAs in various oncological and non-oncological disorders.
2. Non-Coding RNAs: A Brief History
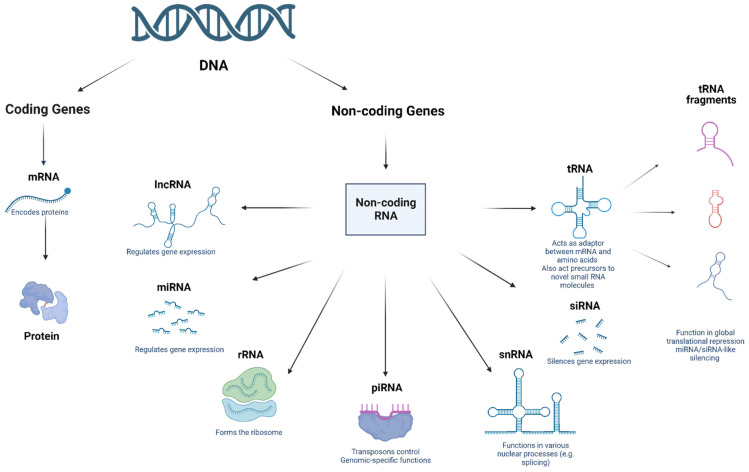
The emergence of small regulatory ncRNAs in the 1980s and 1990s completely shifted the pre-conceived notion of ncRNAs towards functional regulatory molecules [39,40,41,42]. Fire & Mello et al. indicated that double stranded RNAs (dsRNAs) could post-transcriptionally silence the complementary mRNAs in the nematode Caenorhabditis elegans via a process known as RNA interference [43,44]. Shortly afterwards, endogenous dsRNAs, such as small interfering RNAs (siRNAs) and microRNAs (miRNAs), were detected in a plethora of organisms, including plants, flies, and mammals [45]. The most thoroughly investigated classes of regulatory RNAs to date are piwi-associated RNAs, tRNAs, miRNAs, lncRNAs, and circRNAs (Figure 1) [45,46,47,48,49,50,51,52,53,54,55]. The non-coding portion of the mammalian transcriptome outweighs the protein-coding portion. This has been showcased in recently conducted studies, where only a mere 1.9% of the genome encodes for proteins [19,30,56,57,58]. In the coming sections of the review, the authors will focus on introducing the most well-studied ncRNAs.
Figure 1.
Different types of non-coding RNAs.
2.1. MicroRNAs (miRNAs)
In 1993, lin-4 was the first miRNA discovered in C. elegans [42,59]. Lin-4 was found to target lin-4 mRNA [42,59]. However, its discovery did not attract much attention in the scientific community, as lin-4 was exclusively present in worms [60]. The second miRNA, let-7, was also discovered in C. elegans. In line with lin-4, let-7 plays a role in cell differentiation and proliferation. However, unlike lin-4, let-7 is specific to certain species, and it is evolutionarily conserved, highlighting its crucial roles in many organisms [61]. miRNAs are a class of single-stranded RNAs of 19–24 nucleotides (nt) [62,63]. More than 60% of mRNAs contain miRNA target sites in their 3′UTR region [29,64,65]. In addition, several miRNAs have shown themselves to be able to target an abundance of mRNAs, implying a complex and intricate mechanism of action for regulating mRNA [26,66]. Studies describing the implication of miRNAs in the development of several diseases [22,67,68], including cancers [20,67,69,70], cardiac diseases such as hypertrophy and ischemia [64,71,72], and mental disorders such as schizophrenia and major depression [73], were previously reviewed in one of our earlier publications [15].
2.2. Piwi-Interacting RNAs (piRNAs)
They are a group of small non-coding RNAs (sncRNAs) that was proved to exert defense against transposable elements (TEs) in germ lines [17,74]. Lately, ubiquitously expressed piRNAs have been reported in somatic and germ human cells, providing new insights about the diverse potential role of piRNAs [74]. piRNAs are composed of 26–31 nt [75]. It is worth mentioning that piRNAs could be classified into two sub-clusters according to their biogenesis: (1) the pachytene piRNA cluster, which is produced during meiosis and continuously expressed until the haploid spermatid stage, and (2) the pre-pachytene piRNA cluster, which mainly manifests in premeiotic germ cells [75]. Both gene clusters are transcribed into long primary RNAs that are further processed to create primary piRNAs, thereby acting as a guide for the genesis of secondary piRNAs [39]. Primary piRNAs biosynthesis machinery remains to be clearly elucidated; however, secondary piRNAs arise via an amplification mechanism known as the Ping-Pong amplification loop to augment piRNA sequences acting on active elements [76].
Concerning piRNAs function in mammals, piRNAs seem to primarily function in the germline, where they are thought to play a crucial role in repressing transposable and repetitive elements and diminishing their mobility in order to maintain the genomic integrity and stability of germline stem cells [53,77,78]. TEs are abundant repetitive DNA segments that have the capacity to replicate and insert themselves into new locations. This poses a huge threat to genomic stability, as the new insertions can cause DNA breaks and, consequently, the arrest of cell cycle, gene disruption, and higher chances of non-homologous recombination [79]. The extent of this looming threat and the criticality of piRNAs’ role were validated by Siomi et al., where their study showed that the absence of piwi family proteins in mice led to the inactivation of retrotransposons in the male germ line; consequently, this issue halted gametogenesis and resulted in male sterility [53].
2.3. Long ncRNAs (lncRNAs)
They are a class of diversified ncRNAs that are more than 200 nt in length and mainly found in the nucleus or cytoplasm, and as denoted by its name, it lacks protein-coding potential [80,81,82]. LncRNAs are grouped into five distinct categories: sense, antisense, bidirectional, intronic, and intergenic lncRNA [24,83]. Focusing more on their origin, lncRNAs can arise from varying sources, including [84] (a) the disruption of the translational reading frame of a protein-encoding gene (b), the reorganization of chromosomes, (c) the replication of a non-coding gene through retrotransposition, (d) non-encoding RNAs containing adjacent repeats, and (e) the insertion of transposable elements into a gene in such a fashion that they generate a functional, transcribed non-encoding RNA [14,18,23]. Despite the dissimilarity in their origin, studies have interestingly shown how lncRNAs share the same ultimate goal, i.e., to modulate gene expression [25,70,80,83].
2.4. Circular RNAs (circRNAs)
They are covalently closed circular RNAs that lack 5′-3′ polarity or a polyadenylated tail [45,85]. CircRNAs were first identified in 1976 [86] and have since been detected in humans, rats, mice, fungi, and other organisms [87,88,89,90,91,92]. Due to their structural specificity, unknown molecular role, and scarcity [93], circRNAs were initially thought to be ancient conserved molecules produced as splicing by-products and, hence, did not attract much attention at the time [16,94]. However, advances in the field of bioinformatics and high-throughput detection methods have led to the discovery of numerous circRNAs and helped us to gain more insight into their characteristics and functions [94]. It is worth noting that circRNAs are highly conserved molecules that are usually expressed in a tissue-specific and developmental stage-specific manner [95,96,97]. With respect to their function, circRNAs were found to act as miRNA sponges to deter mRNA translation [98,99] and induce gene expression by modulating splicing [100,101] or transcription, as well as interacting with RNA-binding proteins (RBPs) [102]. They are reportedly involved in multiple processes associated with disease progression, and, therefore, these molecules can pave the way for new potential opportunities and might serve as diagnostic and/or prognostic biomarkers [16,94].
3. Endogenous Hydrogen Sulfide (H2S) Biosynthesis
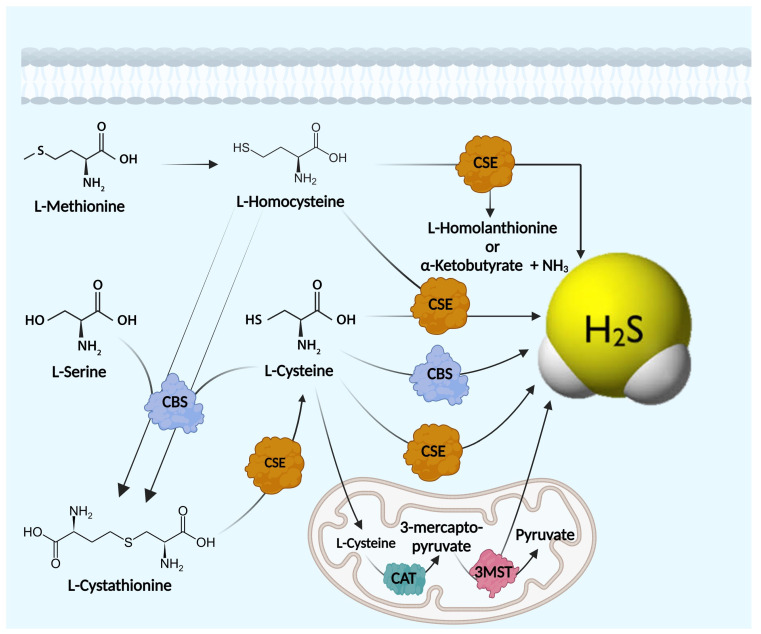
The endogenous generation of H2S mainly occurs through two main routes: enzymatic and non-enzymatic pathways. The enzymatic synthesis of H2S by mammalian tissues depends on two cytosolic pyridoxal-5′-phosphate-dependent enzymes: cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), as well as a pyridoxal-5′-phosphate- independent mitochondrial enzyme known as 3-mercaptopyruvate sulfur-transferase (3-MST) [8]. CBS, CSE, and 3-MST are constitutive enzymes of varying expression levels in different tissues and organs; however, their levels are altered in various pathological conditions [103].
The expression level and activity of the aforementioned enzymes have been reported to be organ- and tissue-specific. For instance, CBS is mostly found in the brain, nervous system, and liver [2], while CSE is predominantly found in the liver and vascular system; however, 3-MST can be detected in the brain and vasculature [104]. It is also worth noting that 3-MST, located in the mitochondria, produces H2S together with cysteine aminotransferase (CAT) [105,106,107,108,109] (Figure 2). The literature shows that these enzymes coordinately regulate the trans-sulfuration activity and, thus, control physiological H2S levels in a complex and overlapping manner [8].
Figure 2.
Hydrogen sulfide biosynthesis.
Apart from enzymatic synthetic pathways, endogenous H2S can also be produced through other non-enzymatic processes, as previously described [110]. To make it more clear, the non-enzymatic production of H2S occurs from either glucose via glycolysis or from phosphogluconate via NADPH oxidase [8]. H2S is also produced through the direct reduction of glutathione and elemental sulfur. The reduction of elemental sulfur to H2S is mediated by reducing equivalents of the glucose oxidation pathway like NADH or NADPH [111]. H2S formation from thiosulfate results from a reductive reaction involving pyruvate, which acts as a hydrogen donor. Thiosulfate is an intermediate of sulfur metabolism from cysteine and a metabolite of H2S that can also lead to the production of H2S [112].
4. H2S Enzymatic Machinery
4.1. Cystathionine-β-Synthase (CBS)
CBS is the most studied H2S-producing enzyme with regard structure and function. CBS gene is located on the long arm of chromosome 21 location 21q22.3. The crystalline structure of the active human CBS constitutes four 63-kDa subunits and is formed of 551 amino acids [113,114]. However, the shorter isozyme (≈48 kDa) was isolated from the liver and linked to increased catalytic activity in the enzyme [115]. Mechanistically, CBS is known to play an important role in amino acid metabolism. It facilitates the condensation of homocysteine with the L-serine amino acid into cystathionine, which is further broken down to form cysteine [116]. In addition to this canonical pathway, CBS has been found to synthesize H2S, depending on L-cysteine amino acid as a substrate and pyridoxal phosphate (PLP) as a coenzyme (Figure 2) [115]. CBS mainly resides in the cytosol; however, certain cell types have CBS located in the nucleus and mitochondria. The translocation of CBS from the cytosol to mitochondria can occur during states of hypoxia [117] or nucleolar stress [118]. As a H2S-synthesizing enzyme, CBS is implicated in numerous physiological events. Hence, many pathophysiological events have been linked to CBS dysregulation, including tumorigenesis [31,37,119].
4.2. Cystathionine-γ-Lyase (CSE)
CSE enzyme is encoded by the CTH gene on the short arm of chromosome 1 (1p31.1) and consists of 12 exons [120]. Another shorter transcription variant with lower activity was reported to be lacking exon 5 of the coding region [121]. CSE is a 405-amino acid polypeptide with a 45 kDa molecular weight. Similar to CBS, CSE catalytic activity is also PLP-dependent and depends on L-cysteine amino acid as a precursor for H2S synthesis [121]. However, unlike CBS, CSE is less investigated, and its regulation is less understood [122]. The pivotal role of CSE was markedly linked to the cardiovascular system [123]. In fact, endothelial cells and smooth-muscle cells in the blood vessels primarily synthesize H2S by CSE [122]. Yet, CSE dysregulation was also found to be associated with tumor progression. Amassing evidence shows that CSE plays crucial roles in numerous types of cancer cells. For instance, the inhibition or knockdown of CSE by shRNA halts the proliferation and migration of human colon cancer cells [124].
4.3. Mercaptopyruvate Sulfurtransferase (3-MST)
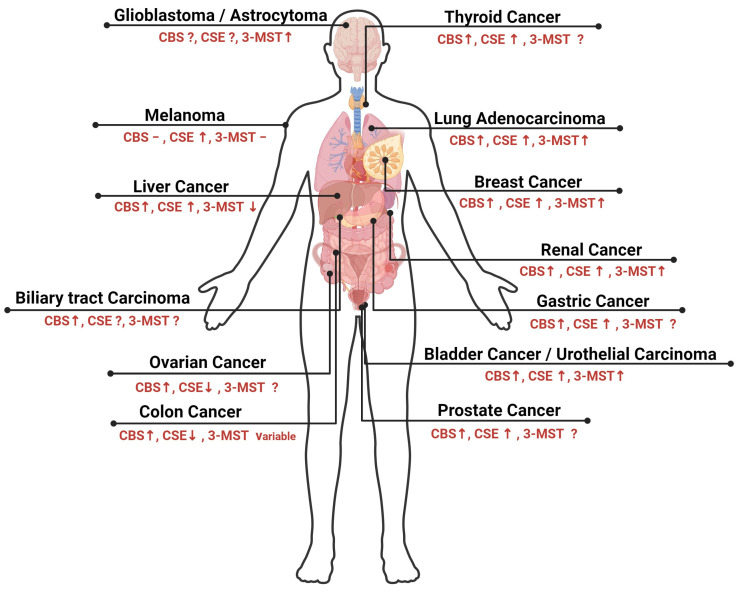
The human 3MST enzyme is encoded by the MPST gene on the long arm of chromosome 22 in the subtelomeric region q13.1 [125]. The MPST gene is 5.6 kbp in length with only two exon regions [126]. Unlike CBS and CSE, 3MST synthesizes H2S using 3-mercaptopyruvate (3-MP)—a product of L-cysteine metabolism by the cysteine aminotransferase (CAT) enzyme in the presence of α-ketoglutarate [127]. One more significant characteristic of 3MST is that it can be detected not only in the cytoplasm in a similar manner to CBS and CSE but also in the mitochondria [127]. Physiologically, 3MST plays a critical role in cellular metabolism and bioenergetics [128]. In recent years, there has been growing interest in the role of 3MST in cancer progression [129]. Some studies have shown that 3MST is upregulated in various types of cancers, as will be discussed later in this review. The expression levels of the three H2S-synthesizing enzymes in several types of cancer are displayed in Figure 3 and Table 1.
