Abstract
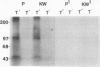
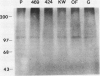
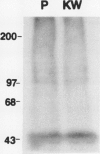
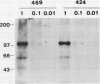
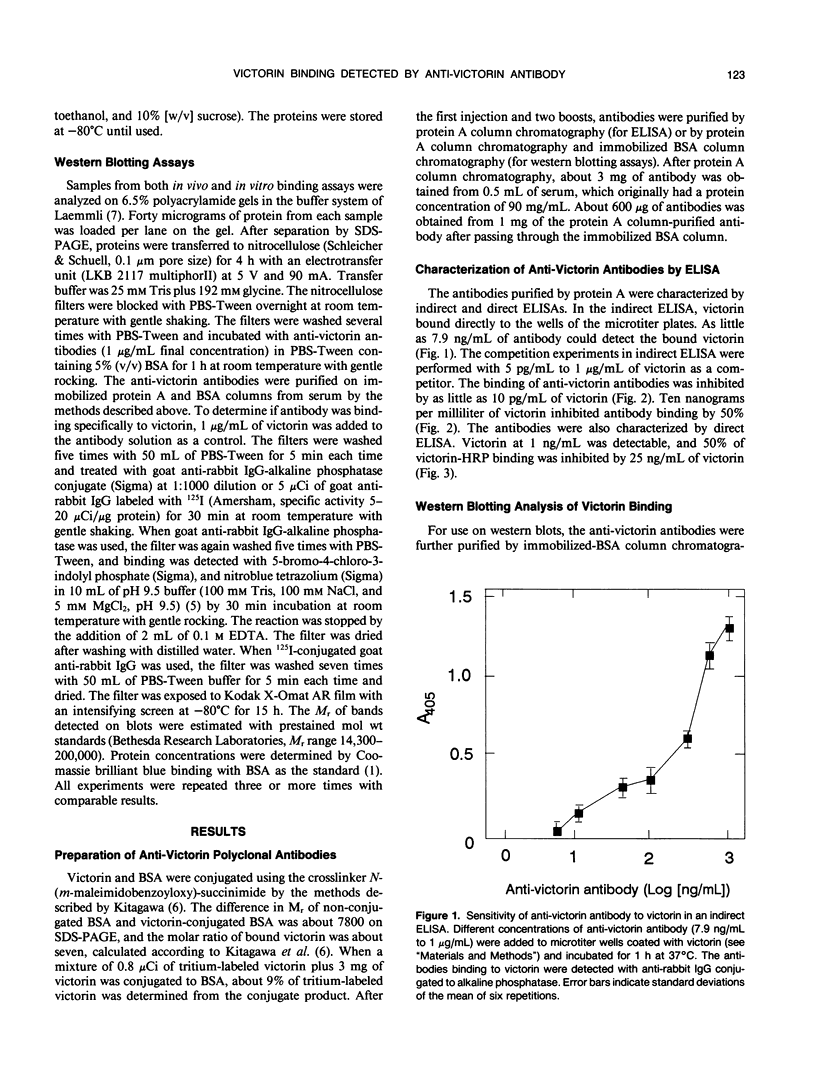
Polyclonal antibodies against victorin, the host-specific toxin produced by Cochliobolus victoriae, were raised in rabbits immunized with a victorin-bovine serum albumin conjugate. The antibodies were purified from serum by protein A column chromatography and characterized by indirect and direct enzymelinked immunosorbent assays (ELISA). The concentration of victorin that inhibited anti-victorin antibody binding by 50% was 10 nanograms per milliliter in an indirect ELISA. The lowest concentration of victorin detectable was 10 picograms per milliliter. In a direct ELISA, 25 nanograms per milliliter of victorin inhibited binding of victorin-horseradish peroxidase conjugate by 50%. In vivo and in vitro covalent binding of victorin to proteins in susceptible and resistant oat (Avena sativa) tissue was examined by western blotting assays using anti-victorin antibody and a second antibody conjugated with 125I or alkaline phosphatase. In vivo binding of victorin to proteins of 100 and 45 kilodaltons was observed in both susceptible and resistant cultivars of oats. Victorin also bound in vitro to proteins of 100, 65, and 45 kilodaltons in both susceptible and resistant oats. The data indicate that victorin binds covalently to the same sites in susceptible and resistant genotypes of oats.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Kawasaki T., Munechika H. Enzyme immunoassay of blasticidin S with high sensitivity: a new and convenient method for preparation of immunogenic (hapten-protein) conjugates. J Biochem. 1982 Aug;92(2):585–590. doi: 10.1093/oxfordjournals.jbchem.a133967. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macko K. A., Hodos W. Near point of accommodation in pigeons. Vision Res. 1985;25(10):1529–1530. doi: 10.1016/0042-6989(85)90232-9. [DOI] [PubMed] [Google Scholar]
- Meehan F., Murphy H. C. A New Helminthosporium Blight of Oats. Science. 1946 Nov 1;104(2705):413–414. doi: 10.1126/science.104.2705.413. [DOI] [PubMed] [Google Scholar]
- Scheffer R. P., Livingston R. S. Host-selective toxins and their role in plant diseases. Science. 1984 Jan 6;223(4631):17–21. doi: 10.1126/science.223.4631.17. [DOI] [PubMed] [Google Scholar]
- Wolpert T. J., Macko V., Acklin W., Arigoni D. Molecular Features Affecting the Biological Activity of the Host-Selective Toxins from Cochliobolus victoriae. Plant Physiol. 1988 Sep;88(1):37–41. doi: 10.1104/pp.88.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert T. J., Macko V. Specific binding of victorin to a 100-kDa protein from oats. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4092–4096. doi: 10.1073/pnas.86.11.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]