Abstract
One of the most prevalent primary malignant brain tumors is glioblastoma (GB). About 6 incidents per 100,000 people are reported annually. Most frequently, these tumors are linked to a poor prognosis and poor quality of life. There has been little advancement in the treatment of GB. In recent years, some innovative medicines have been tested for the treatment of newly diagnosed cases of GB and recurrent cases of GB. Surgery, radiotherapy, and alkylating chemotherapy are all common treatments for GB. A few of the potential alternatives include immunotherapy, tumor-treating fields (TTFs), and medications that target specific cellular receptors. To provide new multimodal therapies that focus on the molecular pathways implicated in tumor initiation and progression in GB, novel medications, delivery technologies, and immunotherapy approaches are being researched. Of these, oncolytic viruses (OVs) are among the most recent. Coupling OVs with certain modern treatment approaches may have significant benefits for GB patients. Here, we discuss several OVs and how they work in conjunction with other therapies, as well as virotherapy for GB. The study was based on the PRISMA guidelines. Systematic retrieval of information was performed on PubMed. A total of 307 articles were found in a search on oncolytic viral therapies for glioblastoma. Out of these 83 articles were meta-analyses, randomized controlled trials, reviews, and systematic reviews. A total of 42 articles were from the years 2018 to 2023. Appropriate studies were isolated, and important information from each of them was understood and entered into a database from which the information was used in this article. One of the most prevalent malignant brain tumors is still GB. Significant promise and opportunity exist for oncolytic viruses in the treatment of GB and in boosting immune response. Making the most of OVs in the treatment of GB requires careful consideration and evaluation of a number of its application factors.
Keywords: glioblastoma, malignant brain tumor, neurosurgery, treatment of glioblastoma, oncolytic viruses for treatment of glioblastoma, nanoparticles, viruses
1. Introduction
In the general population, glioblastoma (GB) is one of the most prevalent malignant brain tumors. Most glioblastomas (around 80–90%) arise de novo and without any prior clinical or histologic signs, especially in elderly individuals. [1]. Currently, one of the most aggressive and incurable cancers is GB [2]. The WHO revised the categorization of malignancies of the central nervous system (CNS) in 2021, incorporating genetic and molecular characteristics into the definition of various glioma subtypes alongside histological ones [3]. Less than 5% of people with GB survive more than five years after diagnosis, with the median overall survival for those with the disease falling between 15 and 20 months [4,5]. Radiation therapy and chemotherapy are usually used after a surgical resection for GB [6]. GB can be treated with temozolomide, bevacizumab, lomustine, intravenous carmustine, and carmustine wafer plants, among other chemotherapy medications [7].
Key risk factors for GB include obesity, cytomegalovirus (CMV), high radiation dosages, and a family history of cancer [8,9,10,11]. GB has been described as having considerable intratumoral heterogeneity, tumor-induced immunosuppression of the microenvironment, and minimal infiltrating immunity [12,13,14]. The blood–brain barrier (BBB) [15], glioma stem cells (GSC) [16,17], and a low tumor mutational load [18,19] are also present.
Recent developments in molecular pathogenesis have led to a better understanding of the GB microenvironment, including its interactions with the human immune system and its genetic, epigenomic, transcriptomic, and proteomic characterization [20]. Among the most promising therapies for GB and brain tumors are novel therapeutic approaches including oncolytic viral therapy [21].
2. Overview Glioblastoma
2.1. Introduction of Glioblastoma
Initially, it was believed that GB only came from glial cells, but research now reveals that they may also come from other cell types that have characteristics of neural stem cells. These cells are in various phases of development from stem cells to neurons to glia, with molecular changes in signaling pathways acting largely as a determinant of phenotypic diversity rather than diverse cell types of origin [22].
2.2. Molecular Description
More than 600 genes were sequenced from more than 200 human tumor samples as a result of genomic profiling and the Cancer Genome Atlas project by Parsons et al., 2008, which revealed the complex genetic profile of GB and established a set of three core signaling pathways that are frequently activated (namely, the tumor protein p53 (p53) pathway, the receptor tyrosine kinase/Ras/phosphoinositide 3-kinase signaling pathway, and the retinoblastoma pathway) [23]. Epidermal growth factor receptor (EGFR) overexpression, mutations in the PTEN gene, and deletion of chromosome 10q are among the genetic changes common to primary GBM. Chromosome 19q deletion, p53 mutations, and isocitrate dehydrogenase 1 (IDH1) mutations are typically observed in secondary GB [24,25,26]. Imaging genomics is a young field of study that investigates relationships between molecular profiles and radiologic characteristics with the potential to one day provide a non-invasive method for identifying, predicting, and correlating genetic variations [27].
Transcriptome studies have become significant methods for grouping cancers into molecular subgroups that differ in terms of their clinical behavior and reaction to treatment [28]. However, when taking into account the extremely aggressive isocitrate dehydrogenase (IDH) wild-type group, the transcriptome categorization has not been able to predict prognosis and pharmacologic vulnerability for specific cancers, such as GBM [29,30]. Specifically, the absence of correlation between survival and physiologically defined subgroups of IDH wild-type GBM has impeded the quest to identify the distinct mechanisms that maintain tumor development in patient subgroups. The transcriptome subgroups utilized to define GBM are preferentially concentrated in tumor cells displaying unique lineage-specific biological states, according to recent results in single cells. The possibility of using the basic biological processes of individual GBM cells to create a clinically meaningful categorization of bulk tumors is still unproven. We reviewed the developed computational approach to extract the core tumor-cell-intrinsic biological states of individual GBM cells from GBM single-cell RNA-sequencing (scRNA-seq) data [31,32,33] and bulk tumors because pathway-based classifications of transcriptomic cancer data have demonstrated higher stability of biological activities and better performance than gene-based classifiers [34]. A novel categorization for GBM was produced by the analysis, which converged on four stable cellular states that include developmental (neuronal and proliferative/progenitor) and metabolic (mitochondrial and glycolytic/plurimetabolic) features. The mitochondrial subtype classifies individuals with better clinical outcomes and is dependent on oxidative phosphorylation (OXPHOS). The mitochondrial group of GBM differs from the glycolytic/plurimetabolic subgroup, which has a poor prognosis and is maintained by the simultaneous activation of numerous energy-producing programs that provide protection against oxidative stress and metabolic variety. This was discovered by multiomics analysis. Targeted metabolic therapy may be beneficial for some GBM patients, as demonstrated by the distinct susceptibility of the mitochondrial subgroup to inhibitors of mitochondrial metabolism.
2.3. Risk Factors
One of the few recognized risk factors that has been proven to increase the likelihood of developing gliomas is exposure to ionizing radiation [35]. Radiation-induced GB is generally diagnosed years after receiving therapeutic radiation that was prescribed for another tumor or illness. [36]. Other environmental risk factors for the growth of gliomas include exposure to vinyl chloride, pesticides, smoking, petroleum refining, and the production of synthetic rubber. It has not been demonstrated that exposure to electromagnetic fields, formaldehyde, or nonionizing radiation from cell phones causes GB [37]. Less than 1% of people with glioma have a recognized hereditary disease; however, some specific genetic diseases, such as neurofibromatosis 1 and 2, tuberous sclerosis, Li–Fraumeni syndrome, retinoblastoma, and Turcot syndrome, are associated with an elevated risk of glioma development [38].
2.4. Clinical Presentation and Imaging
The anatomical components of the affected brain and the size and location of the tumor can all have a significant impact on how a patient with newly diagnosed GB presents [39,40]. Patients frequently have headaches and localized or progressive neurologic impairments as signs of elevated intracranial pressure. Up to 25% of patients have a seizure as their first symptom, and up to 50% of patients can have one later on in the course of the disease [41,42]. Antiepileptic medicines (AEDs) are already part of the standard of care for patients who present with seizures, although routine use of AEDs in individuals without seizures is not advised [43,44]. At the time of diagnosis, corticosteroids are frequently prescribed to patients to assist in reducing vasogenic edema and relieve related signs and symptoms.
A computed tomography (CT) or magnetic resonance imaging (MRI) scan could be used as part of the initial diagnostic imaging process. Nearly all GB are seen on gadolinium contrast-enhanced MRI, which reveals an irregularly shaped mass with a dense ring of enhancement and a hypointense necrosis center [45].
2.5. Current Treatment Options
The current standard of care is for concomitant radiotherapy with temozolomide and the greatest amount of safe surgical resection possible [46]. Because these tumors are commonly invasive and typically located in expressive regions of the brain, such as regions that regulate speech, motor function, and the senses, extensive and full surgical resection of GBM is challenging. Radical removal of the initial tumor mass is not curative due to the high degree of invasiveness, and infiltrating tumor cells typically stay in the nearby brain, causing the disease to develop or return in the future [47].
When feasible, aggressive surgical resection has been shown to be important, and there are trends toward better outcomes in individuals who have undergone more extensive resection [48,49]. Numerous studies [50,51,52,53] have found statistically significant correlations between larger resection depth and longer progression-free survival (PFS) and overall survival (OS). More extensive resections can now be accomplished while maintaining function and quality of life because of advancements in surgical and preoperative mapping procedures [54,55]. Table 1 describes the treatments for new-onset glioblastoma and recurrent glioblastoma.
Table 1.
Treatments for new-onset glioblastoma and recurrent glioblastoma according to The American Society of Clinical Oncology (ASCO) and the Society of Neuro-Oncology (SNO).
| New Onset Glioblastoma | Recurrent Gliobastoma |
|---|---|
| Patients with freshly diagnosed glioblastoma, IDH-wildtype, should be offered concomitant TMZ and RT. | Magnetic resonance imaging (MRI) augmented with gadolinium contrast is advised. |
| Patients who have undergone concurrent RT with TMZ should be offered adjuvant TMZ for six months. | 18-FDG is not advised for use in regular diagnostic procedures. |
| After chemoradiation treatment, individuals may receive adjuvant TMZ in addition to alternating electric field therapy. | Patients with symptomatic pGBM are advised to undergo cytoreductive surgery. |
| Treatment with bevacizumab is not advised. | It is not recommended to reevaluate the methylation status of 06-methylguanine-DNA methyltransferase (MGMT) and the state of isocitrate dehydrogenase (IDH). |
| Hypofractionated radiation therapy combined with TMZ is a suitable choice when the projected survival benefits of a six-week radiation treatment combined with TMZ may not exceed the hazards. | The activity of the mismatch repair enzyme (MRE) 1/programmed death ligand (PDL) is not a helpful part of routine diagnostic testing. |
| When treating patients who are older, in poor performance status, or who have concerns regarding toxicity or prognosis, hypofractionated RT alone or TMZ alone are reasonable options. | Epidermal growth factor receptor (EGFR) amplification may be useful for diagnosis if it has never been tested before. |
| No therapeutic approach is recommended or discouraged; instead, if at all feasible, these patients should be directed to take part in a clinical study. | Patients who are interested in or eligible for clinical trials or molecularly guided treatment may have their large panel sequencing needs taken into consideration. |
| Treatment with TMZ may be beneficial, particularly if it is continued for more than five months after stopping the medication. | |
| When an aged patient’s MGMT promoter status is methylated, fotemustine is recommended. | |
| For adult patients, tumor treatment fields (TTFs) with additional chemotherapy may be taken into consideration. |
2.6. Role of Immunosuppressive Mechanism in Glioblastoma and Resistance to Immunotherapy
One typical feature of GBM that limits a favorable prognosis is recurrence. Not all patients had access to second-line therapy at this time (about 50% did not receive any treatment while their condition progressed) [56,57]. Numerous studies demonstrate that GBM is associated with an immunosuppressive microenvironment as a result of an increase in factors generated by tumor cells, including FASL, PD-1, indolamine 2, 3dioxygenase (IDO), and STAT3. Additionally, microglia cells have the ability to create IL-1 and TGF-B, which in turn regulate local myeloid and lymphatic immune cells and support systemic immunosuppression [58]. By modifying the expression of several extracellular and intracellular mediators, myeloid cells promote the tumor by ensuring an immunosuppressive microenvironment [59]. These variables all alter the cytotoxic T lymphocyte (CTL) phenotype, which raises the quantities of immunosuppressive markers like PD-1. Several research aims to boost anticancer immune responses by utilizing these approaches. For example, vaccination therapy or anti-PD-1 and anti-CTLA-4 treatments are used to kill tumor cells that have GBM-associated antigens like EGFRvIII [60]. On the other hand, a treatment called viral oncolytic therapy applies a virus that can stimulate the immune system against the tumor. Attenuated oncolytic viruses propagate into tumor cells by taking advantage of the absence of a viral defense system [61].