Figure 3.
Differential expression of H2S-synthesizing enzymes in solid malignancies.
Table 1.
Expression levels of H2S-synthesizing enzymes in several oncological phenotypes.
| Type of Cancer | Expression Level of CBS | Expression Level of CSE | Expression Level of 3-MST | Cancer Cell Lines/Cancer Tissues | References |
|---|---|---|---|---|---|
| Colon cancer | High | Low | Variable | Human tissues HCT116 HT29 |
[130,131] |
| Breast cancer | High | High | High | Human tissues | [31,132] |
| Ovarian cancer | High | Low | Not investigated | OV167 OV202 SKOV3 A2780 |
[133] |
| Melanoma | Not altered | High | Not altered | A375 Sk-Mel-5 Sk-Mel-28 PES 43 |
[134] |
| Liver cancer | High | High | Low | HepG2 SMMC-7721 QGY-7703 BEL-7402 BEL-7404 MHCC-LM3 Huh7 Hep3B |
[135,136,137] |
| Lung adenocarcinoma | High | High | High | A549 H522 H1944 |
[138] |
| Bladder cancer | High | High | High | Human tissues | [139] |
| Leukemia | High | Not altered | Not altered | CML-derived K562 cells | [140] |
| Multiple Myeloma | High | Not investigated | Not investigated | Human tissues | [141] |
| Prostate cancer | High | High | Not investigated | LNCap PC3 LNCaP-B |
[142,143,144] |
| Gastric cancer | High | High | Not investigated | SGC-7901 AGS |
[145,146] |
| Renal cancer | High | High | High | RCC4 | [147,148] |
| Glioblastoma | Not investigated | Not investigated | High | C6, U87 |
[37,149,150] |
| Thyroid cancer | High | High | Not investigated | TPC1 TT ARO |
[151,152] |
| Urothelial carcinoma | High | High | High | 5637 UM-UC-3 EJ |
[129,139,147,153] |
| Biliary tract carcinoma | High | Not investigated | Not investigated | GB-H3 GB-D1 TFK-1 HUCCT-1 |
[154] |
| Astrocytoma | Not investigated | Not investigated | High | U373 | [150] |
| Neuroblastoma | Not investigated | Not investigated | High | SH-SY5Y | [155,156] |
| Oral squamous cell carcinoma | High | High | High | OSCC | [157] |
5. Role of H2S in Oncological Disorders and Its Regulation by ncRNAs
Most of the reported literature supports the pro-carcinogenic competence of endogenous H2S. Different oncogenic cascades have been drawn downstream for H2S, such as hastening of cell cycle progression, propagating anti-apoptotic signals and the induction of angiogenesis, thus supporting the novel concept of inhibiting H2S production as a promising strategy for cancer treatment [158].
5.1. Hastening of Cell Cycle
The cell cycle represents a series of highly regulated waves that control the transition of cells from the quiescent state (G0) to proliferative status and ensure the high conformity of the genetic transcript material [159]. Most adult mammalian cells are in the quiescent state, and their re-entry into the cell cycle requires the inactivation of the retinoblastoma protein (pRB) and the transcription of genes required for DNA replication and chromosome segregation [160]. Any disturbance in the regulation of cell cycle is a common feature in the development of human cancers [159]. A recent study reported that the knocking down of CSE in HCC cell lines induced G1/G0 phase arrest and decreased the cell population in S phase, while the cell population in S phase was induced via the administration of 100 µM of NaHS for 24 h [161]. NaHS is an inorganic sulfide salt that has been widely used as an H2S equivalent in many biological studies. Furthermore, NaHS has been widely used in studies investigating the role of exogenous H2S in cancer progression, as it rapidly releases H2S via simple hydrolysis under laboratory conditions [162]. Similarly, another study indicated that exogenous H2S (NaHS, 200–500 µM) served as a pro-proliferative factor that accelerates cell cycle progression in oral squamous cell carcinoma through the increased phosphorylation of Akt and extracellular signal-regulated kinase (ERK) [163]. Likewise, exogenous H2S picked up the pace of the cell cycle in HCT116 via another mechanism of action, where it induced the repression of the tumor suppressor protein (p21), one of the cyclin-dependent kinase (CDK) inhibitory proteins, and, thus, decreased the G0/G1 population and increased the cell population at the S phase [164].
5.2. Propagation of Anti-Apoptotic Signals
Apoptosis is a tightly nuanced cellular suicide program that is critical for the normal development and maintenance of tissue homeostasis in multi-cellular organisms. In cancer cells, apoptosis is suppressed, which is the main reason for the uncontrolled cellular proliferation and resistance to treatment [165]. The CSE/H2S pathway played a vital role in maintaining the cancerous behavior of hepatoma cells, and the inhibition of this pathway could significantly suppress the uncontrolled growth of hepatoma cells by stimulating mitochondrial apoptosis and suppressing cell growth signal transduction [166]. In the same way, another study reported that the treatment of PLC/PRF/5 hepatoma cells with 500 µM NaHS for 24 h markedly increased cellular viability and decreased the number of apoptotic cells [167]. In the same pattern, the exogenous application of 100–1000 µM NaHS for 10 min or the overexpression of CBS hindered apoptosis-induced 6-Hydroxydopamine in a human neuroblastoma cell line [168].
5.3. Induction of Angiogenesis
Angiogenesis, or the sprouting of new blood vessels from the existing vasculature, mediated by several growth factors, such as VEGF, EGF, IGF, and angiogenin [169], was reported to be a fundamental step in the process of metastasis [170]. A growing body of evidence highlights H2S as a vital stimulator of the malignant transformation process, starting from its support for the tumor cellular proliferation and ending with its invigoration of angiogenesis [171]. Recent studies indicate that H2S possesses pro-angiogenic effects mainly by increasing VEGF expression [172,173]. In colon and ovarian cancers, CBS knockdown reduces the metastatic potential of cancer cells and decreases the number of sprouted blood vessels, thus resulting in the attenuation of tumor growth [133,174]. Taken together, these results support the possibility of utilizing CBS, CSE and 3-MST as potential molecular targets for cancer treatment.
5.4. Colon Cancer
Also Szabo group highlighted that CBS is selectively upregulated in colon cancer tissues compared with non-malignant colonic epithelial tissues [130,174]. Through a series of studies involving the knocking in/out of CBS expression or CBS activity (allosteric activation via SAM or inhibition using amino-oxyacetic acid (AOAA) in the HCT116 colon cancer cell line, it was validated that CBS encourages tumor cell advancement [174]. Additionally, several human colon cancer cells displayed elevated CBS expression in comparison to normal non-malignant colon cancer cell lines (NCM356). In 2013, Szabo et al. reported that CBS silencing or inhibition resulted in the suppression of cell proliferation, as well as migration and invasion in HCT116 cells [174]. In line with these findings, SAM, an allosteric activator of CBS, was tested at low concentrations in HCT116, and it resulted in an increase in cell proliferation [175].
The induction of the Akt/PI3K signaling pathway is likely to trigger the pro-proliferative and migratory effects of CBS-synthesized H2S. These findings were validated in an in vivo model, where the growth rate in mice bearing xenografts of either HCT116 cells or patient-derived tumor tissue were significantly reduced following the silencing of CBS expression and/or inhibition of CBS expression via AOAA [176]. In addition, AOAA reduced the metastatic spread of HCT116 cells from the cecum to the liver in an orthotopic xenograft model of nude mice [177]. A recent study conducted by Guo et al. 2021 showed that the stable knockout of CBS using the CRISPR/Cas9 system in colon cancer cells caused the suppression of invasion and metastasis of cells. This study also showed that CBS knockdown reduced the expression of angiogenesis-related proteins such as VEGF [178].
Chen et al. studied the effects of miRNAs on the acquired resistance of 5-fluorouracil. The study revealed that the expression of miR-215-5p is altered following the knockdown of CBS, resulting in the increased sensitivity of HCT116 cells to 5-flurouracil. This effect was also noticed in an in vivo model [179]. Potential targets of miR-215-5p include epiregulin (EREG) and thymidylate synthetase (TYMS), whose expressions were attenuated via the inhibition of H2S synthesis, contributing to the reversal of acquired resistance to 5-flurouracil in HCT116 cell lines [179]. Regarding CSE, Olah et al. highlighted that in most colon cancer patients, CSE expression was quite low in both human biopsies. With respect to 3-MST, it displayed variable levels of expression. Nearly 50% of the individual sample pairs were of a higher expression level in the tumor compared to the normal counterparts, while the rest of the samples (50%) showed a low expression profile [131]. It is worth mentioning that upon performing literature screening, neither CSE nor 3MST regulation by ncRNAs in colon cancer had been previously investigated.
5.5. Breast Cancer
Several studies have shown that human breast cancer tissues and cells lines (MDA-MB-468, MCF-7, and Hs578T) reveal the marked overexpression of CBS, CSE, and 3MST compared to normal breast cells [31,132,180]. Recently, our research group has highlighted the potential regulation of CBS and CSE in breast cancer cell lines by miR-4317 [31] and miR-939-5p [181,182]. It is worth mentioning that, recently, miR-548 and miR-193 have been found by our research group to simultaneously target the three synthesizing enzymes in breast cancer: CBS, CSE and 3MST (Table 2) [132,183].
Digging deeper into the intricate crosstalk and potential interplays between H2S and different types of ncRNAs, our research group investigated the role of the lncRNA MALAT-1 in regulating STAT-3-regulated CSE in BC. Our results show that upon the knockdown of MALAT-1, a marked repression of STAT-3 and CSE was observed, thereby highlighting MALAT-1 as a novel upstream lncRNA regulating the STAT-3/CSE/ H2S axis in breast cancer [184]. Table 2 shows a summary of most of the ncRNAs (miRNAs or lncRNAs) regulating H2S-synthesizing enzymes in different pathological disorders.
5.6. Ovarian Cancer
Similar to results of colon and breast cancers, Bhattacharyya et al. have found that CBS is overexpressed in primary epithelial ovarian cancer human biopsies and cell lines, particularly in serous carcinoma, the most common histologic variant. The majority of ovarian cancer cells showed high CBS expression (both on the mRNA and protein levels) compared to normal ovarian surface epithelial cells [133]. In the same study, the authors also studied the regulatory role of CBS-derived H2S in modulating ovarian cancer cell proliferation, migration, and invasion in vitro and in vivo. In the in vitro model, a variety of genetically mediated stable silencing and pharmacological inhibition of CBS were used where either the silencing of CBS or CBS inactivation by AOAA was found to inhibit cellular viability and proliferation [133]. In the in vivo models, the silencing of CBS led to a significant decrease in tumor weight and the number of tumor nodules, while the inhibition of peritumor angiogenesis was confirmed to cause a marked reduction in CD31 staining [133]. It is worth mentioning that upon performing literature screening, the regulation of H2S-synthesizing enzymes by ncRNAs in ovarian cancer had not been previously investigated.
5.7. Melanoma
Penza et al. [134] evaluated the involvement of the H2S pathway in melanoma. An interesting study was performed involving more than 100 human samples that showed that elevated CSE expression increased from nevi to primary melanoma, decreased in tissue metastases, and was absent in lymph node metastases. On the other hand, the screening of the CBS expression level in the same cohort of patients revealed that CBS was absent in dysplastic nevi. Positive CBS expression was found in only 25% of the primary melanomas analyzed. Therefore, as opposed to other types of cancer reviewed in this review, CBS does not appear to play an dominant role in human melanoma. It is also worth noting that 3-MST expression showed a variable alteration in the analyzed human specimens (from nevi to metastasis) [134]. It is also worth noting that upon performing literature screening, H2S-synthesizing enzymes regulation by ncRNAs in melanoma had not been previously investigated.
5.8. Liver Cancer
Jia et al. [137] showed that CBS protein was highly expressed in the human hepatoma cell lines HepG2 and SMMC-7721. This observation paved the way for the idea that CBS/H2S is involved in HCC proliferation. This hypothesis was validated upon the silencing of the CBS using CBS siRNAs and the inactivation of CBS activity using AOAA, which evidently repressed the proliferation of HCC cell lines. Also, CBS siRNAs induced the apoptosis of SMMC-7721 cells, revealing the involvement of the CBS-induced H2S production in regulating hepatoma cell proliferation. In addition, an increase in the Bax/Bcl ratio and a significant upregulation of caspase-3 and PARP activities were evident following CBS knockdown [137].
5.9. Lung Cancer
Significantly higher protein levels of CBS, CSE, and 3-MST were detected in human lung adenocarcinoma samples compared to normal counterparts. Similarly, elevated expression of CBS, CSE, and 3-MST and H2S production were also reported in multiple lung adenocarcinoma cell lines (A549, H522 and H1944) compared to non-malignant lung epithelial cells (BEAS 2B) [138].
5.10. Bladder Cancer
The expression of H2S-producing enzymes in human bladder cancer tissues compared to their normal counterparts was investigated. Our results showed that H2S levels, as well as CBS, CSE, and 3-MST expression, were higher in bladder cancer biopsies compared to their normal counterparts. Notably, the expression of all three enzymes was correlated to different stages of bladder cancer [139]. Another study showed that the apoptosis of bladder cancer cells was heightened after the inhibition of H2S production by propargylglycine (PAG) and inhibited upon adding exogenous H2S. This was validated in in vitro, as well as in vivo, models. The stimulation of the Erk1/2 signaling pathway and the blockade of mitochondrial apoptosis were suggested as the probable mechanisms behind these results [185]. Exogenous H2S supplementation has also been shown to affect bladder cancer cells. It was found that preconditioning cells with sodium hydrosulfide (NaHS) boosted cells’ proliferation and invasion capacity. This was evident in the expression of matrix metalloproteinases (MMP) 2 and 9, which are crucial for the digestion of collagen IV and hydrolysis of extracellular matrix during invasion, as they increased in a dose-dependent manner after the treatment of bladder cancer cells with NaHS [186].
