Abstract
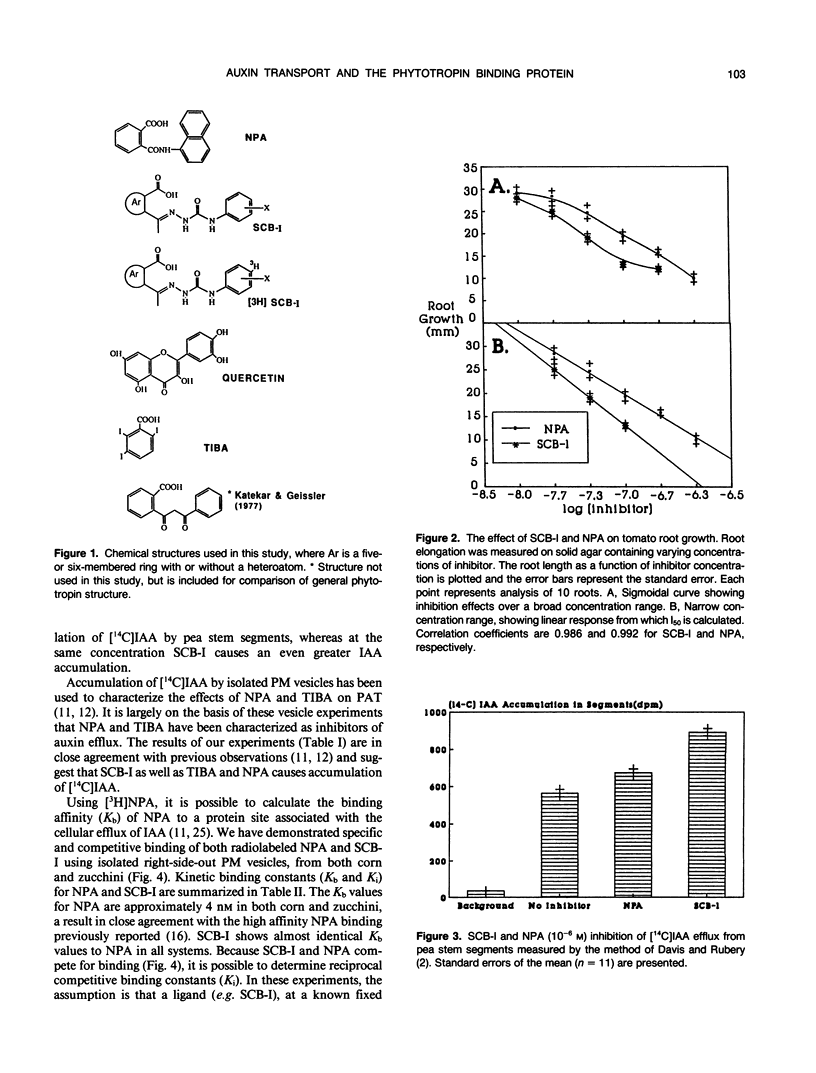
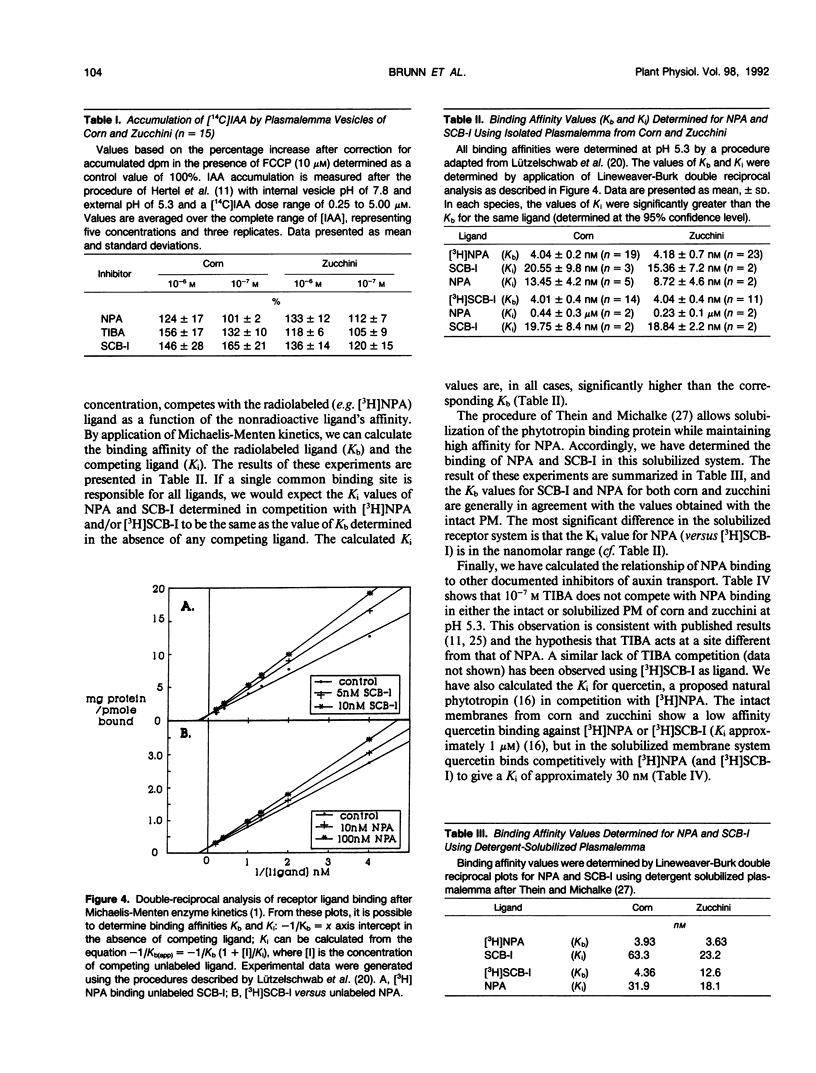
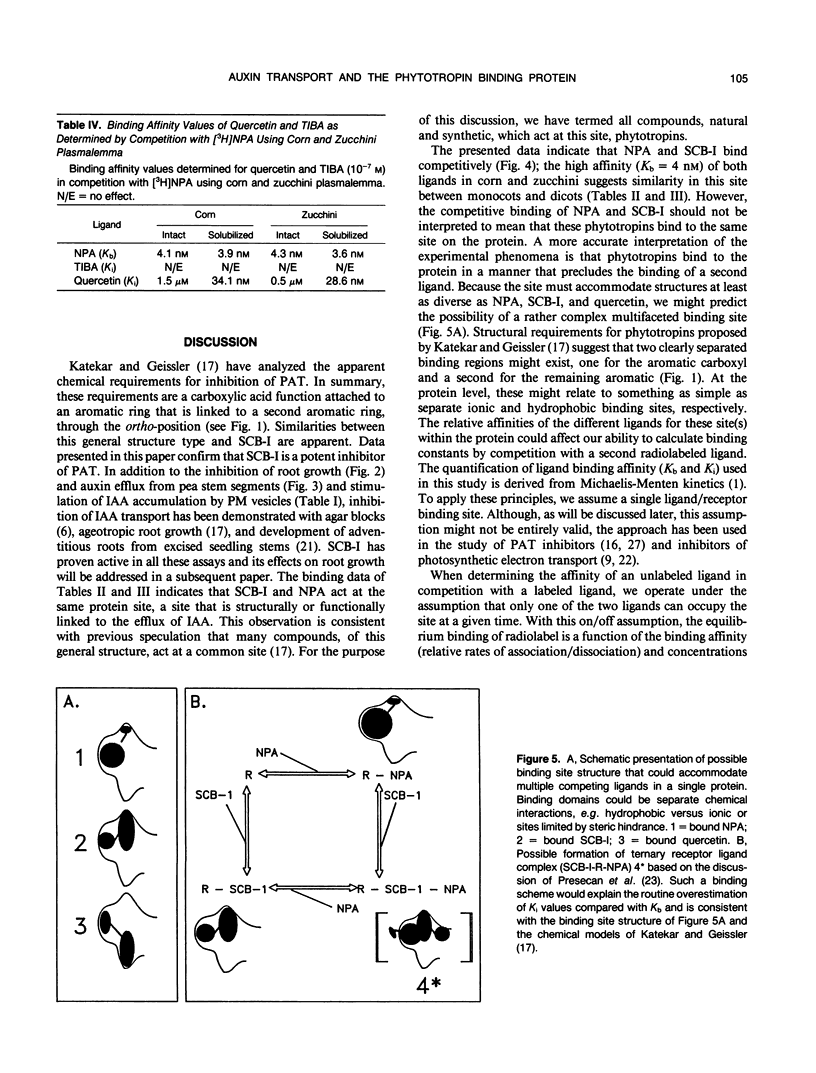
We have described the inhibition of polar auxin transport by several phytotropins including 1-N-naphthylphthalamic acid (NPA) and quercetin. Semicarbazones (substituted phenylsemicarbazones of 2-acetylarylcarboxylic acids) are inhibitors consistent with previously predicted general structural requirements for auxin transport inhibitors. The best semicarbazone derivative tested to date, hereafter called SCB-I, binds to the NPA binding protein with high affinity, Kb = 4 nanomolar. Quantification of the binding of various phytotropins allows us to make some general statements concerning the structure/properties of the NPA binding protein. The data suggest that the ligand binding region of this protein is multifaceted, a conclusion supported by the chemical predictions of Katekar and Geissler ([1977] Plant Physiol 60: 826-829). Although the data do not allow us to make specific conclusions on the structure of the binding site, they do show that both NPA and SCB-I could each occupy two regions of the protein. At least one of these binding regions appears to be common for both inhibitors of auxin transport. We suggest that the diversity of the binding site structure reflects the possible existence of more than one type of natural ligand controlling the process of auxin transport.