Although they induce inflammation, elevated intracranial pressure, and CNS neurotoxicity, CAR T lymphocytes—modified chimeric antigen receptor T cells—offer an additional experimental strategy to elicit the anticancer immune response. As a result, this treatment approach is extremely constrained and intricate [62,63,64].
The poor immunogenicity of GBM and the abundance of immunosuppressive stresses in the microenvironment are the causes of resistance to immunotherapies [65].
In recent times, the Hippo pathway has been extensively researched as a molecular mechanism to regulate angiogenesis, invasion, migration, and proliferation of tumors. Numerous investigations demonstrate that YAP may establish contact between immune cells and tumors, especially with TAMs [66]. Indeed, the recruitment and activation of many inflammatory cytokines, such as IL-6, which regulate the tumor immune response and tumor development, is made possible by the presence of YAP in the nucleus. Moreover, TAMs seen in gliomas generate and secrete IL-6, which has the ability to stimulate the growth of glioma stem cells and trigger the build-up of TAMs in a feed-forward loop.
Consequently, the discovery of the Hippo pathway molecular target and viral oncolytic therapeutics might lessen GBM’s immunosuppressive and chemoresistance characteristics, making the molecular route of interest for research purposes.
3. Novel Oncolytic Viral Therapy for Treatment of Glioblastoma
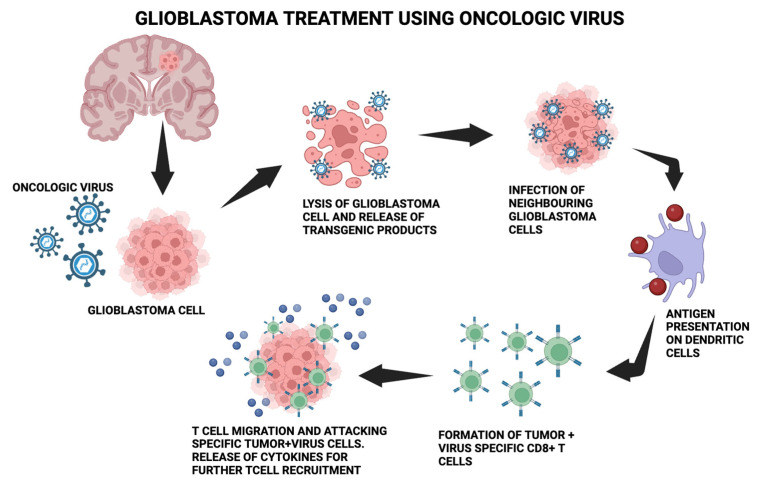
OVs are useful in treating GB because of their ability to replicate virally quickly in rapidly proliferating cells, their absence of distant metastases, and their alignment with the brain environment [67,68]. The anticancer immune response begins by converting “cold tumors” that are immunosuppressed by the microenvironment into “hot tumors” that are sensitive to the immune system [69,70,71,72]. Inducing an immunological response to inadvertently kill cancer cells through several mechanisms, including apoptosis, necrosis, and autophagy, is known as immunogenic cell death (ICD) [73,74,75]. Releases of damage-associated molecular patterns (DAMPs), viral pathogen-associated molecular patterns (PAMPs), tumor-associated antigens (TAAs), and several other cytokines are indicative of this [76,77]. Oncolytic viruses enhance the function of antigen-presenting cells (APCs), which reach lymph nodes to recruit cytotoxic CD8+ T lymphocytes (CTLs) and attract them to the infection site, where they destroy tumor-inducing cells [78,79,80]. Figure 1 depicts the molecular process mentioned.
Figure 1.
The molecular process behind using the oncologic virus for glioblastoma treatment. The oncolytic virus is introduced into glioblastoma cells, which causes lysis and the release of transgenic products. Neighboring glioblastoma cells get infected, leading to antigen presentation on dendritic cells and further leading to the formation of tumor + virus-specific CD8+T cells. T-cell migration and attacking of specific tumor + virus cells occurs.
Virotherapy is currently thought to be a promising immunotherapy for GB. The two types of OVs are replication-competent OVs, which only reproduce in cancer cells, and replication-deficient viral vectors, which are employed as carriers for additional therapeutic genes. Viruses that are produced through genetic engineering and naturally occurring viruses make up the first group [81,82]. The first group includes Newcastle disease viruses (NDV), reoviruses, and parvoviruses. The genetic modification of adenoviruses (Ad), herpes simplex virus (HSV), vaccine viruses (VV), vesicular stomatitis viruses (VSV), polioviruses, and measles viruses (MV) can decrease their pathogenicity and increase their tumor selectivity. To facilitate virus propagation and subsequent stimulation of the antitumor immune response, certain OVs use specialized receptors expressed on tumor cells.
In clinical studies for the treatment of GB, more than 20 oncolytic viruses have been examined. HSV-1 [83,84,85], adenovirus [86], reovirus [87], MVs [88,89], NDVs [90], and poliovirus [91] are a few of them. Novel techniques for OV distribution are being developed to overcome the BBB limitation. One such technique is the convection-enhanced distribution (CED) of the recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO) [92]. CED is a cutting-edge novel technology that transfers therapeutic chemicals in the interstitial regions of the CNS using a pressure gradient in a catheter [92]. For virotherapy to be successful, oncolytic viruses must be delivered effectively and safely. Intratumoral delivery was selected as the main method due to the difficulty of delivering viruses to the CNS and the immune system’s ability to eliminate them [93]. However, it is preferable for the oncolytic virus to be administered consistently to both primary and metastatic tumor locations [94]. Thankfully, glioblastoma seldom spreads metastatically outside of the central nervous system [95]. The oncolytic viral studies and their results are described in Table 2.
Table 2.
Oncolytic viruses in clinical trials for GB patients [96].
| Oncolytic Virus | Outcomes |
|---|---|
| HSV-1 MVR-C5252 (C5252) that has been genetically altered | Safety and tolerability dose-limiting toxicities (DLT) and maximum tolerated dose (MTD) |
| Modified genetically HSV-1 M032 | MTD |
| G207 administered once via catheters into tumors | Safety and tolerability |
| Oncolytic viral vector rQNestin34.5v.2 HSV | MTD |
| DNX-2440, a genetically modified adenovirus | Safety, overall survival, and objective response rate |
| Adenoviral Nsc-crad-s-pk7 | - |
| Adenovirus DNX-2401 | MTD and incidence of adverse event |
| H-1 parvovirus (H-1PV) | Safety and tolerability |
| PVSRIPO, a recombinant nonpathogenic polio-rhinovirus chimera, is injected into a tumor via CED | MTD, dose-limiting toxicities, recommended phase 2 dose |
| PVSRIPO | 14 days following PVSRIPO therapy, toxicity |
| PVSRIPO delivered into a tumor via CED | objective radiographic response, duration of objective radiographic response at 24 and 36 months |
| Live, replication-competent wild-type reovirus REOLYSIN | MTD, DLTs, and six-month response rate |
| Combination of modified vaccinia virus TG6002 and 5-FC | DLTs and the six-month course of the tumor |
3.1. DNA Viruses
3.1.1. Herpes Simplex Virus Type I
The HSV-based oncolytic virus was the first modified viral strain assessed for experimental treatment in a murine GB model [97]. A double-stranded DNA virus belonging to the Herpesviridae family, HSV-1 has double-stranded DNA. Nectin-1, a cell surface protein that is widely expressed in GB, serves as a binding site for HSV-1 [98]. The US Food and Drug Administration (FDA) approved talimogene laherparepvec (T-VEC) in 2015 to treat advanced, unresectable melanoma that is not amenable to surgery [98,99]. T-VEC expresses granulocyte-macrophage colony-stimulating factor (GM-CSF). GB patients are participating in clinical studies with many modified HSV constructs, including G207, HSV-1716, M032, and MVR-C252. Tumor selectivity was improved in all HSV recombinants by removing both copies of the RL1 gene, which codes for the viral protein (ICP34.5 producing neurovirulence) [100,101]. HSV G207, a recombinant strain with a malfunctioning viral ribonucleotide reductase (RR), was unable to reproduce in healthy cells. By producing the homologic gene, tumor cells make up for the loss of RR [102]. Third-generation oncolytic HSV-1 strain G47, sometimes referred to as DELYTACT, has demonstrated encouraging results in phase II clinical studies [103]. G47 was created by modifying the ICP47 gene with a deletion to improve the immune system’s ability to recognize tumors utilizing MHC class I [103]. For 19 adult patients with glioblastoma that was either residual or recurrent, a phase II single-arm study using G47Δ was initiated. Up to six intratumoral doses of G47Δ were given. A one-year survival rate of 84.2% indicates that the stated endpoint was achieved. The most frequent side effect was fever in 17 out of 19 patients. The patients’ biopsies revealed CD4+/CD8+ cells that had infiltrated the tumors [104]. A second phase I/II study of G47 in patients with recurrent or progressive glioblastoma found a median overall survival of 7.3 months and a 38.5% one-year survival rate [105]. This research led to the conditional approval of G47Δ in Japan in 2021. Tumor-specific promotors (such as Nestin-1) are used in the recombinant virus rQNestin34.5 to control ICP34.5 expression for enhanced cytolytic activity in tumor cells [106]. M032 is a recombinant HSV that produces human interleukin 12 (IL-12) to increase interferon-gamma (IFN-) production and anticancer activities [107]. The ICP6 and ICP34.5 genes have been deleted in NG34, a novel variant of rQNestin34.5 produced by the oncolytic HSV (oHSV). Although less hazardous in vivo than its predecessor, NG34 demonstrated comparable efficacy [108]. Another oHSV, rRp450, has an insertion of CYP2B1 and a deletion of ICP6, making it possible to activate the prodrug cyclophosphamide (CP) [109]. This virus boosted effectiveness and survival in tumor-bearing mice once the CP agent was introduced. OV-CDH1 is a modified herpes simplex virus (HSV) that produces E-cadherin to improve viral proliferation in tumors by increasing the oncolytic impact and reducing NK-mediated immunity in the infected cells [110,111]. By producing matrix metalloproteinase 9 (MMP), which targets the EGFRvIII mutant antigen specific to the tumor, another oHSV promotes the tumor’s spread [112]. Furthermore, this virus possesses a unique recognition site for the miR-124 miRNA, which enhances the miRNA’s selectivity for tumor brain cells and suppresses the vital viral protein ICP4 in healthy glial cells [113]. The Flt3L-expressing oHSV demonstrated complete GB clearance in preclinical studies [114]. Another oHSV that produces TRAIL, a protein that triggers TNF-CD95L and encourages apoptosis, exhibits a cytotoxic impact in GB models in mice with prolonged survival rates [115]. It was possible to reverse the effects of oHSV treatment with PD-1 antibody against GB mice models [116]. Whether administered intravenously or intratumorally, there are currently several flaws in the use of ohsv that restrict its therapeutic impact. Although it is difficult for virus particles to move to the lesion region outside of the injection place, intramoral injection can guarantee that they reach the lesion directly. The virus may infect all cancer cells by intravenous injection, which is very useful for treating metastatic lesions.