5.11. Leukemia
CBS was found to be elevated in bone marrow mononuclear cells isolated from newly diagnosed children with CML compared to age- and gender-matched control groups. Meanwhile, the CSE and 3-MST levels showed no significant differences between the two groups. At the in vitro level, in CML-derived K562 cells, the upregulation of CBS expression was demonstrated. CSE and 3-MST displayed no significant differences in their expression levels [140]. The silencing or pharmacological inhibition of CBS using AOAA induced a decrease in the cell proliferation of both K562 cells and bone marrow mononuclear cells from CML patients. This was mediated via the mitochondria-related apoptosis pathway [140].
5.12. Multiple Myeloma
The CBS expression level was significantly higher in patients with multiple myeloma than healthy donors [141]. The pharmacologic inhibition of H2S production using AOAA successfully inhibited the proliferation of the U266 myeloma cell line in a concentration-dependent manner. Exogenous NaHS has a pro-tumorigenic effect on U266 cells by enhancing cell cycle progression, which was diminished by using AOAA. AOAA treatment resulted in a decrease in the expression of anti-apoptotic Bcl-2 and an increase in caspase-3, thus promoting apoptosis [141].
Table 2.
Non-coding RNAs regulating H2S-synthesizing enzymes.
| H2S Synthesizing Enzyme | miRNA | LncRNA | Cell Lines Investigated |
|---|---|---|---|
| CBS | miR-4317 [31] miR-6852 [187] miR-21 [32] miR-376a [188] miR-125b-5p [189] miR-203 [190] miR-215-5p [179] miR-939-5p [38] miR-548 [132] miR-193 [183] |
LINC00336 [187] lncRNA CBSLR [191] LncRNA SNHG1 [188] |
MDA-MB-231 MCF-7 A549 SPC-A-1 MGECs HCMIEC/D3 PC-12 SH-SY5Y HT-29 DLD-1 MKN45 MKN28 |
| CSE | miR-4317 [31] miR-21 [32] miR-216a [192] miR-186 [193] miR-30a-5p [194] miR-328-3p [195] miR-30b-5p [196] miR-22 [197] miR-939-5p [38] miR-548 [132] miR-193 [183] |
LncRNA Oprm1 [196] lncRNA MALAT-1 [184] |
MDA-MB-231 MCF-7 HEK-293FT Primary cultured neonatal cardiomyocytes Mouse Airway Epithelial Cells (MAECs) THP-1 macrophages Vascular Smooth Muscle Cells (SMCs) |
| 3-MST | miR-548 [132] miR-193 [183] |
Not available | MDA-MB-231 |
6. Role of H2S in Non-Oncological Disorders and Its Possible Regulation by ncRNAs
6.1. Digestive System
It has been reported that the exogenous supplementation of H2S prevents ethanol-induced gastric damage in murine models and plays a shielding role against oxidative stress in rat gastric mucosal epithelium [198,199]. Takeuchi et al. reported that H2S is involved in the regulation of acid-induced bicarbonate secretion and mucosal protection in the duodenum [200]. H2S, along with other precursors comprising a dithiolethione moiety, are effective inducers of the antioxidant enzyme heme oxygenase-1 (HO-1). Notably, clinical evidence suggested that HO-1 promotes ulcer healing [201]. Other mechanisms thought to effectively contribute to the gastroprotective effect of H2S are increased epithelial secretion and mucosal blood flow; the reduction of leukocyte adhesion/infiltration; the downregulation of TNF-α, IL-1β and IFN-γ expression; and the scavenging of oxidative species [202]. H2S plays a potential role in resolving intestinal dysmotility in irritable bowel syndrome (IBS). A study conducted by Lin. et al. mentions the importance of CBS/H2S axis in stress-induced colonic hypermotility [203]. This is demonstrated using a stress-induced rat model of IBS, where CBS expression levels were significantly lower than the control and SAM administration groups, an activator of CBS [203].
Another study highlighted the importance of the H2S pathway in IBD, as the 3-MST expression level was significantly decreased in colonic tissues isolated from patients with ulcerative colitis or Crohn’s disease with prominent inflammatory responses. Low levels of 3-MST raised the levels of proinflammatory cytokines, ROS, and apoptosis. On the other hand, 3-MST overexpression alleviated these effects [204]. Additionally, 3-MST modulates apoptosis by increasing AKT expression and decreasing its phosphorylation.
6.2. Liver and Kidneys
The administration of H2S or H2S donor have been found to exert a protective role against ischemia-reperfusion damage in the liver and kidneys [205,206] and reduce the severity of liver injury [207]. In rats with ischemia-reperfusion injury, CSE and H2S levels are upregulated. It was found that the use of exogenous NaHS alleviated ischemia-reperfusion injury in liver hepatocytes in young rat models by activating Nrf2 pathway and reduction in miR-34a expression [208].
H2S has a vital nephroprotective role in diabetic kidney disease by alleviating renal glycative injury [206], inducing renal blood flow, increasing the glomerular filtration rate and urinary sodium excretion [209], and harnessing hyperhomocysteinemia-associated chronic renal failure [210]. Similarly, H2S was found to play a significant hepatoprotective role by diminishing stress-mediated liver injury and hepatic mitochondrial dysfunction in acutely ethanol-exposed mice [211], and it evidently relieves acetaminophen-induced hepatotoxicity in mice [212]. Likewise, H2S had protective role in liver fibrosis. There is evidence that CSE knockout increases cytokines, oxidative stress, inflammation, aggravating hepatitis, and liver fibrosis [213,214].
H2S also provided evidence for protection from cisplatin-induced acute kidney injury in dogs [215]. The administration of NaHS prior to cisplatin injection alleviated cisplatin-induced increase in the level of inflammatory mediators such as NF-κB, TNF-α, IL-1β in canine kidney tissues [215].
miR-21 was found to be upregulated in acute kidney injury (AKI) and may play a pathological role in the development of kidney disease associated with the H2S pathway [32]. For anti-miR-21, as well as exogenous H2S, GYY4137 reversed the changes in AKI through the enhancement of renal vascular density and blood flow in aged mice. Anti-miR-21 also induced the upregulation of CBS and CSE expression, as well as decreases in metalloproteinase-9 and collagen IV expression [32].
6.3. Diabetes Mellitus and Metabolism
Endogenous H2S acts as an antioxidant molecule protecting murine pancreatic β cells from oxidative stress and/or glucotoxicity-induced apoptosis. On the other hand, exogenous H2S through NaHS administration repressed reactive oxygen species (ROS) production by activating Akt signaling [216,217,218]. It seems that H2S biosynthesis decreases as the severity of the disease increases over time, and therapies based on the exogenous supplementation of H2S via administration of H2S donors might prove to be quite promising [219,220,221,222].
Similarly, the administration of NaHS to alleviate diabetic cardiomyopathy was recently studied in murine models [223]. Untreated diabetic models (type 2) showed decreased intracellular H2S levels, reduced CSE expression, altered cardiac function, and the interruption of calcium homeostasis, which activates the mitochondrial apoptosis pathway. Our results suggest that exogenous H2S improves myocardial systolic–diastolic function and reduces apoptosis in diabetic cardiomyopathy [223].
A study was conducted to understand the importance of dietary methionine restriction for improving metabolic health and boosting insulin sensitivity in mice models with high-fat-diet-induced obesity [195]. In this study, Wu et al. recommended that the reduction of dietary methionine enhances protein efficiency in mice and causes an increase in endogenous H2S production. An elevation in endogenous H2S is postulated to occur via miR-328-3p, which directly target CSE. This resulted in alleviating oxidative stress and ER stress, promoting protein homeostasis and metabolic efficiency in mice models with diet-induced obesity [195].
A common complication of diabetes mellitus is cardiovascular disorders, such as microcirculation dysfunction of the lower limbs associated with impaired angiogenesis. The impairment of angiogenesis is mainly caused by vascular endothelial dysfunction associated with hyperglycemia. H2S and miR-126-3p were recognized for their pro-angiogenesis effects [224]. The influence of H2S on miR-126-3p regulation was studied under high-glucose conditions. It was revealed that miR-126-3p expression is modulated by both diabetes and H2S. H2S upregulated miR-126-3p by inhibiting DNA methylation, as well as downregulating the DNMT1 (anti-angiogenic protein) level, which is primarily increased based on a high glucose level. In another context, CSE overexpression in human umbilical vein endothelial cells (HUVECs) has been shown to elevate miR-126-3p, thereby improving the function of endothelial cells and promoting angiogenesis [224]. In another study, Zhou Y et al. concluded that H2S promotes angiogenesis through the downregulation of the expression of miR-640 and by increasing the levels of HIF1α through the VEGFR2-mTOR pathway. Therefore, miR-640 plays a pivotal role in mediating the proangiogenic effect of H2S [225].
6.4. Cardiovascular System
Accumulated evidence highlights that H2S has potent effects on the myocardium and CSE overexpression is highly protective from I/R injury in the heart [226,227].
Exogenous H2S intake showed markedly positive outcomes in the pathological settings of atherosclerosis, myocardial I/R injury, chronic heart failure, and cardiopulmonary resuscitation in small animal models. The observed protective and improved outcomes are associated with enhanced cardiac mechanics, attenuated myocardial inflammation, Nrf2 activation, and reduced cardiomyocyte apoptosis [226,228,229,230,231].
MiR-30 family members are found to be upregulated and negatively correlated with the reported downregulation of CSE in the infarct and border zones following myocardial infarction (MI) in rat hearts. The dual-luciferase reporter assay confirmed that miR-30 directly targets CSE, and the fact that the inhibition of miR-30 can protect the MI heart by upregulating CSE was also confirmed in vivo [194] (Table 2).
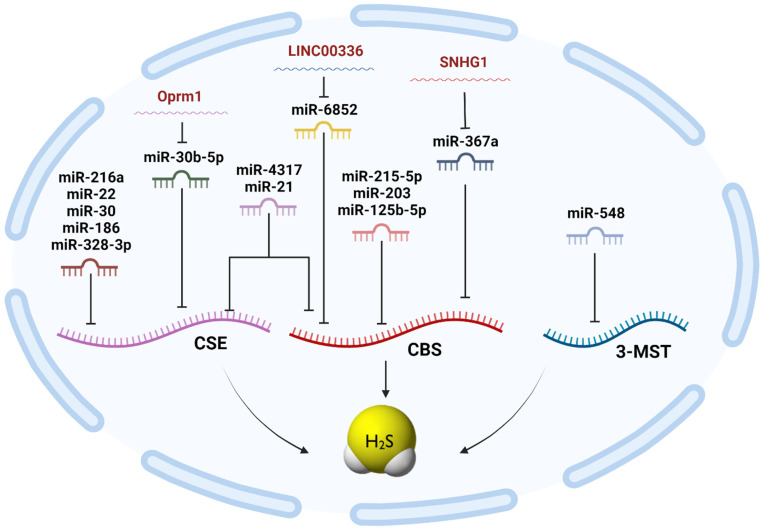
As illustrated in the current review, it has come to our attention that miRNAs in particular are not only drawn as upstream regulators for the H2S-synthesizing enzymes but have also been reported to be drawn downstream for H2S, where either exogenously administered H2S or endogenously produced H2S affect an array of miRNAs as part of H2S-altered signaling cascades. For instance, exogenous H2S treatment upregulates cardioprotective miR-133a in primary cultures of neonatal rat cardiomyocytes and suppresses cardiomyocyte hypertrophy [232,233]. In addition, it attenuates myocardial ischemic and inflammatory injury via the induction of miR-21 [234]. Hu et al. investigated the effects of lncRNA Oprm1 on myocardial I/R injury and its underlying mechanism. Their findings revealed that lncRNA Oprm1 was significantly repressed in the I/R injury in an experimental animal model. Additionally, it exerts a stimulatory effect on CSE expression and H2S level. The dual-luciferase reporter gene assay unraveled the lncRNA/miRNA interplay between Oprm1 and miR-30b-5p that ultimately downregulates the expression of CSE, as shown in Figure 2. In addition, the results of the same study revealed that high levels of endogenous H2S together with the concomitant administration of Oprm1 in I/R injury can reduce apoptosis and protect the myocardium through the activation of the PI3K/Akt pathway and inhibition of HIF1-α activity [196].
Several experimental studies have reported that oxidant-induced leukocyte–endothelial cell interactions are mainly responsible for the microvascular dysfunction brought about via reperfusion [235,236]. In atherosclerosis, monocyte adherence to endothelial cells is stimulated by oxidized sulfur species [237]. Notably, H2S has wide-reaching anti-inflammatory and cytoprotective effects [105] and is an exceedingly potent inhibitor of leukocyte adherence to the vascular endothelium [238]. Thus, H2S might interfere in the inflammatory processes and reduce the tissue injury induced via neutrophils by stimulating apoptosis and/or the scavenging of neutrophil-derived hypochlorous acid (HOCl) [140]. Importantly, H2S is capable of downregulating numerous pro-inflammatory cytokines, including NF-κB, TNF-α, IL-1β, IL-6, and IL-8 [227,239,240,241], to modulate leukocyte adhesion and leukocyte-regulated inflammation, in order to mediate the cardio protection induced via ischemic postconditioning [242] and protect against NF-κB- and TNF-α-mediated endotoxic shock [243].
Moreover, a recent study demonstrated that treatment with GYY4137, a slow-releasing H2S donor, alleviated the endothelial dysfunction induced by TNF-α. The reduction of the activity pro-caspase 3, enhancement of mitochondrial function, and prevention of TNF-α-induced apoptosis are contributing factors involved in this improvement [244]. GYY4137 also upregulates miR-21 expression, which led to the activation of the Akt pathway [245].
It was reported that estrogen (E2) induces the transcription of CSE upon binding to the estrogen receptor α, which constitutively increases specificity protein-1 (SP1) activity. SP1 directly binds to the CSE promoter region to upregulate CSE transcription and H2S synthesis [197]. Moreover, the ectopic expression of miR-22 inhibits estrogen receptor α and, consequently, SP1 and CSE transcription [197]. This implies that miR-22 regulates H2S production in females, and consequently, this could be crucial in the treatment of females with cardiovascular disease [246].