Full text
PDF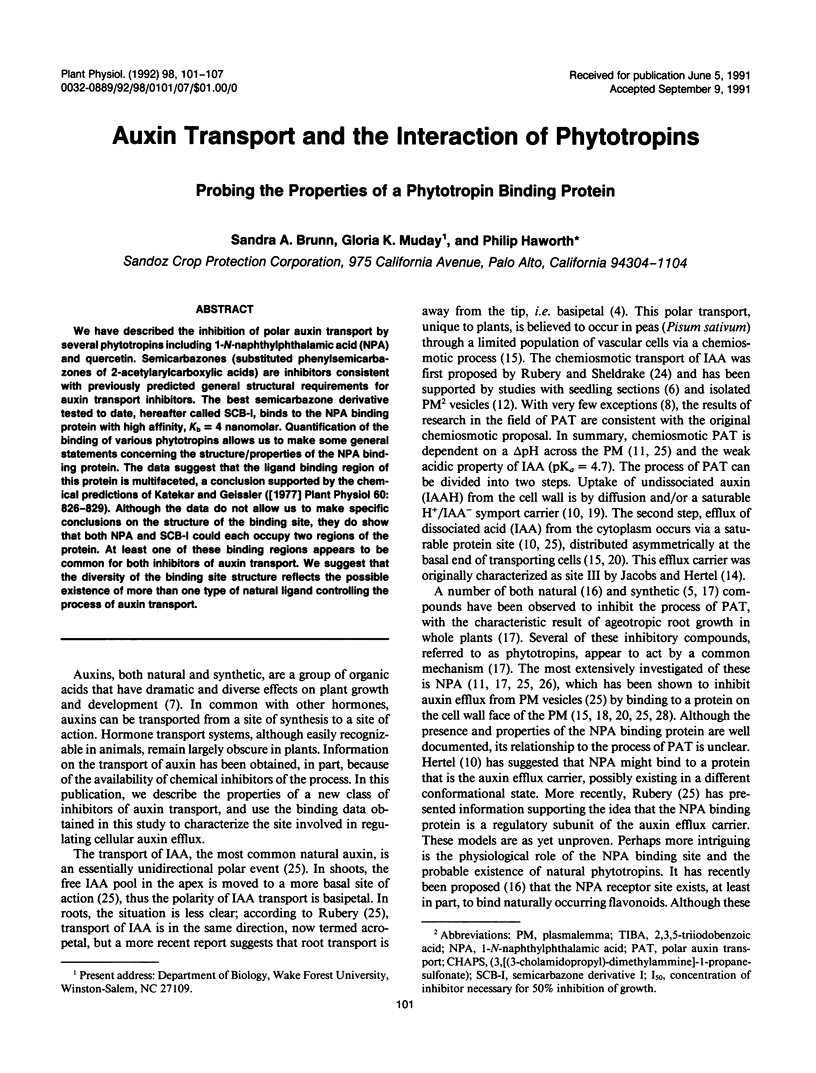



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans M. L. Gravitropism: interaction of sensitivity modulation and effector redistribution. Plant Physiol. 1991;95:1–5. doi: 10.1104/pp.95.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein K. H., Rayle D. Cell wall pH and auxin transport velocity. Plant Physiol. 1984 Sep;76(1):65–67. doi: 10.1104/pp.76.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth P., Steinback K. E. Interaction of Herbicides and Quinone with the Q(B)-Protein of the Diuron-Resistant Chlamydomonas reinhardtii Mutant Dr2. Plant Physiol. 1987 Apr;83(4):1027–1031. doi: 10.1104/pp.83.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Katekar G. F., Geissler A. E. Auxin Transport Inhibitors: III. Chemical Requirements of a Class of Auxin Transport Inhibitors. Plant Physiol. 1977 Dec;60(6):826–829. doi: 10.1104/pp.60.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax T. L., Mehlhorn R. J., Briggs W. R. Active auxin uptake by zucchini membrane vesicles: quantitation using ESR volume and delta pH determinations. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6541–6545. doi: 10.1073/pnas.82.19.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Radosevich S. R., Arntzen C. J. Modification of Herbicide Binding to Photosystem II in Two Biotypes of Senecio vulgaris L. Plant Physiol. 1979 Dec;64(6):995–999. doi: 10.1104/pp.64.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presecan E., Porumb H., Lascu I. Potential misinterpretation of the competitive binding assays. Trends Biochem Sci. 1989 Nov;14(11):443–444. doi: 10.1016/0968-0004(89)90100-x. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 1979;63:437–467. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]