3.1.2. Adenovirus
Adenoviruses are non-enveloped, double-stranded DNA viruses that have an icosahedral shape [117]. Conditionally replicative adenoviruses (CRads) have been developed in a number of variations and have demonstrated promising anti-GB activity in clinical settings [118]. Furthermore, individuals with GB alone or in conjunction with other ICIs are now undergoing clinical studies with a genetically engineered adenovirus (Table 1). Reportedly, malignant gliomas have been effectively treated with an alternative gene-mediated cytotoxic treatment approach [119]. The phase II clinical study employed adenovirus glatimagene besadenovec (AdV-tk), which possesses the HSV thymidine kinase gene and kills cancer cells upon interacting with alacyclovir [120]. Removing the viral replication genes is one way to stop off-targets in normal cells, which can still proliferate in tumor cells. China has approved H101 (Oncorine), which is similar to oncolytic adenovirus ONYX-015, for the treatment of head and neck cancer [121,122]. A loss in the E1B-55K gene limited the recombinant adenovirus ONYX-015 oncolytic’s capacity to replicate to tumors with p53 abnormalities [123]. Two genetic changes were found in DNX-2401, an OV based on serotype 5 Ad (Ad5) [124]. When the E1A gene is removed and an RGD-4C motif is added to the fiber’s HI loop, the virus switches to replicate in cells with impaired pRB pathways that generate v3- and v5-integrins, both of which are indicators of glioma cells [125]. Due to this alteration, adenoviruses may infiltrate cells even in the presence of trace levels of their major receptor, the cox-sackie-adenovirus receptor, on brain tumor cells [126]. The OX40L gene is produced by the second generation of DNX-2401, DNX-2440 (also known as Delta-24-RGDOX), to improve T-cell-mediated immunity by encouraging the proliferation of CD8+ specific-tumor T cells [127,128]. To further stimulate T cells, the oncolytic adenovirus Delta-24-ACT produces the 4-1BB ligand in animal glioma models [129]. With the help of glucocorticoid-induced TNFR-family-related gene ligand (GITRL), another oncolytic adenovirus called Delta-24-RGD was able to increase mice survival and stop further exposure to glioma cells [130]. Furthermore, utilizing an alternative oncolytic adenovirus that expresses the co-stimulator OX40 ligand (OX40L) resulted in the activation of cancer-specific immunity in vivo as well as the expansion of CD8+ T cells [131]. Although it is still in its early stages, the clinical usage of treatments based on oncolytic adenoviruses has shown great promise. It is particularly appealing in the therapeutic setting due to its broad compatibility with currently approved medicines (without increasing toxicity), its various cell-killing activities, and its ability to induce immunogenic cell death.
3.1.3. Parvoviruses
The Parvoviridae family of single-stranded icosahedral DNA viruses includes parvoviruses. Various animal species can be infected by one of about 134 different parvovirus serotypes [132]. A small oncolytic virus called H-1 parvovirus has shown anticancer efficacy against GB [133]. Additionally, H-1PV causes glioma cells to undergo apoptosis and breaks down their resistance to a number of chemotherapeutic drugs [134]. Human U87-MG glioma models in rats showed tumor shrinkage in preclinical studies with the H-1PV [135]. As a result, the ParvOryx01 trial for individuals with recurrent GB (NCT01301430) was started. ParvOryx01 proposed that tumor-infiltrating lymphocytes (TILs) were responsible for inducing immune responses in the removed tumor tissues of GB patients [136]. In high-grade human gliomas, radiation promotes H-1PV viral oncolysis, which may be considered in animal glioma models [119]. Bevacizumab with H-1PV improved the mean survival to 15.4 months in five patients with recurrent GB and produced remission in three of them [137]. These results are associated with the synergistic effect of bevacizumab and H-1PV in controlling GB TME and decreasing VEGF [138]. The first clinical evidence of using H-1PV in combination with bevacizumab and an immune checkpoint inhibitor (nivolumab) was shown in a multimodal clinical study including three patients with recurrent GB. Every participant achieved clinical improvement and confirmed tumor shrinkage, with 78% of cases showing complete or partial remission [139]. All of these results suggest that parvoviruses may be useful in immunotherapies against GB. New clinical trials have confirmed PVs’ safety and tolerability and offered a proxy for confirmation of therapy effectiveness for cancer. Despite the hopeful nature of these data, patient responses following treatment with H-1PV did not match the remarkable outcomes shown in preclinical assessment. Consequently, oncolytic PV treatment still has to be optimized and developed further. Like any medication, oncolytic PVs need a suitable economic climate for clinical study, which is now a significant roadblock in their development.
3.1.4. Myxoma Virus
A part of the poxvirus family with double-stranded DNA is the myxoma virus (MYXV) [140,141]. MYXV can cause an oncolytic impact when it replicates in cells like GB that lack an interferon system [142]. The deletion of the viral antiapoptotic protein M011L in the M011L-deficient MYXV virus boosted apoptosis in malignant glioma cells [143]. A prospective candidate OV that has shown promise in several preclinical cancer models is MYXV. Furthermore, MYXV is an appealing OV platform due to its remarkable safety profile outside of rabbits, its extremely selective tropism for a wide variety of cancer cell types, and the limitation of viral multiplication in original non-transformed human cells.
3.1.5. Vaccinia Virus (VV)
The Poxviridae family includes the double-stranded DNA virus known as a vaccine. Smallpox was eradicated with the aid of VV. Because VV may infect any kind of cell by membrane fusion with a non-integrative replication cycle, it is a suitable platform for oncolytic viral engineering against GB [144]. The only recombinant VV that has shown therapeutic benefits in brain tumor patients is TG6002 [145]. The TG6002 genome contains two additional gene deletions for the RR and thymidine kinase (TK) genes. By introducing the FCU1 gene, the chemotherapy prodrug 5-flucytosine (5-FC) was also converted into 5-fluorouracil (5-FU) [146]. Prior research has demonstrated that systemic PD-1 blockade medication and local injection of oncolytic VV together are more effective than either treatment alone. As an alternative, oncolytic VV or HSV that was engineered to express PD-1 blockades demonstrated an anticancer effect comparable to that of using OV and PD-1 blockades together. In this work, the authors showed for the first time that arming VV with a scFv against TIGIT dramatically improved the parental VV’s antitumor effectiveness by altering the TME’s immunological state. For the first time, it was shown that the antitumor effectiveness of VV equipped with the scFv against TIGIT was further increased by the additional combination of PD-1 or LAG-3 inhibition.
3.2. RNA Viruses
3.2.1. Measles Virus
The measles virus (MV) is a single-stranded RNA virus with a negative sense that belongs to the Paramyxoviridae family [147]. The MV enters cells via engaging with the overexpressed CD46 cell receptor on tumor cells as well as the viral hemagglutinin (H) protein [148]. Recombinant MVs entered clinical trials after glioma xenografts showed strong anticancer efficacy in them [149,150]. To monitor viral expression in cells, such recombinants express the human sodium iodide symporter (NIS) or the human carcinoembryonic antigen (CEA) [151]. NIS allows for the monitoring of viruses using various isotopes and has the potential to enhance viral cytopathogenic effects [152,153]. The present studies deliver the maximum practicable dosages as a result of the observed dose–response correlations. Even with the introduction of technologies like synthesis in serum-free cell culture, tangential flow filtration, and diafiltration, the necessary high-titer, highly purified good manufacturing practice (GMP)-grade recombinant MV remains difficult to produce on a wide scale. However, considering the diversity of MV as a platform for oncolytic vectors, the high safety record of MV vaccinations, the genetic stability and biosafety profile of recombinant MV, and the biosafety profile of MV, these efforts appear to be justified.
3.2.2. Vesicular Stomatitis Virus (VSV)
The VSV is a single-stranded, negative-sense RNA virus that belongs to the Rhabdoviridae family. The spike glycoprotein (G) of the VSV is linked to the low-density lipoprotein receptor (LDL-R), a cell receptor that is broadly dispersed [137]. The VSV is employed as an oncolytic drug against various malignancies by replicating in tumor cells via the abnormalities in their interferon system [138,139]. rVSV (GP) and VSV-EBOV are terms for engineered VSVs that have had the envelope glycoprotein (GP) substituted with GP from the Ebola virus and the non-neurotropic lymphocytic choriomeningitis virus, respectively [140,141]. Despite entering a phase I clinical trial, an oncolytic VSV is suppressed by viral-mediated production of interferon (IFN)β, which has been demonstrated to increase the virus’s safety. Other methods to increase the safety of VSV include changing the tropism by pseudotyping with a heterologous virus’s glycoprotein. In light of this, it has been demonstrated that rVSV-GP, a pseudotyped vaccine vector containing the glycoprotein of the lymphocytic choriomeningitis virus, is both safe and effective.
3.2.3. Reoviruses
When the Ras-signaling pathway is triggered in glioma cells, reoviruses—double-stranded RNA non-enveloped viruses—can multiply in the cells [139]. Reovirus RNA genome mutations occur quite quickly. This offers a degree of flexibility that may be used to choose reovirus variants with higher levels of oncolytic activity. The reovirus genome may also be genetically altered, providing further possibilities for boosting the oncolytic activity. One such method is the insertion of tiny therapeutic transgenes [139]. Reoviruses having the benefit of not being linked to any severe human diseases, and there are now more and more clinical trials including the use of reovirotherapy to treat cancer. With the modest effectiveness of reovirus as a monotherapy, the emphasis has shifted to combination regimens thus far. Apart from genetic alteration, conventional bioselection is an additional mechanism that may be employed to augment the oncolytic capabilities of reoviruses. Recognizing that reoviruses are not oncolytic agents is a positive thing. Thus, the reovirus’s capacity for environmental adaptation may aid in the selection of more potent forms. The jin reoviruses are one type of such mutation. The wild-type reovirus was driven to evolve a way around the JAM-A reliance due to its extended proliferation on cells lacking JAM-A on their surface [139]. This idea can be investigated for tumor forms that are resistant to infection by reoviruses at other phases of the viral replication cycle, including cell lysis or endosomal escape. Certain malignancies have developed defense mechanisms against cancer treatments that depend on inducing apoptosis and avoiding signaling pathways involved in cell death.
3.2.4. Newcastle Disease Virus (NDV)
The Paramyxoviridae family includes the negative-sense, single-stranded RNA-enveloped NDV virus [137]. Interferon-stimulated genes (ISGs) are expressed by the NDV, which is largely an avian virus that preferentially replicates in tumor cells and triggers the type I interferon response in humans [148,149]. Studies suggest that NDV may be useful against GB [150]. The highly contagious avian disease NDV is a member of the Paramyxoviridae family of viruses. It results in a sickness that causes large financial losses and for which the World Organization for Animal Health (OIE) must be notified in writing. NDV is also recognized as an oncolytic virus, able to cause organelle-specific autophagy in the Golgi apparatus, peroxisomes, and mitochondria while also replicating specifically in tumor cells. NDV is a useful model for future cell biology studies because of this. The authors postulated that SIRT3 can function as a crucial gatekeeper for the balance between glycolytic and mitochondrial oxidative metabolism in the control of energy production for viral replication because of its critical involvement in cell metabolism [150].