Angiogenesis, the process of the formation of new capillaries from existing ones [247], plays a critical role in tumor growth and development [248,249]. A study by Cai et al. identified H2S as a promoter of angiogenesis [250]. Other studies have also reported the proangiogenic role of H2S; this was evident when NaHS demonstrated its capability for stimulating the proliferation and tube formation of endothelial cells (ECs), both in vitro and in vivo [173,250]. However, the signaling mechanisms behind it remain relatively elusive. miRNAs were suggested to be among the key players. The deletion of the RNA endonuclease Dicer, responsible for mediating miRNA maturation in the cytosol, and the subsequent aberrant vessel growth in mice models were the first pieces of experimental evidence that miRNAs have control over angiogenesis [251]. Despite establishing this correlation, many researchers are still intrigued with discovering miRNAs that specifically regulate H2S-mediated angiogenesis. Zhou et al. conducted a study highlighting the crosstalk between H2S and miR-640, as shown in Figure 4. This was evident upon the supplementation of vascular ECs with H2S, as miR-640 levels decreased. Alternately, inducing the expression of miR-640 inhibited the proangiogenic effect of H2S [225]. In addition, the knockdown of VEGFR2 or mTOR diminished the repression of miR-640, as well as the proangiogenic effect induced by H2S. It is worth noting that mTOR plays a significant role in modulating miR-640 [225]. Furthermore, miR-640 was found to suppress HIF1A expression by binding to the 3-UTR of its mRNA. It was also reported that HIF1A suppression could be overturned by treating the cells with H2S [225].
Figure 4.
A snapshot of different classes of non-coding RNAs regulating H2S machinery.
6.5. Nervous System
H2S is avital neuromodulator and neuroprotective agent in the central nervous system [252]. Guo et al. highlighted that H2S can protect the brain from I/R injury by maintaining mitochondrial function, repressing pro-inflammatory factors, attenuating ROS, and hampering apoptosis [253]. Li et al. indicated that H2S protected the spinal cord and stimulated autophagy via miR-30c in a rat model of spinal cord I/R injury [254]. Although accumulating evidence crystallized the importance of H2S as a neuroprotectant, the underlying mechanisms of action of this gasotransmitter in spinal cord ischemia-reperfusion injury (SCII) were still indistinct [255]. This prompted Liu et al. to investigate whether cancer susceptibility candidate 7 (CASC7), a dual-localized lncRNA, was implicated in the neuroprotective effects of H2S in SCII. Their results have shown that in SCII rat, CasC7 expression was significantly reduced, while miR-30c expression levels were increased. In contrast to these observations, upon NaSH (H2S donor) preprocessing, CasC7 expression was elevated, while that of miR-30c was decreased [255]. These results proposed that preconditioning with NaSH reduced the spinal cord infarct zone and reversed the CasC7 and mir-30c usual expression pattern. Therefore, this study unveiled the regulatory role exerted by H2S in modulating the expression of CasC7 and miR-30c in SCII rat models, as well as validated the neuroprotective role of hydrogen sulfide in the spinal cord [255].
Emerging experimental data suggest that H2S might carry therapeutic potential for Alzheimer’s patients, since it reduces the mRNA and protein levels of β-amyloid precursor protein-cleaving enzyme 1 [256,257].
Another study demonstrated the neuroprotective role of H2S in hypoxic injury by upregulating the expression of heat shock protein (HSP) 90, a ubiquitous molecule that is associated with cell survival [258,259]. Osborne et al. reported that the slow-releasing H2S prodrug ACS67 diminishes retinal ischemic damage following the elevation of retinal pressure in rats [260]. The authors of this study pointed out that the neuroprotective effect of ACS67 possibly involves the stimulation of GSH formation among several other mechanisms. Biermann et al. also recently reported that H2S preconditioning visibly diminishes retinal ganglion cells (RGCs) apoptosis following retinal ischemia-reperfusion injury [261]. In addition, Mikami et al. displayed the ability of H2S to protect the retina from light-induced damage via the regulation of intracellular calcium by activating vacuolar type H+-ATPase [262]. It has been found that hyperhomocysteinemia can most commonly cause mental illness, seizures, and Alzheimer’s disease through Hcy-induced oxidative stress. Tyagi et al. nominated H2S as a form of shield for the brain that could potentially be a promising therapeutic candidate for the treatment of hyperhomocysteinemia-associated pathologies, such as stroke and neurologic disorders [263].
Chen Z et al. identified the miR-485-5p/TRADD axis as a key player in H2S protection against TNF-α-induced neuronal cell apoptosis. Using a luciferase-reporting gene assay, Western blot, and qRT-PCR, Chen Z et al. confirmed that TRADD is a direct target for miR-485-5p, while the miR-485-5p/TRADD axis can be drawn downstream for H2S in neuronal cells [264].
Liu Y et al. shows that H2S significantly attenuated the increase in Rho-associated protein kinase 2 (ROCK2) and the decrease in miR-135a-5p by MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) in neuronal cells [265].
MiR-125b-5p has been identified as the culprit in an array of neurological disorders, such as Parkinson’s disease [265], Alzheimer’s disease (AD) [266], and pregnancy-related complications [267]. However, few studies have attempted to study its role in ischemic stroke, especially the interaction with H2S in cerebral hypoxia-induced injury [189]. In a study by Shen et al., the relationship between miR-125b-5p and H2S production, as shown in Figure 4, as well as the regulatory mechanism exerted by miR-125b-5p on H2S generation in oxygen/glucose deprivation (OGD)-induced injury in neuron-like rat pheochromocytoma (PC-12), was investigated. The overexpression of miR-125b-5p reduced CBS expression and H2S generation, which consequently exacerbated OGD injury. In contrast, the downregulation of miR-125b-5p increased CBS expression and H2S generation and prohibited PC-12 cells from OGD injury. These results suggested that miR-125b-5p might participate in cerebral hypoxia injury by modulating H2S levels [189].
7. Conclusions and Future Recommendations
In conclusion, the exploration of H2S and its synthesizing enzymes has revealed a multifaceted landscape of cellular regulation. The intricate balance of H2S production, facilitated by CBS, CSE, and 3-MST, emerges as a critical factor in coordinating various physiological and pathological processes. This review demonstrates the comprehensive stratification of the expression pattern of those enzymes in both oncological and non-oncological contexts and the pivotal role of ncRNAs in orchestrating the web of interactions between H2S and its synthesizing enzymes.
In essence, the convergence of research on H2S, its synthesizing enzymes, and the regulatory role of ncRNAs signifies a paradigm shift in our comprehension of cellular signaling. As we continue to unravel the complexities of these interactions, the potential for groundbreaking advancements in therapeutic strategies targeting H2S-associated pathways becomes increasingly promising, offering hope for developing more effective and tailored treatments in the realm of numerous disorders.
It is also worth noting that until now, miRNAs and lncRNAs have been the main candidates from the ncRNA family reported to modulate the H2S biosynthesis under different physiological and pathological conditions. However, other classes of ncRNAs such as circRNAs and piwi RNAs should be evaluated in terms of regulating H2S and its synthesizing enzymes in different pathological conditions. Nonetheless, crosstalk between different classes of ncRNAs should also be probed to explore ceRNA circuits that could be regulating the H2S-synthesizing enzymes in several oncological and non-oncological contexts. On a therapeutic level, understanding the roles of miRNAs and lncRNAs in H2S-regulated pathways may lead to the identification of novel therapeutic targets for all highlighted diseases associated with H2S dysregulation in this review. Future research could explore the development of the RNA-based therapeutics or small molecules targeting these ncRNAs for disease intervention.
Author Contributions
Writing—original draft preparation: R.A.Y., D.A.H., N.K. and S.H. Figures preparation: A.D., K.E. and H.N. Editing and revising the manuscript: C.B., R.M.A.-K. and M.Z.G. Conceptualization: M.Z.G. and R.A.Y. Principal investigators of project, funding, and grant management: C.B., M.Z.G., R.M.A.-K. and R.A.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was funded by the Swiss National Science Foundation (SNSF), grant number SNF IZSTZ0_198887.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Powell C.R., Dillon K.M., Matson J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018;149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 4.Wang R. Hydrogen sulfide: The third gasotransmitter in biology and medicine. Antioxid. Redox Signal. 2010;12:1061–1064. doi: 10.1089/ars.2009.2938. [DOI] [PubMed] [Google Scholar]
- 5.Barr L.A., Calvert J.W. Discoveries of hydrogen sulfide as a novel cardiovascular therapeutic. Circ. J. 2014;78:2111–2118. doi: 10.1253/circj.CJ-14-0728. [DOI] [PubMed] [Google Scholar]
- 6.Hellmich M.R., Coletta C., Chao C., Szabo C. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 2015;22:424–448. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 8.Kolluru G.K., Shen X., Bir S.C., Kevil C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitfield N.L., Kreimier E.L., Verdial F.C., Skovgaard N., Olson K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 10.Kimura Y., Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 11.Zhong G., Chen F., Cheng Y., Tang C., Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Renieris G., Renieris G., Katrini K., Damoulari C., Akinosoglou K., Psarrakis C., Kyriakopoulou M., Dimopoulos G., Lada M., Koufargyris P., et al. Serum Hydrogen Sulfide and Outcome Association in Pneumonia by the SARS-CoV-2 Coronavirus. Shock. 2020;54:633–637. doi: 10.1097/SHK.0000000000001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escaffre O., Szaniszlo P., Törő G., Vilas C.L., Servantes B.J., Lopez E., Juelich T.L., Levine C.B., McLellan S.L.F., Cardenas J.C., et al. Hydrogen Sulfide Ameliorates SARS-CoV-2-Associated Lung Endothelial Barrier Disruption. Biomedicines. 2023;11:1790. doi: 10.3390/biomedicines11071790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youness R.A., Gad M.Z. Long non-coding RNAs: Functional regulatory players in breast cancer. Noncoding RNA Res. 2019;4:36–44. doi: 10.1016/j.ncrna.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ElKhouly A.M., Youness R.A., Gad M.Z. MicroRNA-486-5p and microRNA-486-3p: Multifaceted pleiotropic mediators in oncological and non-oncological conditions. Noncoding RNA Res. 2020;5:11–21. doi: 10.1016/j.ncrna.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawoud A., Zakaria Z.I., Rashwan H.H., Braoudaki M., Youness R.A. Circular RNAs: New layer of complexity evading breast cancer heterogeneity. Noncoding RNA Res. 2023;8:60–74. doi: 10.1016/j.ncrna.2022.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Aziz M.K., Dawoud A., Kiriacos C.J., Fahmy S.A., Hamdy N.M., Youness R.A. Decoding hepatocarcinogenesis from a noncoding RNAs perspective. J. Cell. Physiol. 2023;238:1982–2009. doi: 10.1002/jcp.31076. [DOI] [PubMed] [Google Scholar]
- 18.Abdallah R.M., Elkhouly A.M., Soliman R.A., El Mechawy N., El Sebaei A., Motaal A.A., El-Askary H., Youness R.A., Assal R.A. Hindering the Synchronization Between miR-486-5p and H19 lncRNA by Hesperetin Halts Breast Cancer Aggressiveness Through Tuning ICAM-1. Anticancer Agents Med. Chem. 2022;22:586–595. doi: 10.2174/1871520621666210419093652. [DOI] [PubMed] [Google Scholar]
- 19.Mekky R.Y., Ragab M.F., Manie T., Attia A.A., Youness R.A. MALAT-1: Immunomodulatory lncRNA hampering the innate and the adaptive immune arms in triple negative breast cancer. Transl. Oncol. 2023;31:101653. doi: 10.1016/j.tranon.2023.101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youness R.A., Rahmoon M.A., Assal R.A., Gomaa A.I., Hamza M.T., Waked I., El Tayebi H.M., Abdelaziz A.I. Contradicting interplay between insulin-like growth factor-1 and miR-486-5p in primary NK cells and hepatoma cell lines with a contemporary inhibitory impact on HCC tumor progression. Growth Factors. 2016;34:128–140. doi: 10.1080/08977194.2016.1200571. [DOI] [PubMed] [Google Scholar]
- 21.Youness R.A., El-Tayebi H.M., Assal R.A., Hosny K., Esmat G., Abdelaziz A.I. MicroRNA-486-5p enhances hepatocellular carcinoma tumor suppression through repression of IGF-1R and its downstream mTOR, STAT3 and c-Myc. Oncol. Lett. 2016;12:2567–2573. doi: 10.3892/ol.2016.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaalan Y.M., Handoussa H., Youness R.A., Assal R.A., El-Khatib A.H., Linscheid M.W., El Tayebi H.M., Abdelaziz A.I. Destabilizing the interplay between miR-1275 and IGF2BPs by Tamarix articulata and quercetin in hepatocellular carcinoma. Nat. Prod. Res. 2018;32:2217–2220. doi: 10.1080/14786419.2017.1366478. [DOI] [PubMed] [Google Scholar]
- 23.Selem N.A., Youness R.A., Gad M.Z. What is beyond LncRNAs in breast cancer: A special focus on colon cancer-associated Transcript-1 (CCAT-1) Noncoding RNA Res. 2021;6:174–186. doi: 10.1016/j.ncrna.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soliman A.H., Youness R.A., Sebak A.A., Handoussa H. Phytochemical-derived tumor-associated macrophage remodeling strategy using Phoenix dactylifera L. boosted photodynamic therapy in melanoma via H19/iNOS/PD-L1 axis. Photodiagnosis Photodyn. Ther. 2023;44:103792. doi: 10.1016/j.pdpdt.2023.103792. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Latif M., Riad A., Soliman R.A., Elkhouly A.M., Nafae H., Gad M.Z., Motaal A.A. MALAT-1/p53/miR-155/miR-146a ceRNA circuit tuned by methoxylated quercitin glycoside alters immunogenic and oncogenic profiles of breast cancer. Mol. Cell. Biochem. 2022;477:1281–1293. doi: 10.1007/s11010-022-04378-4. [DOI] [PubMed] [Google Scholar]
- 26.El Kilany F.H., Youness R.A., Assal R.A., Gad M.Z. miR-744/eNOS/NO axis: A novel target to halt triple negative breast cancer progression. Breast Dis. 2021;40:161–169. doi: 10.3233/BD-200454. [DOI] [PubMed] [Google Scholar]
- 27.El Din G.S., Youness R.A., Assal R.A., Gad M.Z. miRNA-506-3p Directly Regulates rs10754339 (A/G) in the Immune Checkpoint Protein B7-H4 in Breast Cancer. Microrna. 2020;9:346–353. doi: 10.2174/2211536609666201209152949. [DOI] [PubMed] [Google Scholar]
- 28.Soliman R.-A., Youness R.A., Manie T.M., Khallaf E., El-Shazly M., Abdelmohsen M., Handoussa H., Gad M.Z. Uncoupling tumor necrosis factor-α and interleukin-10 at tumor immune microenvironment of breast cancer through miR-17-5p/MALAT-1/H19 circuit. Biocell. 2022;46:769–783. doi: 10.32604/biocell.2022.016636. [DOI] [Google Scholar]
- 29.ZeinElAbdeen Y.A., AbdAlSeed A., Youness R.A. Decoding Insulin-Like Growth Factor Signaling Pathway From a Non-coding RNAs Perspective: A Step Towards Precision Oncology in Breast Cancer. J. Mammary Gland. Biol. Neoplasia. 2022;27:79–99. doi: 10.1007/s10911-022-09511-z. [DOI] [PubMed] [Google Scholar]
- 30.El-Daly S.M., Talaat R.M., Braoudaki M., Youness R.A., Cho W.C. Editorial: Recent breakthroughs in the decoding of circulating nucleic acids and their applications to human diseases. Front. Mol. Biosci. 2023;10:1203495. doi: 10.3389/fmolb.2023.1203495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youness R.A., Gad A.Z., Sanber K., Ahn Y.J., Lee G.-J., Khallaf E., Hafez H.M., Motaal A.A., Ahmed N., Gad M.Z. Targeting hydrogen sulphide signaling in breast cancer. J. Adv. Res. 2021;27:177–190. doi: 10.1016/j.jare.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pushpakumar S., Kundu S., Weber G., Sen U. Exogenous hydrogen sulfide and miR-21 antagonism attenuates macrophage-mediated inflammation in ischemia reperfusion injury of the aged kidney. Geroscience. 2021;43:1349–1367. doi: 10.1007/s11357-020-00299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sameri M.J., Savari F., Hoseinynejad K., Danyaei A., Mard S.A. The hepato-protective effect of H2S-modified and non-modified mesenchymal stem cell exosomes on liver ischemia-reperfusion injury in mice: The role of MALAT1. Biochem. Biophys. Res. Commun. 2022;635:194–202. doi: 10.1016/j.bbrc.2022.09.111. [DOI] [PubMed] [Google Scholar]
- 34.Lu W., Wen J. H2S-mediated inhibition of RhoA/ROCK pathway and noncoding RNAs in ischemic stroke. Metab. Brain Dis. 2023;38:163–176. doi: 10.1007/s11011-022-01130-1. [DOI] [PubMed] [Google Scholar]
- 35.Zhai Y., Tyagi S.C., Tyagi N. Cross-talk of MicroRNA and hydrogen sulfide: A novel therapeutic approach for bone diseases. Biomed. Pharmacother. 2017;92:1073–1084. doi: 10.1016/j.biopha.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia S.M., Matheson B., Morales-Loredo J.H., Jernigan N.L., Kanagy N.L., Resta T.C., Clark R.M., Shekarriz R., Bosc L.V.G. Hydrogen sulfide and miR21 are suitable biomarkers of hypoxic exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022;323:R900–R909. doi: 10.1152/ajpregu.00199.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahmy S.A., Dawoud A., Zeinelabdeen Y.A., Kiriacos C.J., Daniel K.A., Eltahtawy O., Abdelhalim M.M., Braoudaki M., Youness R.A. Molecular Engines, Therapeutic Targets, and Challenges in Pediatric Brain Tumors: A Special Emphasis on Hydrogen Sulfide and RNA-Based Nano-Delivery. Cancers. 2022;14:5244. doi: 10.3390/cancers14215244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nafea H., Youness R.A., Dawoud A., Khater N., Manie T., Abdel-Kader R., Bourquin C., Szabo C., Gad M.Z. Dual targeting of H2S synthesizing enzymes; cystathionine β-synthase and cystathionine γ-lyase by miR-939-5p effectively curbs triple negative breast cancer. Heliyon. 2023;9:e21063. doi: 10.1016/j.heliyon.2023.e21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hombach S., Kretz M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 40.Andersen J., Delihas N., Ikenaka K., Green P.J., Pines O., Ilercil O., Inouye M. The isolation and characterization of RNA coded by the micF gene in Escherichia coli. Nucleic Acids Res. 1987;15:2089–2101. doi: 10.1093/nar/15.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen J., Forst S.A., Zhao K., Inouye M., Delihas N. The function of micF RNA. micF RNA is a major factor in the thermal regulation of OmpF protein in Escherichia coli. J. Biol. Chem. 1989;264:17961–17970. doi: 10.1016/S0021-9258(19)84666-5. [DOI] [PubMed] [Google Scholar]
- 42.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 43.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 44.Rocheleau C.E., Downs W.D., Lin R., Wittmann C., Bei Y., Cha Y.-H., Ali M., Priess J.R., Mello C.C. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/S0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- 45.Abaza T., El-Aziz M.K.A., Daniel K.A., Karousi P., Papatsirou M., Fahmy S.A., Hamdy N.M., Kontos C.K., Youness R.A. Emerging Role of Circular RNAs in Hepatocellular Carcinoma Immunotherapy. Int. J. Mol. Sci. 2023;24:16484. doi: 10.3390/ijms242216484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carthew R.W., Sontheimer E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dueck A., Meister G. Assembly and function of small RNA—Argonaute protein complexes. Biol. Chem. 2014;395:611–629. doi: 10.1515/hsz-2014-0116. [DOI] [PubMed] [Google Scholar]
- 49.Farazi T.A., Juranek S.A., Tuschl T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development. 2008;135:1201–1214. doi: 10.1242/dev.005629. [DOI] [PubMed] [Google Scholar]
- 50.Meister G., Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 51.Mendell J.T., Olson E.N. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siomi H., Siomi M.C. On the road to reading the RNA-interference code. Nature. 2009;457:396–404. doi: 10.1038/nature07754. [DOI] [PubMed] [Google Scholar]
- 53.Siomi M.C., Sato K., Pezic D., Aravin A.A. PIWI-interacting small RNAs: The vanguard of genome defence. Nat. Rev. Mol. Cell Biol. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- 54.Keam S.P., Hutvagner G. tRNA-Derived Fragments (tRFs): Emerging New Roles for an Ancient RNA in the Regulation of Gene Expression. Life. 2015;5:1638–1651. doi: 10.3390/life5041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akins R.B., Ostberg K., Cherlin T., Tsiouplis N.J., Loher P., Rigoutsos I. The Typical tRNA Co-Expresses Multiple 5′ tRNA Halves Whose Sequences and Abundances Depend on Isodecoder and Isoacceptor and Change with Tissue Type, Cell Type, and Disease. Non-Coding RNA. 2023;9:69. doi: 10.3390/ncrna9060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.International Human Genome Sequencing Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 58.Mattick J.S. Non-coding RNAs: The architects of eukaryotic complexity. EMBO Rep. 2001;2:986–991. doi: 10.1093/embo-reports/kve230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wightman B., Ha I., Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 60.Yang J.X., Rastetter R.H., Wilhelm D. Non-coding RNAs: An Introduction. Adv. Exp. Med. Biol. 2016;886:13–32. doi: 10.1007/978-94-017-7417-8_2. [DOI] [PubMed] [Google Scholar]
- 61.Reinhart B.J., Slack F.J., Basson M., Pasquinelli A.E., Bettinger J.C., Rougvie A.E., Horvitz H.R., Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 62.Ruvkun G. Molecular biology. Glimpses of a tiny RNA world. Science. 2001;294:797–799. doi: 10.1126/science.1066315. [DOI] [PubMed] [Google Scholar]
- 63.Awad A.R., Youness R.A., Ibrahim M., Motaal A.A., El-Askary H.I., Assal R., Gad M.Z. An acetylated derivative of vitexin halts MDA-MB-231 cellular progression and improves its immunogenic profile through tuning miR-20a-MICA/B axis. Nat. Prod. Res. 2021;35:3126–3130. doi: 10.1080/14786419.2019.1686372. [DOI] [PubMed] [Google Scholar]
- 64.Sayed D., Abdellatif M. MicroRNAs in development and disease. Physiol. Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 65.Youssef S.S., Youness R.A., Abbas E.A.E.-R., Osman N.M., Elfiky A., El-Kassas M. miR-516a-3P, a potential circulating biomarker in hepatocellular carcinoma, correlated with rs738409 polymorphism in PNPLA3. Pers. Med. 2022;19:483–493. doi: 10.2217/pme-2022-0005. [DOI] [PubMed] [Google Scholar]
- 66.Friedman R.C., Farh K.K., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 68.Lin S., Gregory R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rahmoon M.A., Youness R.A., Gomaa A.I., Hamza M.T., Waked I., El Tayebi H.M., Abdelaziz A.I. MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Factors. 2017;35:76–87. doi: 10.1080/08977194.2017.1354859. [DOI] [PubMed] [Google Scholar]
- 70.Ramzy A., ElSafy S., A Elshoky H., Soliman A., Youness R., Mansour S., Sebak A. Drugless nanoparticles tune-up an array of intertwined pathways contributing to immune checkpoint signaling and metabolic reprogramming in triple-negative breast cancer. Biomed. Mater. 2022;18:015023. doi: 10.1088/1748-605X/aca85d. [DOI] [PubMed] [Google Scholar]
- 71.Liu N., Olson E.N. MicroRNA regulatory networks in cardiovascular development. Dev. Cell. 2010;18:510–525. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solly E.L., Dimasi C.G., Bursill C.A., Psaltis P.J., Tan J.T.M. MicroRNAs as Therapeutic Targets and Clinical Biomarkers in Atherosclerosis. J. Clin. Med. 2019;8:2199. doi: 10.3390/jcm8122199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Issler O., Chen A. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 2015;16:201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- 74.Huang S., Yoshitake K., Asakawa S. A Review of Discovery Profiling of PIWI-Interacting RNAs and Their Diverse Functions in Metazoans. Int. J. Mol. Sci. 2021;22:11166. doi: 10.3390/ijms222011166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei J.W., Huang K., Yang C., Kang C.S. Non-coding RNAs as regulators in epigenetics (Review) Oncol. Rep. 2017;37:3–9. doi: 10.3892/or.2016.5236. [DOI] [PubMed] [Google Scholar]
- 76.Toth K.F., Pezic D., Stuwe E., Webster A. The piRNA Pathway Guards the Germline Genome against Transposable Elements. Adv. Exp. Med. Biol. 2016;886:51–77. doi: 10.1007/978-94-017-7417-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luteijn M.J., Ketting R.F. PIWI-interacting RNAs: From generation to transgenerational epigenetics. Nat. Rev. Genet. 2013;14:523–534. doi: 10.1038/nrg3495. [DOI] [PubMed] [Google Scholar]
- 78.Malone C.D., Hannon G.J. Molecular evolution of piRNA and transposon control pathways in Drosophila. Cold Spring Harb. Symp. Quant. Biol. 2009;74:225–234. doi: 10.1101/sqb.2009.74.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slotkin R.K., Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- 80.Cao X., Yeo G., Muotri A.R., Kuwabara T., Gage F.H. Noncoding RNAs in the mammalian central nervous system. Annu Rev. Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 81.Mercer T.R., Dinger M.E., Mattick J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 82.Nafea H., Youness R.A., Abou-Aisha K., Gad M.Z. LncRNA HEIH/miR-939-5p interplay modulates triple-negative breast cancer progression through NOS2-induced nitric oxide production. J. Cell. Physiol. 2021;236:5362–5372. doi: 10.1002/jcp.30234. [DOI] [PubMed] [Google Scholar]
- 83.Selem N.A., Nafae H., Manie T., Youness R.A., Gad M.Z. Let-7a/cMyc/CCAT1/miR-17-5p Circuit Re-sensitizes Atezolizumab Resistance in Triple Negative Breast Cancer through Modulating PD-L1. Pathol. Res. Pract. 2023;248:154579. doi: 10.1016/j.prp.2023.154579. [DOI] [PubMed] [Google Scholar]
- 84.Ponting C.P., Oliver P.L., Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 85.Chen L.L., Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanger H.L., Klotz G., Riesner D., Gross H.J., Kleinschmidt A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Capel B., Swain A., Nicolis S., Hacker A., Walter M., Koopman P., Goodfellow P., Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–4130. doi: 10.1016/0092-8674(93)90279-Y. [DOI] [PubMed] [Google Scholar]
- 88.Cocquerelle C., Daubersies P., Majerus M.A., Kerckaert J.P., Bailleul B. Splicing with inverted order of exons occurs proximal to large introns. EMBO J. 1992;11:1095–1098. doi: 10.1002/j.1460-2075.1992.tb05148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976;8:547–555. doi: 10.1016/0092-8674(76)90223-3. [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto Y., Fishel R., Wickner R.B. Circular single-stranded RNA replicon in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1990;87:7628–7632. doi: 10.1073/pnas.87.19.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zaphiropoulos P.G. Exon skipping and circular RNA formation in transcripts of the human cytochrome P-450 2C18 gene in epidermis and of the rat androgen binding protein gene in testis. Mol. Cell. Biol. 1997;17:2985–2993. doi: 10.1128/MCB.17.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaphiropoulos P.G. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: Correlation with exon skipping. Proc. Natl. Acad. Sci. USA. 1996;93:6536–6541. doi: 10.1073/pnas.93.13.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nigro J.M., Cho K.R., Fearon E.R., Kern S.E., Ruppert J.M., Oliner J.D., Kinzler K.W., Vogelstein B. Scrambled exons. Cell. 1991;64:607–613. doi: 10.1016/0092-8674(91)90244-S. [DOI] [PubMed] [Google Scholar]