3.2.5. Seneca Valley Virus Isolate 001 (SVV-001)
A member of the Picornaviridae family of positive-sense single-stranded RNA, the SVV-001 [151] has shown oncolytic activity against solid tumors, with a particular affinity for cells expressing the endothelium receptor TEM8/ANTXR1 [152]. The transmembrane glycoprotein adhesion molecule TEM8/ANTXR1 is more prevalent in some cancer types and mediates cell motility and its interactions with the extracellular matrix (ECM) [153]. TEM8/ANTXR1 is the first biomarker for SVV-based oncolytic viral treatment [154]. SVV-001 given intravenously has anticancer properties and is able to pass the BBB [155]. As the first oncolytic virus ever tested in children globally, SVV-001 is also the first to be examined in a phase I study for recurrent/refractory cancers in both adults and pediatrics. The use of this virus in tumors showing neuroendocrine traits is supported by favorable preclinical evidence in SCID mice; however, objective clinical responses were absent in the phase I studies conducted in adults and children. Considering how powerful this virus was when it first emerged in SCID mice models, completely eliminating tumors with a single SVV-001 injection, this is a little unexpected. Given that these mice were Rag2 SCID models, they were severely immunocompromised and hence unable to establish an immune response involving T-regulatory cells and/or create neutralizing antibodies against SVV-001, as shown in human investigations. This may have contributed to some of the observed effects. Nevertheless, without the need to determine the highest dosage that may be tolerated, systemic delivery of the virus did show promise for safety in both pediatric and adult populations.
3.2.6. Polioviruses
The Picornaviridae family of positive-sense single-strand RNA viruses includes polioviruses [156]. The CD155/PVR receptor, which is typically overexpressed on cancerous cells, is used by polioviruses to infect cells [156].
The internal ribosome entry site (IRES) of an attenuated poliovirus type 1 (Sabin) vaccination strain was substituted with an IRES from a human rhinovirus type 2 in order to reduce the potential neurovirulence [157,158]. In the phase I study (NCT01491893) looking at the intratumoral CED of PVSSRIPO in patients with recurrent GB, the safety and absence of neurovirulence were established. Consequently, the PVSRIPO was granted a breakthrough therapeutic classification by the FDA in 2016 [159]. Additionally, the figures demonstrate that the trial’s survival rate was higher than that of the historical controls, with rates at 24 and 36 months rising by 21%. We anxiously await the results of a phase II study (NCT02986178) investigating PVSRIPO alone or in combination with lomustine in patients with GB [159].
Human rhinovirus type 30 has been inserted into the IRES of the novel recombinant poliovirus type 3 vaccination strain RVP3, which replicates only in tumor cells and leaves healthy cell lines unaffected [160]. On primary glioma cells from various patients as well as various glioma models, RVP3 demonstrated oncolytic efficacy [161].
A study carried out in this patient group, assessing intratumoral convection-enhanced transport of the recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO), found that all 61 patients who got PVSRIPO had a median overall survival of 12.5 months, longer than the historical control group’s 11.3 months [162].
3.2.7. Sindbis Virus
The Togaviridae family of positive-sense single-stranded RNA viruses includes the Sindbis virus [163]. Via binding to the laminin receptor (LAMR), Sindbis infects cancer cells and causes death in glioma cells [164,165] via tyrosine phosphorylating protein kinase C delta. The Semliki forest virus (SFV4miRT) contains target sequences for miR124, miR125, and miR134 inserted into it; it is expressed more in healthy CNS cells than in glioma cells [166]. As a result, this virus has a decreased neurotropism, oncolytic effectiveness, and safer profile [167,168]. According to recently published research [169,170], the Zika virus (ZIKV) can infect GB stem cells (GSCs) and exhibit oncolytic activity on them.
This suggests that modifying ZIKV to more precisely target GB in the absence of normal neuronal cells may enhance treatment results [171,172]. ZIKV-LAV was created by a 10-nucleotide deletion in the 3′ untranslated region (3-UTR) of the genome. It exhibits lower neurovirulence and higher GB oncolytic activity [173,174].
Clinical studies for GB have typically demonstrated that oncolytic viruses are safe and efficient against glioma cells, albeit few of these trials have progressed to phase III. Sitimagene ceradenovec, an adenoviral vector expressing the HSV thymidine kinase gene, was studied in the phase III clinical study “ASPECT” in combination with intravenous ganciclovir. However, there was no appreciable effect on overall survival [175]. A phase III trial for Toca511, a retroviral vector containing the gene for cytosine deaminase (CD), has begun. Toca511’s CD gene transforms the cancer-killing compound 5-flucytosine into 5-fluracil [176,177]. This trial was also stopped for undisclosed reasons. It is crucial to remember that while OVs have been shown to be safe and beneficial in preclinical research, clinical efficacy has not yet attained the intended degree [178,179]. Table 3 discusses all the oncolytic viruses mentioned in this section along with their advantages.
Table 3.
Oncolytic viruses and their advantages.
| Oncolytic Virus | Advantages |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4. Discussion
One of the worst and most lethal diseases is still GB. The use of oncolytic viruses to treat and stimulate the immune system against GB has considerable promise. A number of issues need to be resolved in order to maximize the benefits of OVs. Considerations include the OV’s ability to multiply and infect tumor cells, the condition of the tumor microenvironment, the degree of immune cell infiltration, and the possibility of inducing an anticancer response. This study reviewed the present state of GB therapy alternatives, including oncolytic viruses and nanoparticles that are currently being utilized or studied in clinical studies. Virotherapy is regarded to be a promising immunotherapy for GB at the moment. There are two types of viral vectors (OVs): replication-competent OVs, which only multiply in cancer cells, and replication-deficient OVs, which are utilized as carriers for additional therapeutic genes. The first group consists of viruses created through genetic engineering and naturally existing viruses. Newcastle disease viruses (NDV), reoviruses, and parvoviruses comprise the first category. To increase tumor selectivity and decrease pathogenicity, genetic modifications can be made to adenoviruses (Ad), herpes simplex virus (HSV), vaccine viruses (VV), vesicular stomatitis viruses (VSV), polioviruses, and measles viruses (MV). Some OVs use specific receptors that are expressed on tumor cells to help spread the virus and then trigger the anti-tumor immune response.
Many OVs are now in clinical studies, and numerous factors, including viral delivery and appropriate dosage, are being investigated. Ex vivo customized models might be constructed to choose the best oncolytic virus for each patient. Several OVs will be evaluated in future clinical studies to see if they connect with tumor heterogeneity and immune state complexity. As new techniques for virotherapy are attempted, new monitoring and evaluation requirements should be implemented. Prospective research endeavors aimed at clarifying the process of OVs in various GB models ought to employ inventive genetic engineering techniques and viral delivery methods. In conclusion, although virotherapy alone may be beneficial, combining immunotherapy and oncolytic viral techniques with customized strategies may lead to a more successful course of treatment for individuals with GB.
5. Conclusions
We can safely state that oncolytic viral therapy is a well-established cancer treatment modality at this point. The efficacy of oncolytic virus therapy is anticipated to increase when coupled with immunotherapy since the common feature that plays a significant role in showing anticancer effects during oncolytic activities is the formation of specific antitumor immunity. Functional transgenes would enable oncolytic viruses to be equipped with a wide range of anticancer capabilities in the future. Based on the kind and stage of cancer, a combination of suitable viruses may then be selected from this panel. Oncolytic viral therapy appears to be the beginning of a new age in cancer treatment, where patients have the freedom to choose this treatment option. For the purpose of creating oncolytic viruses, a reiterative feedback loop—in which the outcomes of clinical trials inform and influence the design of succeeding generations of viruses—is preferred over a unidirectional method. Particularly with regard to virotherapy in the brain, preclinical laboratory research can only partially address the unique challenges this field faces. The biological effects of viruses vary greatly depending on the species under investigation, in contrast to small molecule therapies. Human viruses including poliovirus, AdV, and HSV are greatly attenuated in tumor models found in rodents, but they may be less so when administered to people. On the other hand, non-human infections including PRV, SIN, and VSV can be harmful to mice, which makes preclinical survival research extremely difficult. Oncolytic viruses are distinct from conventional medications in a number of ways. Since they are live viruses, their effective dosages may vary depending on how quickly they multiply in a therapeutic setting. Little information is currently known on the relationship between viral dosage, in vivo replicative capability, and treatment response. To create safe and effective dose guidelines, more research on viral replication and clinical response in pertinent preclinical models and clinical trials is necessary. In several clinical studies carried out across a wide spectrum of malignancies, oncolytic viruses have so far been linked to a generally acceptable safety profile. However, given these agents’ capacity for replication, infection control measures—such as proper handling, storage, preparation, and delivery of the virus—must be taken seriously. The actual risk of infection is contingent upon the type of virus, co-occurring medical problems in patients, close household contacts, and healthcare personnel who may come into touch with the virus. Furthermore, a lot of oncolytic viruses have recombinant DNA elements, and therefore there are theoretical worries about the possible effects of these gene segments and the possibility that they would recombine with wild-type viruses in the environment. A new level of safety concerns arises when these non-human viruses enter clinical trials because the effects of environmental contamination and transmission must be taken into account. Oncolytic viruses provide special manufacturing and regulatory challenges since they are live reproducing viruses. Since tissue cultures are used to proliferate most viruses, techniques for producing high-titer viruses, screening for adventitial infections, and evaluating virus purity and replication capacity are necessary. Therefore, it is necessary to take into account protocols for laboratory safety throughout the manufacturing and vialing processes, product validation and purity, and design quality that arises from producing biologics in cell culture. It has been difficult to produce the very high titer lysates needed for therapeutic dosage for some viruses, which can provide a problem for the biotechnology manufacturing industry. These elements have been examined and require more investigation. Oncolytic viruses, however, have been linked to a highly favorable risk-benefit ratio, and thus more research and development of this novel class of medications is expected, with a focus on combination therapies in particular. A novel class of medications is introduced by oncolytic viral immunotherapy, a very promising method for treating cancer patients.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ohgaki H., Kleihues P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 2.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger T.R., Wen P.Y., Lang-Orsini M., Chukwueke U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022;8:1493–1501. doi: 10.1001/jamaoncol.2022.2844. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert M.R., Wang M., Aldape K.D., Stupp R., Hegi M.E., Jaeckle K.A., Armstrong T.S., Wefel J.S., Won M., Blumenthal D.T., et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan A.C., Ashley D.M., López G.Y., Malinzak M., Friedman H.S., Khasraw M. Management of glioblastoma: State of the art and future directions. CA A Cancer J. Clin. 2020;70:299–312. doi: 10.3322/caac.21613. [DOI] [PubMed] [Google Scholar]
- 6.Davis M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016;20((Suppl. S5)):S2–S8. doi: 10.1188/16.CJON.S1.2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher J.P., Adamson D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines. 2021;9:324. doi: 10.3390/biomedicines9030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander B.M., Cloughesy T.F. Adult Glioblastoma. J. Clin. Oncol. 2017;35:2402–2409. doi: 10.1200/JCO.2017.73.0119. [DOI] [PubMed] [Google Scholar]
- 9.Moore S.C., Rajaraman P., Dubrow R., Darefsky A.S., Koebnick C., Hollenbeck A., Schatzkin A., Leitzmann M.F. Height, Body Mass Index, and Physical Activity in Relation to Glioma Risk. Cancer Res. 2009;69:8349–8355. doi: 10.1158/0008-5472.CAN-09-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph G.P., McDermott R., Baryshnikova M.A., Cobbs C.S., Ulasov I.V. Cytomegalovirus as an Oncomodulatory Agent in the Progression of Glioma. Cancer Lett. 2017;384:79–85. doi: 10.1016/j.canlet.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Rice T., Lachance D.H., Molinaro A.M., Eckel-Passow J.E., Walsh K.M., Barnholtz-Sloan J., Ostrom Q.T., Francis S.S., Wiemels J., Jenkins R.B., et al. Understanding Inherited Genetic Risk of Adult Glioma—A Review. Neuro-Oncol. Pract. 2016;3:10–16. doi: 10.1093/nop/npv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L., et al. Single-Cell RNA-Seq Highlights Intratumoral Heterogeneity in Primary Glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razavi S.M., Lee K.E., Jin B.E., Aujla P.S., Gholamin S., Li G. Immune Evasion Strategies of Glioblastoma. Front. Surg. 2016;3:11. doi: 10.3389/fsurg.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutoit V., Migliorini D., Dietrich P.Y., Walker P.R. Immunotherapy of Malignant Tumors in the Brain: How Different from Other Sites? Front. Oncol. 2016;6:256. doi: 10.3389/fonc.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao S., Wu Q., McLendon R.E., Hao Y., Shi Q., Hjelmeland A.B., Dewhirst M.W., Bigner D.D., Rich J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 16.Liu G., Yuan X., Zeng Z., Tunici P., Ng H., Abdulkadir I.R., Lu L., Irvin D., Black K.L., Yu J.S. Analysis of Gene Expression and Chemoresistance of CD133+ Cancer Stem Cells in Glioblastoma. Mol. Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harder B.G., Blomquist M.R., Wang J., Kim A.J., Woodworth G.F., Winkles J.A., Loftus J.C., Tran N.L. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front. Oncol. 2018;8:462. doi: 10.3389/fonc.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A.J.R., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L., et al. Signatures of Mutational Processes in Human Cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodges T.R., Ott M., Xiu J., Gatalica Z., Swensen J., Zhou S., Huse J.T., de Groot J., Li S., Overwijk W.W., et al. Mutational Burden, Immune Checkpoint Expression, and Mismatch Repair in Glioma: Implications for Immune Checkpoint Immunotherapy. Neuro-Oncology. 2017;19:1047–1057. doi: 10.1093/neuonc/nox026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLendon R., Friedman A., Bigner D., van Meir E.G., Brat D.J., Mastrogianakis G.M., Olson J.J., Mikkelsen T., Lehman N., Aldape K., et al. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terrível M., Gromicho C., Matos A.M. Oncolytic Viruses: What to Expect from Their Use in Cancer Treatment. Microbiol. Immunol. 2020;64:477–492. doi: 10.1111/1348-0421.12753. [DOI] [PubMed] [Google Scholar]
- 22.Phillips H.S., Kharbanda S., Chen R., Forrest W.F., Soriano R.H., Wu T.D., Misra A., Nigro J.M., Colman H., Soroceanu L., et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Parsons D.W., Jones S., Zhang X., Lin J.C., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.M., Gallia G.L., et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alifieris C., Trafalis D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015;152:63–82. doi: 10.1016/j.pharmthera.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Wilson T.A., Karajannis M.A., Harter D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014;5:64. doi: 10.4103/2152-7806.132138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young R.M., Jamshidi A., Davis G., Sherman J.H. Current trends in the surgical management and treatment of adult glioblastoma. Ann. Transl. Med. 2015;3:121. doi: 10.3978/j.issn.2305-5839.2015.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moton S., Elbanan M., Zinn P.O., Colen R.R. Imaging Genomics of Glioblastoma: Biology, Biomarkers, and Breakthroughs. Top. Magn. Reson. Imaging TMRI. 2015;24:155–163. doi: 10.1097/RMR.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 28.Cieslik M., Chinnaiyan A.M. Cancer transcriptome profiling at the juncture of clinical translation. Nat. Rev. Genet. 2018;19:93–109. doi: 10.1038/nrg.2017.96. [DOI] [PubMed] [Google Scholar]
- 29.Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q., Hu B., Hu X., Kim H., Squatrito M., Scarpace L., De Carvalho A.C., Lyu S., Li P., Li Y., et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32:42–56. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neftel C., Laffy J., Filbin M.G., Hara T., Shore M.E., Rahme G.J., Richman A.R., Silverbush D., Shaw M.L., Hebert C.M., et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178:835–849. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu K., Hu Y., Wu F., Guo Q., Qian Z., Hu W., Chen J., Wang K., Fan X., Wu X., et al. Surveying brain tumor heterogeneity by single-cell RNA-sequencing of multi-sector biopsies. Natl. Sci. Rev. 2020;7:1306–1318. doi: 10.1093/nsr/nwaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan J., Levitin H.M., Frattini V., Bush E.C., Boyett D.M., Samanamud J., Ceccarelli M., Dovas A., Zanazzi G., Canoll P., et al. Single-cell transcriptome analysis of lineage diversity in high-grade glioma. Genome Med. 2018;10:57. doi: 10.1186/s13073-018-0567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S., Kon M., De Lisi C. Pathway-based classification of cancer subtypes. Biol. Direct. 2012;7:21. doi: 10.1186/1745-6150-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellor S.V., Pagano-Young T.A., Avgeropoulos N.G. Glioblastoma: Background, standard treatment paradigms, and supportive care considerations. J. Law Med. Ethics A J. Am. Soc. Law Med. Ethics. 2014;42:171–182. doi: 10.1111/jlme.12133. [DOI] [PubMed] [Google Scholar]
- 36.Johnson D.R., Fogh S.E., Giannini C., Kaufmann T.J., Raghunathan A., Theodosopoulos P.V., Clarke J.L. Case-Based Review: Newly diagnosed glioblastoma. Neuro-Oncol. Pract. 2015;2:106–121. doi: 10.1093/nop/npv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkataramani V., Yang Y., Schubert M.C., Reyhan E., Tetzlaff S.K., Wißmann N., Botz M., Soyka S.J., Beretta C.A., Pramatarov R.L., et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell. 2022;185:2899–2917.e31. doi: 10.1016/j.cell.2022.06.054. [DOI] [PubMed] [Google Scholar]
- 38.Moraes F.Y., Lo A., Morgan E.R., Millar B.A., Shultz D.B., Maurice C., Harlos C., Kongkham P., Bernstein M., Zadeh G., et al. Management and Outcomes in the Oldest-Old Population with Glioblastoma. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2018;45:199–205. doi: 10.1017/cjn.2017.278. [DOI] [PubMed] [Google Scholar]
- 39.Lobera A. Imaging in Glioblastoma Multiforme. 2015. [(accessed on 14 June 2022)]. Available online: http://emedicine.medscape.com/article/340870-overview.
- 40.Halani S.H., Babu R., Adamson D.C. Management of Glioblastoma Multiforme in Elderly Patients: A Review of the Literature. World Neurosurg. 2017;105:53–62. doi: 10.1016/j.wneu.2017.04.153. [DOI] [PubMed] [Google Scholar]
- 41.Perry J., Zinman L., Chambers A., Spithoff K., Lloyd N., Laperriere N., Neuro-oncology Disease Site Group. Cancer Care Ontario’s Program in Evidence-Based Care The use of prophylactic anticonvulsants in patients with brain tumours—A systematic review. Curr. Oncol. 2006;13:222–229. doi: 10.3747/co.v13i6.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiff D., Lee E.Q., Nayak L., Norden A.D., Reardon D.A., Wen P.Y. Medical management of brain tumors and the sequelae of treatment. Neuro-Oncology. 2015;17:488–504. doi: 10.1093/neuonc/nou304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glantz M.J., Cole B.F., Forsyth P.A., Recht L.D., Wen P.Y., Chamberlain M.C., Grossman S.A., Cairncross J.G. Practice parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neurology. 2000;54:1886–1893. doi: 10.1212/WNL.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 44.Blissitt P.A., American Association of Neuroscience Nurses Clinical practice guideline series update: Care of the adult patient with a brain tumor. J. Neurosci. Nurs. J. Am. Assoc. Neurosci. Nurses. 2014;46:367–368. doi: 10.1097/JNN.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 45.Nabors L.B., Portnow J., Ammirati M., Baehring J., Brem H., Brown P., Butowski N., Chamberlain M.C., Fenstermaker R.A., Friedman A., et al. Central Nervous System Cancers, Version 1.2015. J. Natl. Compr. CancerNetw. JNCCN. 2015;13:1191–1202. doi: 10.6004/jnccn.2015.0148. [DOI] [PubMed] [Google Scholar]