- 94.Han B., Chao J., Yao H. Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol. Ther. 2018;187:31–44. doi: 10.1016/j.pharmthera.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 95.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rybak-Wolf A., Stottmeister C., Glažar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R., et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 97.Salzman J., Chen R.E., Olsen M.N., Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/annotation/f782282b-eefa-4c8d-985c-b1484e845855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 99.Wang K., Long B., Liu F., Wang J.-X., Liu C.-Y., Zhao B., Zhou L.-Y., Sun T., Wang M., Yu T., et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016;37:2602–2611. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 100.Ashwal-Fluss R., Meyer M., Nagarjuna P., Andranik I., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 101.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 102.Du W.W., Yang W., Liu E., Yang Z., Dhaliwal P., Yang B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Szabo C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016;15:185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calvert J.W., Coetzee W.A., Lefer D.J. Novel insights into hydrogen sulfide—Mediated cytoprotection. Antioxid. Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li L., Rose P., Moore P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 106.Gadalla M.M., Snyder S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miles E.W., Kraus J.P. Cystathionine beta-synthase: Structure, function, regulation, and location of homocystinuria-causing mutations. J. Biol. Chem. 2004;279:29871–29874. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- 108.Pan L.L., Liu X.H., Gong Q.H., Yang H.B., Zhu Y.Z. Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy? Antioxid. Redox Signal. 2012;17:106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 110.Rose P., Moore P.K., Zhu Y.Z. H2S biosynthesis and catabolism: New insights from molecular studies. Cell. Mol. Life Sci. 2017;74:1391–1412. doi: 10.1007/s00018-016-2406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Searcy D.G., Lee S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998;282:310–322. doi: 10.1002/(SICI)1097-010X(19981015)282:3<310::AID-JEZ4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 112.Ubuka T., Abe T., Kajikawa R., Morino K. Determination of hydrogen sulfide and acid-labile sulfur in animal tissues by gas chromatography and ion chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2001;757:31–37. doi: 10.1016/S0378-4347(01)00046-9. [DOI] [PubMed] [Google Scholar]
- 113.Ereno-Orbea J., Majtan T., Oyenarte I., Kraus J.P., Martinez-Cruz L.A. Structural basis of regulation and oligomerization of human cystathionine beta-synthase, the central enzyme of transsulfuration. Proc. Natl. Acad. Sci. USA. 2013;110:E3790–E3799. doi: 10.1073/pnas.1313683110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ereno-Orbea J., Majtan T., Oyenarte I., Kraus J.P., Martinez-Cruz L.A. Structural insight into the molecular mechanism of allosteric activation of human cystathionine beta-synthase by S-adenosylmethionine. Proc. Natl. Acad. Sci. USA. 2014;111:E3845–E3852. doi: 10.1073/pnas.1414545111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zuhra K., Augsburger F., Majtan T., Szabo C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules. 2020;10:697. doi: 10.3390/biom10050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chasse J.F., Paly E., Paris D., Paul V., Sinet P.M., Kamoun P., London J. Genomic organization of the human cystathionine beta-synthase gene: Evidence for various cDNAs. Biochem. Biophys. Res. Commun. 1995;211:826–832. doi: 10.1006/bbrc.1995.1886. [DOI] [PubMed] [Google Scholar]
- 117.Teng H., Wu B., Zhao K., Yang G., Wu L., Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine beta-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA. 2013;110:12679–12684. doi: 10.1073/pnas.1308487110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pagliara V., Saide A., Mitidieri E., di Villa B.R., Sorrentino R., Russo G., Russo A. 5-FU targets rpL3 to induce mitochondrial apoptosis via cystathionine-beta-synthase in colon cancer cells lacking p53. Oncotarget. 2016;7:50333–50348. doi: 10.18632/oncotarget.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Youness R.A., Assal R.A., Motaal A.A., Gad M.Z. A novel role of sONE/NOS3/NO signaling cascade in mediating hydrogen sulphide bilateral effects on triple negative breast cancer progression. Nitric Oxide. 2018;80:12–23. doi: 10.1016/j.niox.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 120.Lu Y., O’Dowd B.F., Orrego H., Israel Y. Cloning and nucleotide sequence of human liver cDNA encoding for cystathionine gamma-lyase. Biochem. Biophys. Res. Commun. 1992;189:749–758. doi: 10.1016/0006-291X(92)92265-Y. [DOI] [PubMed] [Google Scholar]
- 121.Levonen A.L., Lapatto R., Saksela M., Raivio K.O. Human cystathionine gamma-lyase: Developmental and in vitro expression of two isoforms. Pt 1Biochem. J. 2000;347:291–295. doi: 10.1042/bj3470291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 123.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fan K., Li N., Qi J., Yin P., Zhao C., Wang L., Li Z., Zha X. Wnt/beta-catenin signaling induces the transcription of cystathionine-gamma-lyase, a stimulator of tumor in colon cancer. Cell. Signal. 2014;26:2801–2808. doi: 10.1016/j.cellsig.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 125.Rao S.P., Dobariya P., Bellamkonda H., More S.S. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants. 2023;12:603. doi: 10.3390/antiox12030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jarabak R. 3-Mercaptopyruvate sulfurtransferase. Methods Enzym. 1981;77:291–297. doi: 10.1016/s0076-6879(81)77040-x. [DOI] [PubMed] [Google Scholar]
- 127.Pedre B., Dick T.P. 3-Mercaptopyruvate sulfurtransferase: An enzyme at the crossroads of sulfane sulfur trafficking. Biol. Chem. 2021;402:223–237. doi: 10.1515/hsz-2020-0249. [DOI] [PubMed] [Google Scholar]
- 128.Govar A.A., Törő G., Szaniszlo P., Pavlidou A., Bibli S., Thanki K., Resto V.A., Chao C., Hellmich M.R., Szabo C., et al. 3-Mercaptopyruvate sulfurtransferase supports endothelial cell angiogenesis and bioenergetics. Br. J. Pharmacol. 2020;177:866–883. doi: 10.1111/bph.14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Augsburger F., Szabo C. Potential role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H2S) pathway in cancer cells. Pharmacol. Res. 2020;154:104083. doi: 10.1016/j.phrs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 130.Hellmich M.R., Szabo C. Hydrogen Sulfide and Cancer. Handb. Exp. Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oláh G., Módis K., Törö G., Hellmich M.R., Szczesny B., Szabo C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem. Pharmacol. 2018;149:186–204. doi: 10.1016/j.bcp.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dawoud A., Youness R., Nafea H., Manie T., Abdel-Kader R., Gad M. 26P 3MST: A potential workhorse in H2S signaling trimmed by microRNA-548 in breast cancer. ESMO Open. 2023;8:100992. doi: 10.1016/j.esmoop.2023.100992. [DOI] [Google Scholar]
- 133.Bhattacharyya S., Saha S., Giri K., Lanza I.R., Nair K.S., Jennings N.B., Rodriguez-Aguayo C., Lopez-Berestein G., Basal E., Weaver A.L., et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Panza E., De Cicco P., Armogida C., Scognamiglio G., Gigantino V., Botti G., Germano D., Napolitano M., Papapetropoulos A., Bucci M., et al. Role of the cystathionine gamma lyase/hydrogen sulfide pathway in human melanoma progression. Pigment. Cell Melanoma Res. 2015;28:61–72. doi: 10.1111/pcmr.12312. [DOI] [PubMed] [Google Scholar]
- 135.Li M., Song X., Jin Q., Chen Y., Zhang J., Gao J., Cen L., Lin Y., Xu C., He X., et al. 3-Mercaptopyruvate sulfurtransferase represses tumour progression and predicts prognosis in hepatocellular carcinoma. Liver Int. 2022;42:1173–1184. doi: 10.1111/liv.15228. [DOI] [PubMed] [Google Scholar]
- 136.Zhang C.-H., Jiang Z.-L., Meng Y., Yang W.-Y., Zhang X.-Y., Zhang Y.-X., Khattak S., Ji X.-Y., Wu D.-D. Hydrogen sulfide and its donors: Novel antitumor and antimetastatic agents for liver cancer. Cell. Signal. 2023;106:110628. doi: 10.1016/j.cellsig.2023.110628. [DOI] [PubMed] [Google Scholar]
- 137.Jia H., Ye J., You J., Shi X., Kang W., Wang T. Role of the cystathionine beta-synthase/H2S system in liver cancer cells and the inhibitory effect of quinolone-indolone conjugate QIC2 on the system. Oncol. Rep. 2017;37:3001–3009. doi: 10.3892/or.2017.5513. [DOI] [PubMed] [Google Scholar]
- 138.Szczesny B., Marcatti M., Zatarain J.R., Druzhyna N., Wiktorowicz J.E., Nagy P., Hellmich M.R., Szabo C. Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Sci. Rep. 2016;6:36125. doi: 10.1038/srep36125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gai J.W., Qin W., Liu M., Wang H.-F., Zhang M., Li M., Zhou W.-H., Ma Q.-T., Liu G.-M., Song W.-H., et al. Expression profile of hydrogen sulfide and its synthases correlates with tumor stage and grade in urothelial cell carcinoma of bladder. Urol. Oncol. 2016;34:166.e15–166.e20. doi: 10.1016/j.urolonc.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 140.Whiteman M., Cheung N.S., Zhu Y.-Z., Chu S.H., Siau J.L., Wong B.S., Armstrong J.S., Moore P.K. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 141.Zhang M., Li J., Huang B., Kuang L., Xiao F., Zheng D. Cystathionine beta Synthase/Hydrogen Sulfide Signaling in Multiple Myeloma Regulates Cell Proliferation and Apoptosis. J. Environ. Pathol. Toxicol. Oncol. 2020;39:281–290. doi: 10.1615/JEnvironPatholToxicolOncol.2020034851. [DOI] [PubMed] [Google Scholar]
- 142.Liu M., Wu L., Montaut S., Yang G. Hydrogen Sulfide Signaling Axis as a Target for Prostate Cancer Therapeutics. Prostate Cancer. 2016;2016:8108549. doi: 10.1155/2016/8108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gai J.W., Wahafu W., Guo H., Liu M., Wang X.-C., Xiao Y.-X., Zhang L., Xin Z.-C., Jin J. Further evidence of endogenous hydrogen sulphide as a mediator of relaxation in human and rat bladder. Asian J. Androl. 2013;15:692–696. doi: 10.1038/aja.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Akbari M., Sogutdelen E., Juriasingani S., Sener A. Hydrogen Sulfide: Emerging Role in Bladder, Kidney, and Prostate Malignancies. Oxid. Med. Cell. Longev. 2019;2019:2360945. doi: 10.1155/2019/2360945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang L., Qi Q., Yang J., Sun D., Li C., Xue Y., Jiang Q., Tian Y., Xu C., Wang R. An Anticancer Role of Hydrogen Sulfide in Human Gastric Cancer Cells. Oxid. Med. Cell. Longev. 2015;2015:636410. doi: 10.1155/2015/636410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sekiguchi F., Sekimoto T., Ogura A., Kawabata A. Endogenous Hydrogen Sulfide Enhances Cell Proliferation of Human Gastric Cancer AGS Cells. Biol. Pharm. Bull. 2016;39:887–890. doi: 10.1248/bpb.b15-01015. [DOI] [PubMed] [Google Scholar]
- 147.Shackelford R.E., Abdulsattar J., Wei E.X., Cotelingam J., Coppola D., Herrera G.A. Increased Nicotinamide Phosphoribosyltransferase and Cystathionine-β-Synthase in Renal Oncocytomas, Renal Urothelial Carcinoma, and Renal Clear Cell Carcinoma. Anticancer Res. 2017;37:3423–3427. doi: 10.21873/anticanres.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Breza J., Jr., Soltysova A., Hudecova S., Penesova A., Szadvari I., Babula P., Chovancova B., Lencesova L., Pos O., Ondrias K., et al. Endogenous H2S producing enzymes are involved in apoptosis induction in clear cell renal cell carcinoma. BMC Cancer. 2018;18:591. doi: 10.1186/s12885-018-4508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peleli M., Antoniadou I., Rodrigues-Junior D.M., Savvoulidou O., Caja L., Katsouda A., Ketelhuth D.F., Stubbe J., Madsen K., Moustakas A., et al. Cystathionine gamma-lyase (CTH) inhibition attenuates glioblastoma formation. Redox Biol. 2023;64:102773. doi: 10.1016/j.redox.2023.102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bronowicka-Adamska P., Bentke A., Wróbel M. Hydrogen sulfide generation from l-cysteine in the human glioblastoma-astrocytoma U-87 MG and neuroblastoma SHSY5Y cell lines. Acta Biochim. Pol. 2017;64:171–176. doi: 10.18388/abp.2016_1394. [DOI] [PubMed] [Google Scholar]
- 151.Turbat-Herrera E.A., Kilpatrick M.J., Chen J., Meram A.T., Cotelingam J., Ghali G., Kevil C.G., Coppola D., Shackelford R.E. Cystathione β-Synthase Is Increased in Thyroid Malignancies. Anticancer Res. 2018;38:6085–6090. doi: 10.21873/anticanres.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu Y., Ma N., Wei P., Zeng Z., Meng J. Expression of hydrogen sulfide synthases and Hh signaling pathway components correlate with the clinicopathological characteristics of papillary thyroid cancer patients. Int. J. Clin. Exp. Pathol. 2018;11:1818–1824. [PMC free article] [PubMed] [Google Scholar]
- 153.Panza E., Bello I., Smimmo M., Brancaleone V., Mitidieri E., Bucci M., Cirino G., Sorrentino R., Bianca R.D.d.V. Endogenous and exogenous hydrogen sulfide modulates urothelial bladder carcinoma development in human cell lines. Biomed. Pharmacother. 2022;151:113137. doi: 10.1016/j.biopha.2022.113137. [DOI] [PubMed] [Google Scholar]