- 46.Nowak B., Rogujski P., Janowski M., Lukomska B., Andrzejewska A. Mesenchymal stem cells in glioblastoma therapy and progression: How one cell does it all. Biochim. Et Biophys. Acta. Rev. Cancer. 2021;1876:188582. doi: 10.1016/j.bbcan.2021.188582. [DOI] [PubMed] [Google Scholar]
- 47.Kuhnt D., Becker A., Ganslandt O., Bauer M., Buchfelder M., Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro-Oncology. 2011;13:1339–1348. doi: 10.1093/neuonc/nor133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roder C., Bisdas S., Ebner F.H., Honegger J., Naegele T., Ernemann U., Tatagiba M. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: High-field iMRI versus conventional and 5-ALA-assisted surgery. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014;40:297–304. doi: 10.1016/j.ejso.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 49.Keles G.E., Anderson B., Berger M.S. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg. Neurol. 1999;52:371–379. doi: 10.1016/S0090-3019(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 50.Lacroix M., Abi-Said D., Fourney D.R., Gokaslan Z.L., Shi W., DeMonte F., Lang F.F., McCutcheon I.E., Hassenbusch S.J., Holland E., et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 51.Mukherjee D., Quiñones-Hinojosa A. Impact of extent of resection on outcomes in patients with high-grade gliomas. In: Hayat M.A., editor. Tumors of the Central Nervous System. Volume 2. Springer; San Francisco, CA, USA: 2011. pp. 173–179. [Google Scholar]
- 52.Stummer W., Pichlmeier U., Meinel T., Wiestler O.D., Zanella F., Reulen H.J., ALA-Glioma Study Group Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 53.Wollmann G., Ozduman K., van den Pol A.N. Oncolytic virus therapy for glioblastoma multiforme: Concepts and candidates. Cancer J. 2012;18:69–81. doi: 10.1097/PPO.0b013e31824671c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raja J., Ludwig J.M., Gettinger S.N., Schalper K.A., Kim H.S. Oncolytic virus immunotherapy: Future prospects for oncology. J. Immunother. Cancer. 2018;6:140. doi: 10.1186/s40425-018-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakomy R., Kazda T., Selingerova I., Poprach A., Pospisil P., Belanova R., Fadrus P., Vybihal V., Smrcka M., Jancalek R., et al. Real-World Evidence in Glioblastoma: Stupp’s Regimen After a Decade. Front. Oncol. 2020;10:840. doi: 10.3389/fonc.2020.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weller M., Cloughesy T., Perry J.R., Wick W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro-Oncology. 2013;15:4–27. doi: 10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gramatzki D., Dehler S., Rushing E.J., Zaugg K., Hofer S., Yonekawa Y., Bertalanffy H., Valavanis A., Korol D., Rohrmann S., et al. Glioblastoma in the canton of Zurich, Switzerland revisited, 2005 to 2009. Cancer. 2016;122:2206–2215. doi: 10.1002/cncr.30023. [DOI] [PubMed] [Google Scholar]
- 58.Jackson C.M., Kochel C.M., Nirschl C.J., Durham N.M., Ruzevick J., Alme A., Francica B.J., Elias J., Daniels A., Dubensky T.W., et al. Systemic tolerance mediated by melanoma brain tumors is reversible by radiotherapy and vaccination. Clin. Cancer Res. 2016;22:1161–1172. doi: 10.1158/1078-0432.CCR-15-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Topalian S.L., Taube J.M., Anders R.A., Pardoll D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jähnisch H., Füssel S., Kiessling A., Wehner R., Zastrow S., Bachmann M., Rieber E.P., Wirth M.P., Schmitz M. Dendritic cell-based immunotherapy for prostate cancer. Clin. Dev. Immunol. 2010;2010:517493. doi: 10.1155/2010/517493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aurelian L. Oncolytic viruses as immunotherapy, Progress and remaining challenges. OncoTargets Ther. 2016;9:2627–2637. doi: 10.2147/OTT.S63049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jena B., Dotti G., Cooper L.J. Redirecting t-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K., et al. Tandem CAR T cells targeting HER2 and IL13R_2 mitigate tumor antigen escape. J. Clin. Investig. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dapash M., Hou D., Castro B., Lee-Chang C., Lesniak M.S. The Interplay between Glioblastoma and Its Microenvironment. Cells. 2021;10:2257. doi: 10.3390/cells10092257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang W., Yang S., Zhang F., Cheng F., Wang X., Rao J. Influence of the Hippo-YAP signaling pathway on tumor associated macrophages (TAMs) and its implications on cancer immunosuppressive microenvironment. Ann. Transl. Med. 2020;8:399. doi: 10.21037/atm.2020.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartlett D.L., Liu Z., Sathaiah M., Ravindranathan R., Guo Z., He Y., Guo Z.S. Oncolytic viruses as therapeutic cancer vaccines. Mol. Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gujar S., Bell J., Diallo J.S. SnapShot: Cancer Immunotherapy with Oncolytic Viruses. Cell. 2019;176:1240–1240.e1. doi: 10.1016/j.cell.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 68.Angom R.S., Nakka N.M.R., Bhattacharya S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023;13:1536. doi: 10.3390/brainsci13111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asija S., Chatterjee A., Yadav S., Chekuri G., Karulkar A., Jaiswal A.K., Goda J.S., Purwar R. Combinatorial approaches to effective therapy in glioblastoma (GBM): Current status and what the future holds. Int. Rev. Immunol. 2022;41:582–605. doi: 10.1080/08830185.2022.2101647. [DOI] [PubMed] [Google Scholar]
- 70.Tahir M., Ahmad N., Lei D., Ali S. Emerging role of oncolytic viruses and stem cells in gene therapy: Should they be integrated? Drug Discov. Today. 2022;27:2244–2251. doi: 10.1016/j.drudis.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 71.Webb M.J., Sener U., Vile R.G. Current Status and Challenges of Oncolytic Virotherapy for the Treatment of Glioblastoma. Pharmaceuticals. 2023;16:793. doi: 10.3390/ph16060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang B., Li X., Li Y., Zhang J., Zong Z., Zhang H. Current Immunotherapies for Glioblastoma Multiforme. Front. Immunol. 2021;11:603911. doi: 10.3389/fimmu.2020.603911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahmoud A.B., Ajina R., Aref S., Darwish M., Alsayb M., Taher M., AlSharif S.A., Hashem A.M., Alkayyal A.A. Advances in immunotherapy for glioblastoma multiforme. Front. Immunol. 2022;13:944452. doi: 10.3389/fimmu.2022.944452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mihelson N., McGavern D.B. Viral Control of Glioblastoma. Viruses. 2021;13:1264. doi: 10.3390/v13071264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shoaf M.L., Desjardins A. Oncolytic Viral Therapy for Malignant Glioma and Their Application in Clinical Practice. Neurotherapeutics. 2022;19:1818–1831. doi: 10.1007/s13311-022-01256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamynina M., Tskhovrebova S., Fares J., Timashev P., Laevskaya A., Ulasov I. Oncolytic Virus-Induced Autophagy in Glioblastoma. Cancers. 2021;13:3482. doi: 10.3390/cancers13143482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qi Z., Long X., Liu J., Cheng P. Glioblastoma microenvironment and its reprogramming by oncolytic virotherapy. Front. Cell. Neurosci. 2022;16:819363. doi: 10.3389/fncel.2022.819363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haddad A.F., Young J.S., Mummaneni N.V., Kasahara N., Aghi M.K. Immunologic aspects of viral therapy for glioblastoma and implications for interactions with immunotherapies. J. Neuro-Oncol. 2021;152:1–13. doi: 10.1007/s11060-020-03684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu P., Wang Y., Wang Y., Kong Z., Chen W., Li J., Chen W., Tong Y., Ma W., Wang Y. Effects of oncolytic viruses and viral vectors on immunity in glioblastoma. Gene Ther. 2022;29:115–126. doi: 10.1038/s41434-020-00207-9. [DOI] [PubMed] [Google Scholar]
- 80.Ali S., Xia Q., Muhammad T., Liu L., Meng X., Bars-Cortina D., Khan A.A., Huang Y., Dong L. Glioblastoma Therapy: Rationale for a Mesenchymal Stem Cell-based Vehicle to Carry Recombinant Viruses. Stem Cell Rev. Rep. 2022;18:523–543. doi: 10.1007/s12015-021-10207-w. [DOI] [PubMed] [Google Scholar]
- 81.Blitz S.E., Kappel A.D., Gessler F.A., Klinger N.V., Arnaout O., Lu Y., Peruzzi P.P., Smith T.R., Chiocca E.A., Friedman G.K., et al. Tumor-Associated Macrophages/Microglia in Glioblastoma Oncolytic Virotherapy: A Double-Edged Sword. Int. J. Mol. Sci. 2022;23:1808. doi: 10.3390/ijms23031808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou C., Chen Q., Chen Y., Qin C.F. Oncolytic Zika Virus: New Option for Glioblastoma Treatment. DNA Cell Biol. 2023;42:267–273. doi: 10.1089/dna.2022.0375. [DOI] [PubMed] [Google Scholar]
- 83.Marelli G., Howells A., Lemoine N.R., Wang Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Front. Immunol. 2018;9:866. doi: 10.3389/fimmu.2018.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Markert J.M., Medlock M.D., Rabkin S.D., Gillespie G.Y., Todo T., Hunter W.D., Palmer C.A., Feigenbaum F., Tornatore C., Tufaro F., et al. Conditionally Replicating Herpes Simplex Virus Mutant, G207 for the Treatment of Malignant Glioma: Results of a Phase I Trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 85.Markert J.M., Liechty P.G., Wang W., Gaston S., Braz E., Karrasch M., Nabors L.B., Markiewicz M., Lakeman A.D., Palmer C.A., et al. Phase Ib Trial of Mutant Herpes Simplex Virus G207 Inoculated Pre-and Post-Tumor Resection for Recurrent GBM. Mol. Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Markert J.M., Razdan S.N., Kuo H.C., Cantor A., Knoll A., Karrasch M., Nabors L.B., Markiewicz M., Agee B.S., Coleman J.M., et al. A Phase 1 Trial of Oncolytic HSV-1, G207, given in Combination with Radiation for Recurrent GBM Demonstrates Safety and Radiographic Responses. Mol. Ther. 2014;22:1048–1055. doi: 10.1038/mt.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chiocca E.A., Abbed K.M., Tatter S., Louis D.N., Hochberg F.H., Barker F., Kracher J., Grossman S.A., Fisher J.D., Carson K., et al. A Phase I Open-Label, Dose-Escalation, Multi-Institutional Trial of Injection with an E1B-Attenuated Adenovirus, ONYX-015, into the Peritumoral Region of Recurrent Malignant Gliomas, in the Adjuvant Setting. Mol. Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 88.Kicielinski K.P., Chiocca E.A., Yu J.S., Gill G.M., Coffey M., Markert J.M. Phase 1 Clinical Trial of Intratumoral Reovirus Infusion for the Treatment of Recurrent Malignant Gliomas in Adults. Mol. Ther. 2014;22:1056–1062. doi: 10.1038/mt.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen C., Paraskevakou G., Iankov I., Giannini C., Schroeder M., Sarkaria J., Puri R.K., Russell S.J., Galanis E. Interleukin-13 Displaying Retargeted Oncolytic Measles Virus Strains Have Significant Activity Against Gliomas with Improved Specificity. Mol. Ther. 2008;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allen C., Opyrchal M., Aderca I., Schroeder M.A., Sarkaria J.N., Domingo E., Federspiel M.J., Galanis E. Oncolytic Measles Virus Strains Have Significant Antitumor Activity against Glioma Stem Cells. Gene Ther. 2013;20:444–449. doi: 10.1038/gt.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Freeman A.I., Zakay-Rones Z., Gomori J.M., Linetsky E., Rasooly L., Greenbaum E., Rozenman-Yair S., Panet A., Libson E., Irving C.S., et al. Phase I/II Trial of Intravenous NDV-HUJ Oncolytic Virus in Recurrent Glioblastoma Multiforme. Mol. Ther. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 92.Gromeier M., Lachmann S., Rosenfeld M.R., Gutin P.H., Wimmer E. Intergeneric Poliovirus Recombinants for the Treatment of Malignant Glioma. Proc. Natl. Acad. Sci. USA. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Desjardins A., Gromeier M., Herndon J.E., 2nd, Beaubier N., Bolognesi D.P., Friedman A.H., Friedman H.S., McSherry F., Muscat A.M., Nair S., et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018;379:150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehta A.M., Sonabend A.M., Bruce J.N. Convection-Enhanced Delivery. Neurotherapeutics. 2017;14:358–371. doi: 10.1007/s13311-017-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaufman H.L., Kim D.W., Deraffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and Distant Immunity Induced by Intralesional Vaccination with an Oncolytic Herpes Virus Encoding GM-CSF in Patients with Stage IIIc and IV Melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 96.Hsu E., Keene D., Ventureyra E., Matzinger M.A., Jimenez C., Wang H.S., Grimard L. Bone Marrow Metastasis in Astrocytic Gliomata. J. Neuro-Oncol. 1998;37:285–293. doi: 10.1023/A:1005909127196. [DOI] [PubMed] [Google Scholar]