- 154.Fan K., Zhang S., Ni X., Shen S., Wang J., Sun W., Suo T., Liu H., Ni X., Liu H. KRAS G12D mutation eliminates reactive oxygen species through the Nrf2/CSE/H2S axis and contributes to pancreatic cancer growth. Acta Biochim. Biophys. Sin. 2022;54:1731–1739. doi: 10.3724/abbs.2022173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang H., Gao Y., Zhao F.L., Qiao P.F., Yan Y. Hydrogen sulfide-induced processing of the amyloid precursor protein in SH-SY5Y human neuroblastoma cells involves the PI3-K/Akt signaling pathway. Cell. Mol. Neurobiol. 2015;35:265–272. doi: 10.1007/s10571-014-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koike S., Ogasawara Y., Shibuya N., Kimura H., Ishii K. Polysulfide exerts a protective effect against cytotoxicity caused by t-buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett. 2013;587:3548–3555. doi: 10.1016/j.febslet.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 157.Meram A.T., Chen J., Patel S., Kim D.D., Shirley B., Covello P., Coppola D., Wei E.X., Ghali G., Kevil C.G., et al. Hydrogen Sulfide Is Increased in Oral Squamous Cell Carcinoma Compared to Adjacent Benign Oral Mucosae. Anticancer Res. 2018;38:3843–3852. doi: 10.21873/anticanres.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu D., Si W., Wang M., Lv S., Ji A., Li Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide. 2015;50:38–45. doi: 10.1016/j.niox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 159.Schwartz G.K., Shah M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 160.Malumbres M., Barbacid M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 161.Yin P., Zhao C., Li Z., Mei C., Yao W., Liu Y., Li N., Qi J., Wang L., Shi Y., et al. Sp1 is involved in regulation of cystathionine gamma-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell. Signal. 2012;24:1229–1240. doi: 10.1016/j.cellsig.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 162.Bae J., Kumazoe M., Yamashita S., Tachibana H. Hydrogen sulphide donors selectively potentiate a green tea polyphenol EGCG-induced apoptosis of multiple myeloma cells. Sci. Rep. 2017;7:6665. doi: 10.1038/s41598-017-06879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ma Z., Bi Q., Wang Y. Hydrogen sulfide accelerates cell cycle progression in oral squamous cell carcinoma cell lines. Oral Dis. 2015;21:156–162. doi: 10.1111/odi.12223. [DOI] [PubMed] [Google Scholar]
- 164.Cai W.J., Wang M.J., Ju L.H., Wang C., Zhu Y.C. Hydrogen sulfide induces human colon cancer cell proliferation: Role of Akt, ERK and p21. Cell Biol. Int. 2010;34:565–572. doi: 10.1042/CBI20090368. [DOI] [PubMed] [Google Scholar]
- 165.Mathew R., Karantza-Wadsworth V., White E. Role of autophagy in cancer. Nat. Rev. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pan Y., Ye S., Yuan D., Zhang J., Bai Y., Shao C. Hydrogen sulfide (H2S)/cystathionine gamma-lyase (CSE) pathway contributes to the proliferation of hepatoma cells. Mutat. Res. 2014;763–764:10–18. doi: 10.1016/j.mrfmmm.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 167.Zhen Y., Pan W., Hu F., Wu H., Feng J., Zhang Y., Chen J. Exogenous hydrogen sulfide exerts proliferation/anti-apoptosis/angiogenesis/migration effects via amplifying the activation of NF-kappaB pathway in PLC/PRF/5 hepatoma cells. Int. J. Oncol. 2015;46:2194–2204. doi: 10.3892/ijo.2015.2914. [DOI] [PubMed] [Google Scholar]
- 168.Tiong C.X., Lu M., Bian J.S. Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/Akt pathway. Br. J. Pharmacol. 2010;161:467–480. doi: 10.1111/j.1476-5381.2010.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang W., Liu J.N., Tan X.Y. Vaccination with xenogeneic tumor endothelial proteins isolated in situ inhibits tumor angiogenesis and spontaneous metastasis. Int. J. Cancer. 2009;125:124–132. doi: 10.1002/ijc.24362. [DOI] [PubMed] [Google Scholar]
- 171.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z., Jeschke M.G., Branski L.K., Herndon D.N., Wang R., et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Holwerda K.M., Burke S.D., Faas M.M., Zsengeller Z., Stillman I.E., Kang P.M., van Goor H., McCurley A., Jaffe I.Z., Karumanchi S.A., et al. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J. Am. Soc. Nephrol. 2014;25:717–725. doi: 10.1681/ASN.2013030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang M.J., Cai W.-J., Li N., Ding Y.-J., Chen Y., Liu H.-D., Zhang A.-J., Xu J.-J., Yuan S., Kevil C.G., et al. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid. Redox Signal. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 174.Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., Hellmich M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Módis K., Coletta C., Asimakopoulou A., Szczesny B., Chao C., Papapetropoulos A., Hellmich M.R., Szabo C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-beta-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide. 2014;41:146–156. doi: 10.1016/j.niox.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Szabo C., Hellmich M.R. Endogenously produced hydrogen sulfide supports tumor cell growth and proliferation. Cell Cycle. 2013;12:2915–2916. doi: 10.4161/cc.26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yue T., Zuo S., Bu D., Zhu J., Chen S., Ma Y., Ma J., Guo S., Wen L., Zhang X., et al. Aminooxyacetic acid (AOAA) sensitizes colon cancer cells to oxaliplatin via exaggerating apoptosis induced by ROS. J. Cancer. 2020;11:1828–1838. doi: 10.7150/jca.35375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guo S., Li J., Huang Z., Yue T., Zhu J., Wang X., Liu Y., Wang P., Chen S. The CBS-H2S axis promotes liver metastasis of colon cancer by upregulating VEGF through AP-1 activation. Br. J. Cancer. 2022;126:1055–1066. doi: 10.1038/s41416-021-01681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen S., Yue T., Huang Z., Zhu J., Bu D., Wang X., Pan Y., Liu Y., Wang P. Inhibition of hydrogen sulfide synthesis reverses acquired resistance to 5-FU through miR-215-5p-EREG/TYMS axis in colon cancer cells. Cancer Lett. 2019;466:49–60. doi: 10.1016/j.canlet.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 180.Sen S., Kawahara B., Gupta D., Tsai R., Khachatryan M., Roy-Chowdhuri S., Bose S., Yoon A., Faull K., Farias-Eisner R., et al. Role of cystathionine beta-synthase in human breast Cancer. Free. Radic. Biol. Med. 2015;86:228–238. doi: 10.1016/j.freeradbiomed.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 181.Nafea H., Youness R.A., Abou-Aisha K., Gad M. 60P Curbing the interplay between miR-939-5p and HEIH lncRNA by EGCG represses hydrogen sulphide machinery and hinders breast cancer progression. Ann. Oncol. 2020;31:S1235. doi: 10.1016/j.annonc.2020.08.2219. [DOI] [Google Scholar]
- 182.Nafea H., Youness R., Abou-Aisha K., Gad M. Interplay between the Pan-Tumor Suppressor miR-939-5p and the oncogenic lncRNA-HEIH dually curbs Hydrogen Sulphide and Nitric Oxide production in breast cancer cells. Eur. J. Cancer. 2020;138:S70. doi: 10.1016/S0959-8049(20)30714-0. [DOI] [Google Scholar]
- 183.Dawoud A., Youness R.A., Nafea H.M., Bourquin C., Szabo C., Abdel-Kader R.M., Gad M. 2280P MiR-193a-3p: A pan-repressor of H2S synthesizing enzymes regulates tumorigenesis and immunosurveillance in breast cancer. Ann. Oncol. 2023;34:S1169. doi: 10.1016/j.annonc.2023.09.1308. [DOI] [Google Scholar]
- 184.Khater N., Habashy D., Youness R.A., Gad M.Z. 14P MALAT-1: A novel LncRNA modulating STAT-3 regulated cystathionine-γ-lyase (CSE) in breast cancer. Ann. Oncol. 2021;32:S7. doi: 10.1016/j.annonc.2021.01.027. [DOI] [Google Scholar]
- 185.Wahafu W., Gai J., Song L., Ping H., Wang M., Yang F., Niu Y., Xing N. Increased H(2)S and its synthases in urothelial cell carcinoma of the bladder, and enhanced cisplatin-induced apoptosis following H(2)S inhibition in EJ cells. Oncol. Lett. 2018;15:8484–8490. doi: 10.3892/ol.2018.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu H., Chang J., Zhao Z., Li Y., Hou J. Effects of exogenous hydrogen sulfide on the proliferation and invasion of human Bladder cancer cells. J. Cancer Res. Ther. 2017;13:829–832. doi: 10.4103/jcrt.JCRT_423_17. [DOI] [PubMed] [Google Scholar]
- 187.Wang M., Mao C., Ouyang L., Liu Y., Lai W., Liu N., Shi Y., Chen L., Xiao D., Yu F., et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26:2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lv L., Xi H.P., Huang J.C., Zhou X.Y. LncRNA SNHG1 alleviated apoptosis and inflammation during ischemic stroke by targeting miR-376a and modulating CBS/H2S pathway. Int. J. Neurosci. 2021;131:1162–1172. doi: 10.1080/00207454.2020.1782904. [DOI] [PubMed] [Google Scholar]
- 189.Shen Y., Shen Z., Guo L., Zhang Q., Wang Z., Miao L., Wang M., Wu J., Guo W., Zhu Y. MiR-125b-5p is involved in oxygen and glucose deprivation injury in PC-12 cells via CBS/H2S pathway. Nitric Oxide. 2018;78:11–21. doi: 10.1016/j.niox.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 190.Zhang Q., Shen Z., Shen Y., Ma M., Jue H., Zhu Y., Guo W. The regulatory role of MiR-203 in oxidative stress induced cell injury through the CBS/H2S pathway. Nitric Oxide. 2022;118:31–38. doi: 10.1016/j.niox.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 191.Yang H., Hu Y., Weng M., Liu X., Wan P., Hu Y., Ma M., Zhang Y., Xia H., Lv K. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J. Adv. Res. 2022;37:91–106. doi: 10.1016/j.jare.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gong D., Cheng H.-P., Xie W., Zhang M., Liu D., Lan G., Huang C., Zhao Z.-W., Chen L.-Y., Yao F., et al. Cystathionine γ-lyase(CSE)/hydrogen sulfide system is regulated by miR-216a and influences cholesterol efflux in macrophages via the PI3K/AKT/ABCA1 pathway. Biochem. Biophys. Res. Commun. 2016;470:107–116. doi: 10.1016/j.bbrc.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 193.Yao Y., Zhang X., Chen H.-P., Li L., Xie W., Lan G., Zhao Z.-W., Zheng X.-L., Wang Z.-B., Tang C.-K. MicroRNA-186 promotes macrophage lipid accumulation and secretion of pro-inflammatory cytokines by targeting cystathionine γ-lyase in THP-1 macrophages. Atherosclerosis. 2016;250:122–132. doi: 10.1016/j.atherosclerosis.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 194.Shen Y., Shen Z., Miao L., Xin X., Lin S., Zhu Y., Guo W., Zhu Y.Z. miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-γ-lyase expression. Antioxid. Redox Signal. 2015;22:224–240. doi: 10.1089/ars.2014.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu G., Wang Y., Yang Y., Shi Y., Sun J., Xu Y., Luo T., Le G. Dietary Methionine Restriction Upregulates Endogenous H2S via miR-328-3p: A Potential Mechanism to Improve Liver Protein Metabolism Efficiency in a Mouse Model of High-fat-diet-induced Obesity. Mol. Nutr. Food Res. 2019;63:e1800735. doi: 10.1002/mnfr.201800735. [DOI] [PubMed] [Google Scholar]
- 196.Hu X., Liu B., Wu P., Lang Y., Li T. LncRNA Oprm1 overexpression attenuates myocardial ischemia/reperfusion injury by increasing endogenous hydrogen sulfide via Oprm1/miR-30b-5p/CSE axis. Life Sci. 2020;254:117699. doi: 10.1016/j.lfs.2020.117699. [DOI] [PubMed] [Google Scholar]
- 197.Wang L., Tang Z.P., Zhao W., Cong B.H., Lu J.Q., Tang X.L., Li X.H., Zhu X.Y., Ni X. MiR-22/Sp-1 Links Estrogens with the Up-Regulation of Cystathionine γ-Lyase in Myocardium, which Contributes to Estrogenic Cardioprotection against Oxidative Stress. Endocrinology. 2015;156:2124–2137. doi: 10.1210/en.2014-1362. [DOI] [PubMed] [Google Scholar]
- 198.Medeiros J.V.R., Bezerra V.H., Gomes A.S., Barbosa A.L.R., Lima-Júnior R.C.P., Soares P.M.G., Brito G.A.C., Ribeiro R.A., Cunha F.Q., Souza M.H.L.P. Hydrogen sulfide prevents ethanol-induced gastric damage in mice: Role of ATP-sensitive potassium channels and capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 2009;330:764–770. doi: 10.1124/jpet.109.152801. [DOI] [PubMed] [Google Scholar]
- 199.Yonezawa D., Sekiguchi F., Miyamoto M., Taniguchi E., Honjo M., Masuko T., Nishikawa H., Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 200.Takeuchi K., Kita K., Hayashi S., Aihara E. Regulatory mechanism of duodenal bicarbonate secretion: Roles of endogenous prostaglandins and nitric oxide. Pharmacol. Ther. 2011;130:59–70. doi: 10.1016/j.pharmthera.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 201.Sparatore A., Perrino E., Tazzari V., Giustarini D., Rossi R., Rossoni G., Erdman K., Schröder H., Del Soldato P. Pharmacological profile of a novel H2S-releasing aspirin. Free Radic. Biol. Med. 2009;46:586–592. doi: 10.1016/j.freeradbiomed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 202.Wallace J.L. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid. Redox Signal. 2010;12:1125–1133. doi: 10.1089/ars.2009.2900. [DOI] [PubMed] [Google Scholar]
- 203.Lin M., Hu G., Yu B. Dysregulated cystathionine-beta-synthase/hydrogen sulfide signaling promotes chronic stress-induced colonic hypermotility in rats. Neurogastroenterol. Motil. 2023;35:e14488. doi: 10.1111/nmo.14488. [DOI] [PubMed] [Google Scholar]
- 204.Zhang J., Cen L., Zhang X., Tang C., Chen Y., Zhang Y., Yu M., Lu C., Li M., Li S., et al. MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT. Redox Biol. 2022;56:102469. doi: 10.1016/j.redox.2022.102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jha S., Calvert J.W., Duranski M.R., Ramachandran A., Lefer D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lin C.C., Yin M.C. Antiglycative and anti-VEGF effects of s-ethyl cysteine and s-propyl cysteine in kidney of diabetic mice. Mol. Nutr. Food Res. 2008;52:1358–1364. doi: 10.1002/mnfr.200800007. [DOI] [PubMed] [Google Scholar]
- 207.Kang K., Zhao M., Jiang H., Tan G., Pan S., Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl. 2009;15:1306–1314. doi: 10.1002/lt.21810. [DOI] [PubMed] [Google Scholar]