- 97.Zadeh G., Lang F., Daras M., Cloughesy T., Colman H., Ong S., Ramakrishna R., Vogelbaum M., Groves M., Nassiri F., et al. ATIM-24. Interim results of a phase II multicenter study of the conditionally replicative oncolytic adenovirus DNX-2401 with pembrolizumab (keytruda) for recurrent glioblastoma; captive study (KEYNOTE-192) Neuro-Oncology. 2018;20:vi6. doi: 10.1093/neuonc/noy148.019. [DOI] [Google Scholar]
- 98.Hamad A., Yusubalieva G.M., Baklaushev V.P., Chumakov P.M., Lipatova A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses. 2023;15:547. doi: 10.3390/v15020547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martuza R.L., Malick A., Markert J.M., Ruffner K.L., Coen D.M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 100.Krummenacher C., Nicola A.V., Whitbeck J.C., Lou H., Hou W., Lambris J.D., Geraghty R.J., Spear P.G., Cohen G.H., Eisenberg R.J. Herpes Simplex Virus Glycoprotein D Can Bind to Poliovirus Receptor-Related Protein 1 or Herpesvirus Entry Mediator, Two Structurally Unrelated Mediators of Virus Entry. J. Virol. 1998;72:7064–7074. doi: 10.1128/JVI.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bommareddy P.K., Patel A., Hossain S., Kaufman H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017;18:1–15. doi: 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rehman H., Silk A.W., Kane M.P., Kaufman H.L. Into the Clinic: Talimogene Laherparepvec (T-VEC), a First-in-Class Intratumoral Oncolytic Viral Therapy. J. Immunother. Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andtbacka R.H.I., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S., et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 104.Orvedahl A., Alexander D., Tallóczy Z., Sun Q., Wei Y., Zhang W., Burns D., Leib D.A., Levine B. HSV-1 ICP34.5 Confers Neurovirulence by Targeting the Beclin 1 Autophagy Protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 105.Holman H.A., MacLean A.R. Neurovirulent Factor ICP34.5 Uniquely Expressed in the Herpes Simplex Virus Type 1 Δγ134.5 Mutant 1716. J. Neurovirol. 2008;14:28–40. doi: 10.1080/13550280701769999. [DOI] [PubMed] [Google Scholar]
- 106.Mineta T., Rabkin S.D., Yazaki T., Hunter W.D., Martuza R.L. Attenuated Multi–Mutated Herpes Simplex Virus-1 for the Treatment of Malignant Gliomas. Nat. Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 107.Taguchi S., Fukuhara H., Todo T. Oncolytic Virus Therapy in Japan: Progress in Clinical Trials and Future Perspectives. Jpn. J. Clin. Oncol. 2019;49:201–209. doi: 10.1093/jjco/hyy170. [DOI] [PubMed] [Google Scholar]
- 108.Todo T., Martuza R.L., Rabkin S.D., Johnson P.A. Oncolytic Herpes Simplex Virus Vector with Enhanced MHC Class I Presentation and Tumor Cell Killing. Proc. Natl. Acad. Sci. USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Todo T., Ito H., Ino Y., Ohtsu H., Ota Y., Shibahara J., Tanaka M. Intratumoral Oncolytic Herpes Virus G47∆ for Residual or Recurrent Glioblastoma: A Phase 2 Trial. Nat. Med. 2022;28:1630–1639. doi: 10.1038/s41591-022-01897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Todo T., Ino Y., Ohtsu H., Shibahara J., Tanaka M. A Phase I/II Study of Triple-Mutated Oncolytic Herpes Virus G47∆ in Patients with Progressive Glioblastoma. Nat. Commun. 2022;13:4119. doi: 10.1038/s41467-022-31262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kambara H., Okano H., Chiocca E.A., Saeki Y. An Oncolytic HSV-1 Mutant Expressing ICP34.5 under Control of a Nestin Promoter Increases Survival of Animals Even When Symptomatic from a Brain Tumor. Cancer Res. 2005;65:2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 112.Trinchieri G. Interleukin-12 and the Regulation of Innate Resistance and Adaptive Immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 113.Roth J.C., Cassady K.A., Cody J.J., Parker J.N., Price K.H., Coleman J.M., Peggins J.O., Noker P.E., Powers N.W., Grimes S.D., et al. Evaluation of the Safety and Biodistribution of M032, an Attenuated Herpes Simplex Virus Type 1 Expressing HIL-12, After Intracerebral Administration to Aotus Nonhuman Primates. Hum. Gene Ther. Clin. Dev. 2014;25:16–27. doi: 10.1089/humc.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nakashima H., Nguyen T., Kasai K., Passaro C., Ito H., Goins W.F., Shaikh I., Erdelyi R., Nishihara R., Nakano I., et al. Toxicity and Efficacy of a Novel Gadd34-Expressing Oncolytic Hsv-1 for the Treatment of Experimental Glioblastoma. Clin. Cancer Res. 2018;24:2574–2584. doi: 10.1158/1078-0432.CCR-17-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Studebaker A.W., Hutzen B.J., Pierson C.R., Haworth K.B., Cripe T.P., Jackson E.M., Leonard J.R. Oncolytic Herpes Virus RRp450 Shows Efficacy in Orthotopic Xenograft Group 3/4 Medulloblastomas and Atypical Teratoid/Rhabdoid Tumors. Mol. Ther. Oncolytics. 2017;6:22–30. doi: 10.1016/j.omto.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ogbomo H., Zemp F.J., Lun X., Zhang J., Stack D., Rahman M.M., Mcfadden G., Mody C.H., Forsyth P.A. Myxoma Virus Infection Promotes NK Lysis of Malignant Gliomas In Vitro and In Vivo. PLoS ONE. 2013;8:e66825. doi: 10.1371/journal.pone.0066825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu B., Ma R., Russell L., Yoo J.Y., Han J., Cui H., Yi P., Zhang J., Nakashima H., Dai H., et al. An Oncolytic Herpesvirus Expressing E-Cadherin Improves Survival in Mouse Models of Glioblastoma. Nat. Biotechnol. 2018;37:45–54. doi: 10.1038/nbt.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sette P., Amankulor N., Li A., Marzulli M., Leronni D., Zhang M., Goins W.F., Kaur B., Bolyard C., Cripe T.P., et al. GBM-Targeted OHSV Armed with Matrix Metalloproteinase 9 Enhances Anti-Tumor Activity and Animal Survival. Mol. Ther. Oncolytics. 2019;15:214–222. doi: 10.1016/j.omto.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mazzacurati L., Marzulli M., Reinhart B., Miyagawa Y., Uchida H., Goins W.F., Li A., Kaur B., Caligiuri M., Cripe T., et al. Use of MiRNA Response Sequences to Block Off-Target Replication and Increase the Safety of an Unattenuated, Glioblastoma-Targeted Oncolytic HSV. Mol. Ther. 2015;23:99–107. doi: 10.1038/mt.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim C.Y., Jeong M., Mushiake H., Kim B.M., Kim W.B., Ko J.P., Kim M.H., Kim M., Kim T.H., Robbins P.D., et al. Cancer Gene Therapy Using a Novel Secretable Trimeric TRAIL. Gene Ther. 2005;13:330–338. doi: 10.1038/sj.gt.3302658. [DOI] [PubMed] [Google Scholar]
- 121.Tamura K., Wakimoto H., Agarwal A.S., Rabkin S.D., Bhere D., Martuza R.L., Kuroda T., Kasmieh R., Shah K. Multimechanistic Tumor Targeted Oncolytic Virus Overcomes Resistance in Brain Tumors. Mol. Ther. 2013;21:68–77. doi: 10.1038/mt.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Passaro C., Alayo Q., de Laura I., McNulty J., Grauwet K., Ito H., Bhaskaran V., Mineo M., Lawler S.E., Shah K., et al. Arming an Oncolytic Herpes Simplex Virus Type 1 with a Single-Chain Fragment Variable Antibody against PD-1 for Experimental Glioblastoma Therapy. Clin. Cancer Res. 2019;25:290–299. doi: 10.1158/1078-0432.CCR-18-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sharma A., Li X., Bangari D.S., Mittal S.K. Adenovirus Receptors and Their Implications in Gene Delivery. Virus Res. 2009;143:184–194. doi: 10.1016/j.virusres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kiyokawa J., Wakimoto H. Preclinical and Clinical Development of Oncolytic Adenovirus for the Treatment of Malignant Glioma. Oncolytic Virotherapy. 2019;8:27–37. doi: 10.2147/OV.S196403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chiocca E.A., Aguilar L.K., Bell S.D., Kaur B., Hardcastle J., Cavaliere R., McGregor J., Lo S., Ray-Chaudhuri A., Chakravarti A., et al. Phase IB Study of Gene-Mediated Cytotoxic Immunotherapy Adjuvant to up-Front Surgery and Intensive Timing Radiation for Malignant Glioma. J. Clin. Oncol. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wheeler L.A., Manzanera A.G., Bell S.D., Cavaliere R., McGregor J.M., Grecula J.C., Newton H.B., Lo S.S., Badie B., Portnow J., et al. Phase II Multicenter Study of Gene-Mediated Cytotoxic Immunotherapy as Adjuvant to Surgical Resection for Newly Diagnosed Malignant Glioma. Neuro-Oncology. 2016;18:1137–1145. doi: 10.1093/neuonc/now002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liang M. Oncorine, the World First Oncolytic Virus Medicine and Its Update in China. Curr. Cancer Drug Targets. 2018;18:171–176. doi: 10.2174/1568009618666171129221503. [DOI] [PubMed] [Google Scholar]
- 128.Garber K. China Approves World’s First Oncolytic Virus Therapy for Cancer Treatment. J. Natl. Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 129.Blackford A.N., Grand R.J.A. Adenovirus E1B 55-Kilodalton Protein: Multiple Roles in Viral Infection and Cell Transformation. J. Virol. 2009;83:4000–4012. doi: 10.1128/JVI.02417-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang H., Gomez-Manzano C., Aoki H., Alonso M.M., Kondo S., McCormick F., Xu J., Kondo Y., Bekele B.N., Colman H., et al. Examination of the Therapeutic Potential of Delta-24-RGD in Brain Tumor Stem Cells: Role of Autophagic Cell Death. JNCI J. Natl. Cancer Inst. 2007;99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 131.Avβ3 and Avβ5 Integrin Expression in Glioma Periphery: Neurosurgery. [(accessed on 15 November 2022)]. Available online: https://journals.lww.com/neurosurgery/Abstract/2001/08000/_v_3_and__v_5_Integrin_Expression_in_Glioma.22.aspx.