- 208.Huang X., Gao Y., Qin J., Lu S. The role of miR-34a in the hepatoprotective effect of hydrogen sulfide on ischemia/reperfusion injury in young and old rats. PLoS ONE. 2014;9:e113305. doi: 10.1371/journal.pone.0113305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xia M., Chen L., Muh R.W., Li P.L., Li N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J. Pharmacol. Exp. Ther. 2009;329:1056–1062. doi: 10.1124/jpet.108.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sen U., Basu P., Abe O.A., Givvimani S., Tyagi N., Metreveli N., Shah K.S., Passmore J.C., Tyagi S.C. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am. J. Physiol. Renal Physiol. 2009;297:F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zeng T., Zhang C.L., Zhu Z.P., Yu L.H., Zhao X.L., Xie K.Q. Diallyl trisulfide (DATS) effectively attenuated oxidative stress-mediated liver injury and hepatic mitochondrial dysfunction in acute ethanol-exposed mice. Toxicology. 2008;252:86–91. doi: 10.1016/j.tox.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 212.Morsy M.A., Ibrahim S.A., Abdelwahab S.A., Zedan M.Z., Elbitar H.I. Curative effects of hydrogen sulfide against acetaminophen-induced hepatotoxicity in mice. Life Sci. 2010;87:692–698. doi: 10.1016/j.lfs.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 213.Ci L., Yang X., Gu X., Li Q., Guo Y., Zhou Z., Zhang M., Shi J., Yang H., Wang Z., et al. Cystathionine gamma-Lyase Deficiency Exacerbates CCl4-Induced Acute Hepatitis and Fibrosis in the Mouse Liver. Antioxid. Redox Signal. 2017;27:133–149. doi: 10.1089/ars.2016.6773. [DOI] [PubMed] [Google Scholar]
- 214.Robert K., Nehmé J., Bourdon E., Pivert G., Friguet B., Delcayre C., Delabar M., Janel N. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology. 2005;128:1405–1415. doi: 10.1053/j.gastro.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 215.Wang S., Liu X., Liu Y. Hydrogen sulfide protects from acute kidney injury via attenuating inflammation activated by necroptosis in dogs. J. Vet. Sci. 2022;23:e72. doi: 10.4142/jvs.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kaneko Y., Kimura T., Taniguchi S., Souma M., Kojima Y., Kimura Y., Kimura H., Niki I. Glucose-induced production of hydrogen sulfide may protect the pancreatic beta-cells from apoptotic cell death by high glucose. FEBS Lett. 2009;583:377–382. doi: 10.1016/j.febslet.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 217.Taniguchi S., Kang L., Kimura T., Niki I. Hydrogen sulphide protects mouse pancreatic beta-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br. J. Pharmacol. 2011;162:1171–1178. doi: 10.1111/j.1476-5381.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Taniguchi S., Niki I. Significance of hydrogen sulfide production in the pancreatic beta-cell. J. Pharmacol. Sci. 2011;116:1–5. doi: 10.1254/jphs.11R01CP. [DOI] [PubMed] [Google Scholar]
- 219.Brancaleone V., Roviezzo F., Vellecco V., De Gruttola L., Bucci M., Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br. J. Pharmacol. 2008;155:673–680. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Denizalti M., Bozkurt T.E., Akpulat U., Sahin-Erdemli I., Abacioglu N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch. Pharmacol. 2011;383:509–517. doi: 10.1007/s00210-011-0601-6. [DOI] [PubMed] [Google Scholar]
- 221.Lefer D.J. Potential importance of alterations in hydrogen sulphide (H2S) bioavailability in diabetes. Br. J. Pharmacol. 2008;155:617–619. doi: 10.1038/bjp.2008.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Whiteman M., Gooding K.M., Whatmore J.L., Ball C.I., Mawson D., Skinner K., Tooke J.E., Shore A.C. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 223.Peng S., Wang M., Zhang S., Liu N., Li Q., Kang J., Chen L., Li M., Pang K., Huang J., et al. Hydrogen sulfide regulates SERCA2a SUMOylation by S-Sulfhydration of SENP1 to ameliorate cardiac systole-diastole function in diabetic cardiomyopathy. Biomed. Pharmacother. 2023;160:114200. doi: 10.1016/j.biopha.2022.114200. [DOI] [PubMed] [Google Scholar]
- 224.Xue W., Zhang Q., Chen Y., Zhu Y. Hydrogen Sulfide Improves Angiogenesis by Regulating the Transcription of pri-miR-126 in Diabetic Endothelial Cells. Cells. 2022;11:2651. doi: 10.3390/cells11172651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhou Y., Li X.H., Zhang C.C., Wang M.J., Xue W.L., Wu D.D., Ma F.F., Li W.W., Tao B.B., Zhu Y.C. Hydrogen sulfide promotes angiogenesis by downregulating miR-640 via the VEGFR2/mTOR pathway. Am. J. Physiol. Cell Physiol. 2016;310:C305–C317. doi: 10.1152/ajpcell.00230.2015. [DOI] [PubMed] [Google Scholar]
- 226.Calvert J.W., Jha S., Gundewar S., Elrod J.W., Ramachandran A., Pattillo C.B., Kevil C.G., Lefer D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Elrod J.W., Calvert J.W., Morrison J., Doeller J.E., Kraus D.W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Calvert J.W., Elston M., Nicholson C.K., Gundewar S., Jha S., Elrod J.W., Ramachandran A., Lefer D.J. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lynn E.G., Austin R.C. Hydrogen sulfide in the pathogenesis of atherosclerosis and its therapeutic potential. Expert Rev. Clin. Pharmacol. 2011;4:97–108. doi: 10.1586/ecp.10.130. [DOI] [PubMed] [Google Scholar]
- 230.Minamishima S., Bougaki M., Sips P.Y., De Yu J., Minamishima Y.A., Elrod J.W., Lefer D.J., Bloch K.D., Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Predmore B.L., Lefer D.J. Hydrogen sulfide-mediated myocardial pre- and post-conditioning. Expert Rev. Clin. Pharmacol. 2011;4:83–96. doi: 10.1586/ecp.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu J., Hao D.D., Zhang J.S., Zhu Y.C. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. Biochem. Biophys. Res. Commun. 2011;413:342–347. doi: 10.1016/j.bbrc.2011.08.101. [DOI] [PubMed] [Google Scholar]
- 233.Wu Y., Guo Y.Y., Zhang Y.Y., Zhang Y. Effects of hydrogen sulfide (H2S) on cardiac hypertrophy and miRNA-133a-mediated Ca2+/calcineurin/NFATc4 signal pathway in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2018;34:29–34. doi: 10.12047/j.cjap.5492.2018.009. [DOI] [PubMed] [Google Scholar]
- 234.Toldo S., Das A., Mezzaroma E., Chau V.Q., Marchetti C., Durrant D., Samidurai A., Van Tassell B.W., Yin C., Ockaili R.A., et al. Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ. Cardiovasc. Genet. 2014;7:311–320. doi: 10.1161/CIRCGENETICS.113.000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lefer D.J., Scalia R., Campbell B., Nossuli T., Hayward R., Salamon M., Grayson J., Lefer A.M. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J. Clin. Investig. 1997;99:684–691. doi: 10.1172/JCI119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yusof M., Kamada K., Kalogeris T., Gaskin F.S., Korthuis R.J. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H868–H876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zanardo R.C.O., Brancaleone V., Distrutti E., Fiorucci S., Cirino G., Wallace J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 239.Kloesch B., Liszt M., Steiner G., Broll J. Inhibitors of p38 and ERK1/2 MAPkinase and hydrogen sulphide block constitutive and IL-1beta-induced IL-6 and IL-8 expression in the human chondrocyte cell line C-28/I2. Rheumatol. Int. 2012;32:729–736. doi: 10.1007/s00296-010-1682-0. [DOI] [PubMed] [Google Scholar]
- 240.Oh G.S., Pae H.-O., Lee B.-S., Kim B.-N., Kim J.-M., Kim H.-R., Jeon S.B., Jeon W.K., Chae H.-J., Chung H.-T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 241.Pan L.L., Liu X.H., Gong Q.H., Wu D., Zhu Y.Z. Hydrogen sulfide attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS ONE. 2011;6:e19766. doi: 10.1371/journal.pone.0019766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 242.Yong Q.C., Lee S.W., Foo C.S., Neo K.L., Chen X., Bian J.-S. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 243.Li L., Salto-Tellez M., Tan C.H., Whiteman M., Moore P.K. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic. Biol. Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 244.Sanchez L.D., Sanchez-Aranguren L., Wang K., Spickett C.M., Griffiths H.R., Dias I.H.K. TNF-alpha-Mediated Endothelial Cell Apoptosis Is Rescued by Hydrogen Sulfide. Antioxidants. 2023;12:794. doi: 10.3390/antiox12030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lu M., Jiang X., Tong L., Zhang F., Ma L., Dong X., Sun X. MicroRNA-21-Regulated Activation of the Akt Pathway Participates in the Protective Effects of H2S against Liver Ischemia-Reperfusion Injury. Biol. Pharm. Bull. 2018;41:229–238. doi: 10.1248/bpb.b17-00769. [DOI] [PubMed] [Google Scholar]
- 246.Hackfort B.T., Mishra P.K. Emerging role of hydrogen sulfide-microRNA crosstalk in cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H802–H812. doi: 10.1152/ajpheart.00660.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Birbrair A., Zhang T., Wang Z.-M., Messi M.L., Olson J.D., Mintz A., Delbono O., Hayes K.L., Messina L.M., Schwartz L.M., et al. Type-2 pericytes participate in normal and tumoral angiogenesis. Am. J. Physiol. Cell Physiol. 2014;307:C25–C38. doi: 10.1152/ajpcell.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 249.Johnstone C.C., Farley A. The physiological basics of wound healing. Nurs. Stand. 2005;19:59–65; quiz 6. doi: 10.7748/ns.19.43.59.s55. [DOI] [PubMed] [Google Scholar]
- 250.Cai W.J., Wang M.-J., Moore P.K., Jin H.-M., Yao T., Zhu Y.-C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 251.Suárez Y., Fernández-Hernando C., Yu J., Gerber S.A., Harrison K.D., Pober J.S., Iruela-Arispe M.L., Merkenschlager M., Sessa W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tan B.H., Wong P.T., Bian J.S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010;56:3–10. doi: 10.1016/j.neuint.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 253.Guo W., Kan J.-T., Cheng Z.-Y., Chen J.-F., Shen Y.-Q., Xu J., Wu D., Zhu Y.-Z. Hydrogen sulfide as an endogenous modulator in mitochondria and mitochondria dysfunction. Oxid. Med. Cell. Longev. 2012;2012:878052. doi: 10.1155/2012/878052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li L., Jiang H.K., Li Y.P., Guo Y.P. Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. J. Biomed. Sci. 2015;22:50. doi: 10.1186/s12929-015-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liu Y., Pan L., Jiang A., Yin M. Hydrogen sulfide upregulated lncRNA CasC7 to reduce neuronal cell apoptosis in spinal cord ischemia-reperfusion injury rat. Biomed. Pharmacother. 2018;98:856–862. doi: 10.1016/j.biopha.2017.12.079. [DOI] [PubMed] [Google Scholar]
- 256.Lee M., Sparatore A., Del Soldato P., McGeer E., McGeer P.L. Hydrogen sulfide-releasing NSAIDs attenuate neuroinflammation induced by microglial and astrocytic activation. Glia. 2010;58:103–113. doi: 10.1002/glia.20905. [DOI] [PubMed] [Google Scholar]
- 257.Zhang H., Gao Y., Zhao F., Dai Z., Meng T., Tu S., Yan Y. Hydrogen sulfide reduces mRNA and protein levels of beta-site amyloid precursor protein cleaving enzyme 1 in PC12 cells. Neurochem. Int. 2011;58:169–175. doi: 10.1016/j.neuint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 258.Meng J., Mei W., Dong Y., Wang J., Zhao C., Lan A., Yang C., Chen P., Feng J., Hu C. Heat shock protein 90 mediates cytoprotection by H2S against chemical hypoxia-induced injury in PC12 cells. Clin. Exp. Pharmacol. Physiol. 2011;38:42–49. doi: 10.1111/j.1440-1681.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- 259.Tay A.S., Hu L.F., Lu M., Wong P.T., Bian J.S. Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience. 2010;167:277–286. doi: 10.1016/j.neuroscience.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 260.Osborne N.N., Ji D., Majid A.S.A., Fawcett R.J., Sparatore A., Del Soldato P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Investig. Opthalmol. Vis. Sci. 2010;51:284–294. doi: 10.1167/iovs.09-3999. [DOI] [PubMed] [Google Scholar]
- 261.Biermann J., Lagreze W.A., Schallner N., Schwer C.I., Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol. Vis. 2011;17:1275–1286. [PMC free article] [PubMed] [Google Scholar]
- 262.Mikami Y., Shibuya N., Kimura Y., Nagahara N., Yamada M., Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J. Biol. Chem. 2011;286:39379–39386. doi: 10.1074/jbc.M111.298208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tyagi N., Givvimani S., Qipshidze N., Kundu S., Kapoor S., Vacek J.C., Tyagi S.C. Hydrogen sulfide mitigates matrix metalloproteinase-9 activity and neurovascular permeability in hyperhomocysteinemic mice. Neurochem. Int. 2010;56:301–307. doi: 10.1016/j.neuint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chen Z., Zhang Z., Zhang D., Li H., Sun Z. Hydrogen sulfide protects against TNF-alpha induced neuronal cell apoptosis through miR-485-5p/TRADD signaling. Biochem. Biophys. Res. Commun. 2016;478:1304–1309. doi: 10.1016/j.bbrc.2016.08.116. [DOI] [PubMed] [Google Scholar]
- 265.Liu Y., Liao S., Quan H., Lin Y., Li J., Yang Q. Involvement of microRNA-135a-5p in the Protective Effects of Hydrogen Sulfide Against Parkinson’s Disease. Cell. Physiol. Biochem. 2016;40:18–26. doi: 10.1159/000452521. [DOI] [PubMed] [Google Scholar]
- 266.Lugli G., Cohen A.M., Bennett D.A., Shah R.C., Fields C.J., Hernandez A.G., Smalheiser N.R. Plasma Exosomal miRNAs in Persons with and without Alzheimer Disease: Altered Expression and Prospects for Biomarkers. PLoS ONE. 2015;10:e0139233. doi: 10.1371/journal.pone.0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hromadnikova I., Kotlabova K., Hympanova L., Krofta L. Cardiovascular and Cerebrovascular Disease Associated microRNAs are Dysregulated in Placental Tissues Affected with Gestational Hypertension, Preeclampsia and Intrauterine Growth Restriction. PLoS ONE. 2015;10:e0138383. doi: 10.1371/journal.pone.0138383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.