- 132.Asaoka K., Tada M., Sawamura Y., Ikeda J., Abe H. Dependence of Efficient Adenoviral Gene Delivery in Malignant Glioma Cells on the Expression Levels of the Coxsackievirus and Adenovirus Receptor. J. Neurosurg. 2000;92:1002–1008. doi: 10.3171/jns.2000.92.6.1002. [DOI] [PubMed] [Google Scholar]
- 133.Martínez-Vélez N., Garcia-Moure M., Marigil M., González-Huarriz M., Puigdelloses M., Pérez-Larraya J.G., Zalacaín M., Marrodán L., Varela-Guruceaga M., Laspidea V., et al. The Oncolytic Virus Delta-24-RGD Elicits an Antitumor Effect in Pediatric Glioma and DIPG Mouse Models. Nat. Commun. 2019;10:2235. doi: 10.1038/s41467-019-10043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang H., Shin D.H., Nguyen T.T., Fueyo J., Fan X., Henry V., Carrillo C.C., Yi Y., Alonso M.M., Collier T.L., et al. Localized Treatment with Oncolytic Adenovirus Delta-24-RGDOX Induces Systemic Immunity against Disseminated Subcutaneous and Intracranial Melanomas. Clin. Cancer Res. 2019;25:6801–6814. doi: 10.1158/1078-0432.CCR-19-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Puigdelloses M., Garcia-Moure M., Labiano S., Laspidea V., Gonzalez-Huarriz M., Zalacain M., Marrodan L., Martinez-Velez N., de La Nava D., Ausejo I., et al. CD137 and PD-L1 Targeting with Immunovirotherapy Induces a Potent and Durable Antitumor Immune Response in Glioblastoma Models. J. Immunother. Cancer. 2021;9:e002644. doi: 10.1136/jitc-2021-002644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rivera-Molina Y., Jiang H., Fueyo J., Nguyen T., Shin D.H., Youssef G., Fan X., Gumin J., Alonso M.M., Phadnis S., et al. GITRL-Armed Delta-24-RGD Oncolytic Adenovirus Prolongs Survival and Induces Anti-Glioma Immune Memory. Neurooncol. Adv. 2019;1:vdz009. doi: 10.1093/noajnl/vdz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marchini A., Bonifati S., Scott E.M., Angelova A.L., Rommelaere J. Oncolytic Parvoviruses: From Basic Virology to Clinical Applications. Virol. J. 2015;12:6. doi: 10.1186/s12985-014-0223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Angelova A.L., Barf M., Geletneky K., Unterberg A., Rommelaere J. Immunotherapeutic Potential of Oncolytic H-1 Parvovirus: Hints of Glioblastoma Microenvironment Conversion towards Immunogenicity. Viruses. 2017;9:382. doi: 10.3390/v9120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.di Piazza M., Mader C., Geletneky K., Herrero y Calle M., Weber E., Schlehofer J., Deleu L., Rommelaere J. Cytosolic Activation of Cathepsins Mediates Parvovirus H-1-Induced Killing of Cisplatin and TRAIL-Resistant Glioma Cells. J. Virol. 2007;81:4186–4198. doi: 10.1128/JVI.02601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geletneky K., Kiprianova I., Ayache A., Koch R., Herrero Y., Calle M., Deleu L., Sommer C., Thomas N., Rommelaere J., et al. Regression of Advanced Rat and Human Gliomas by Local or Systemic Treatment with Oncolytic Parvovirus H-1 in Rat Models. Neuro-Oncology. 2010;12:804–814. doi: 10.1093/neuonc/noq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Geletneky K., Hajda J., Angelova A.L., Leuchs B., Capper D., Bartsch A.J., Neumann J.O., Schöning T., Hüsing J., Beelte B., et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017;25:2620–2634. doi: 10.1016/j.ymthe.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Geletneky K., Hartkopf A.D., Krempien R., Rommelaere J., Schlehofer J.R. Improved Killing of Human High-Grade Glioma Cells by Combining Ionizing Radiation with Oncolytic Parvovirus H-1 Infection. J. Biomed. Biotechnol. 2010;2010:350748. doi: 10.1155/2010/350748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Geletneky K., Angelova A., Leuchs B., Bartsch A., Capper D., Hajda J., Rommelaere J. Atnt-07favorable Response of Patients with Glioblastoma at Second or Third Recurrence to Repeated Injection of Oncolytic Parvovirus H-1 in Combination with Bevacicumab. Neuro-Oncology. 2015;17:v11. doi: 10.1093/neuonc/nov205.07. [DOI] [Google Scholar]
- 144.Angelova A., Ferreira T., Bretscher C., Rommelaere J., Marchini A. Parvovirus-Based Combinatorial Immunotherapy: A Reinforced Therapeutic Strategy against Poor-Prognosis Solid Cancers. Cancers. 2021;13:342. doi: 10.3390/cancers13020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Idbaih A., Erbs P., Foloppe J., Chneiweiss H., Kempf J., Homerin M., Schmitt C., Them L.N., Delattre J.-Y. TG6002: A Novel Oncolytic and Vectorized Gene pro-Drug Therapy Approach to Treat Glioblastoma. J. Clin. Oncol. 2017;35:e13510. doi: 10.1200/JCO.2017.35.15_suppl.e13510. [DOI] [Google Scholar]
- 146.Wang F., Ma Y., Barrett J.W., Gao X., Loh J., Barton E., Virgin H.W., IV, McFadden G. Disruption of Erk-Dependent Type I Interferon Induction Breaks the Myxoma Virus Species Barrier. Nat. Immunol. 2004;5:1266–1274. doi: 10.1038/ni1132. [DOI] [PubMed] [Google Scholar]
- 147.Lun X., Yang W., Alain T., Shi Z.Q., Muzik H., Barrett J.W., McFadden G., Bell J., Hamilton M.G., Senger D.L., et al. Myxoma Virus Is a Novel Oncolytic Virus with Significant Antitumor Activity against Experimental Human Gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pisklakova A., McKenzie B., Zemp F., Lun X., Kenchappa R.S., Etame A.B., Rahman M.M., Reilly K., Pilon-Thomas S., McFadden G., et al. M011L-Deficient Oncolytic Myxoma Virus Induces Apoptosis in Brain Tumor-Initiating Cells and Enhances Survival in a Novel Immunocompetent Mouse Model of Glioblastoma. Neuro-Oncology. 2016;18:1088–1098. doi: 10.1093/neuonc/now006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Al Yaghchi C., Zhang Z., Alusi G., Lemoine N.R., Wang Y. Vaccinia Virus, a Promising New Therapeutic Agent for Pancreatic Cancer. Immunotherapy. 2015;7:1249–1258. doi: 10.2217/imt.15.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lam P.Y., Breakefield X.O. Potential of gene therapy for brain tumors. Hum. Mol. Genet. 2001;10:777–787. doi: 10.1093/hmg/10.7.777. [DOI] [PubMed] [Google Scholar]
- 151.Foloppe J., Kempf J., Futin N., Kintz J., Cordier P., Pichon C., Findeli A., Vorburger F., Quemeneur E., Erbs P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics. 2019;14:1–14. doi: 10.1016/j.omto.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geletneky K., Weiss C., Bernhard H., Capper D., Leuchs B., Marchini A. Rommelaere, J. ATIM-29. First clinical observation of improved anti-tumor effects of viro-immunotherapy with oncolytic parvovirus H-1 in combination with PD-1 checkpoint blockade and bevacicumab in patients with recurrent glioblastoma. Neuro-Oncology. 2016;18:vi24. doi: 10.1093/neuonc/now212.094. [DOI] [Google Scholar]
- 153.Anderson B.D., Nakamura T., Russell S.J., Peng K.W. High CD46 Receptor Density Determines Preferential Killing of Tumor Cells by Oncolytic Measles Virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 154.McDonald C.J., Erlichman C., Ingle J.N., Rosales G.A., Allen C., Greiner S.M., Harvey M.E., Zollman P.J., Russell S.J., Galanis E. A Measles Virus Vaccine Strain Derivative as a Novel Oncolytic Agent against Breast Cancer. Breast Cancer Res. Treat. 2006;99:177–184. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 155.Blechacz B., Splinter P.L., Greiner S., Myers R., Peng K.W., Federspiel M.J., Russell S.J., LaRusso N.F. Engineered Measles Virus as a Novel Oncolytic Viral Therapy System for Hepatocellular Carcinoma. Hepatology. 2006;44:1465–1477. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- 156.Liu C., Sarkaria J.N., Petell C.A., Paraskevakou G., Zollman P.J., Schroeder M., Carlson B., Decker P.A., Wu W., James C.D., et al. Combination of Measles Virus Virotherapy and Radiation Therapy Has Synergistic Activity in the Treatment of Glioblastoma Multiforme. Clin. Cancer Res. 2007;13:7155–7165. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- 157.Opyrchal M., Allen C., Iankov I., Aderca I., Schroeder M., Sarkaria J., Galanis E. Effective Radiovirotherapy for Malignant Gliomas by Using Oncolytic Measles Virus Strains Encoding the Sodium Iodide Symporter (MV-NIS) Hum. Gene Ther. 2011;23:419–427. doi: 10.1089/hum.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Msaouel P., Iankov I.D., Allen C., Aderca I., Federspiel M.J., Tindall D.J., Morris J.C., Koutsilieris M., Russell S.J., Galanis E. Noninvasive Imaging and Radiovirotherapy of Prostate Cancer Using an Oncolytic Measles Virus Expressing the Sodium Iodide Symporter. Mol. Ther. 2009;17:2041–2048. doi: 10.1038/mt.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nikolic J., Belot L., Raux H., Legrand P., Gaudin Y., Albertini A.A. Structural Basis for the Recognition of LDL-Receptor Family Members by VSV Glycoprotein. Nat. Commun. 2018;9:1029. doi: 10.1038/s41467-018-03432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nikitina A.S., Lipatova A.V., Goncharov A.O., Kliuchnikova A.A., Pyatnitskiy M.A., Kuznetsova K.G., Hamad A., Vorobyev P.O., Alekseeva O.N., Mahmoud M., et al. Multiomic Profiling Identified EGF Receptor Signaling as a Potential Inhibitor of Type I Interferon Response in Models of Oncolytic Therapy by Vesicular Stomatitis Virus. Int. J. Mol. Sci. 2022;23:5244. doi: 10.3390/ijms23095244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang X., Zhang T., Davis J.N., Marzi A., Marchese A.M., Robek M.D., van den Pol A.N. Mucin-Like Domain of Ebola Virus Glycoprotein Enhances Selective Oncolytic Actions against Brain Tumors. J. Virol. 2020;94:e01967-19. doi: 10.1128/JVI.01967-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Muik A., Stubbert L.J., Jahedi R.Z., Geib Y., Kimpel J., Dold C., Tober R., Volk A., Klein S., Dietrich U., et al. Re-Engineering Vesicular Stomatitis Virus to Abrogate Neurotoxicity, Circumvent Humoral Immunity, and Enhance Oncolytic Potency. Cancer Res. 2014;74:3567–3578. doi: 10.1158/0008-5472.CAN-13-3306. [DOI] [PubMed] [Google Scholar]
- 163.Wilcox M.E., Yang W.Q., Senger D., Rewcastle N.B., Morris D.G., Brasher P.M.A., Shi Z.Q., Johnston R.N., Nishikawa S., Lee P.W.K., et al. Reovirus as an Oncolytic Agent Against Experimental Human Malignant Gliomas. JNCI J. Natl. Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 164.Ganar K., Das M., Sinha S., Kumar S. Newcastle Disease Virus: Current Status and Our Understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bai Y., Chen Y., Hong X., Liu X., Su X., Li S., Dong X., Zhao G., Li Y. Newcastle Disease Virus Enhances the Growth-Inhibiting and Proapoptotic Effects of Temozolomide on Glioblastoma Cells in vitro and in vivo. Sci. Rep. 2018;8:11470. doi: 10.1038/s41598-018-29929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.García-Romero N., Palacín-Aliana I., Esteban-Rubio S., Madurga R., Rius-Rocabert S., Carrión-Navarro J., Presa J., Cuadrado-Castano S., Sánchez-Gómez P., García-Sastre A., et al. Newcastle Disease Virus (NDV) Oncolytic Activity in Human Glioma Tumors Is Dependent on CDKN2A-Type I IFN Gene Cluster Codeletion. Cells. 2020;9:1405. doi: 10.3390/cells9061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang P., Chen G.Z. Comment to “Recurrent Glioblastoma Treated with Recombinant Poliovirus”. Chin. Med. J. 2018;131:2645–2646. doi: 10.4103/0366-6999.244121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Abdullah J.M., Mustafa Z., Ideris A. Newcastle Disease Virus Interaction in Targeted Therapy against Proliferation and Invasion Pathways of Glioblastoma Multiforme. Biomed. Res. Int. 2014;2014:386470. doi: 10.1155/2014/386470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burke M.J. Oncolytic Seneca Valley Virus: Past Perspectives and Future Directions. Oncolytic Virotherapy. 2016;5:81–89. doi: 10.2147/OV.S96915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Evans D.J., Wasinger A.M., Brey R.N., Dunleavey J.M., Croix B.S., Bann J.G. Seneca Valley Virus Exploits TEM8, a Collagen Receptor Implicated in Tumor Growth. Front. Oncol. 2018;8:506. doi: 10.3389/fonc.2018.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Corbett V., Hallenbeck P., Rychahou P., Chauhan A. Evolving Role of Seneca Valley Virus and Its Biomarker TEM8/ANTXR1 in Cancer Therapeutics. Front. Mol. Biosci. 2022;9:868. doi: 10.3389/fmolb.2022.930207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu J., Yang X., Deng Q., Yang C., Wang D., Jiang G., Yao X., He X., Ding J., Qiang J., et al. TEM8 Marks Neovasculogenic Tumor-Initiating Cells in Triple-Negative Breast Cancer. Nat. Commun. 2021;12:4413. doi: 10.1038/s41467-021-24703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Z., Zhao X., Mao H., Baxter P.A., Huang Y., Yu L., Wadhwa L., Su J.M., Adesina A., Perlaky L., et al. Intravenous Injection of Oncolytic Picornavirus SVV-001 Prolongs Animal Survival in a Panel of Primary Tumor–Based Orthotopic Xenograft Mouse Models of Pediatric Glioma. Neuro-Oncology. 2013;15:1173–1185. doi: 10.1093/neuonc/not065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Goetz C., Dobrikova E., Shveygert M., Dobrikov M., Gromeier M. Oncolytic Poliovirus against Malignant Glioma. Future Virol. 2011;6:1045–1058. doi: 10.2217/fvl.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sloan K.E., Stewart J.K., Treloar A.F., Matthews R.T., Jay D.G. CD155/PVR Enhances Glioma Cell Dispersal by Regulating Adhesion Signaling and Focal Adhesion Dynamics. Cancer Res. 2005;65:10930–10937. doi: 10.1158/0008-5472.CAN-05-1890. [DOI] [PubMed] [Google Scholar]
- 176.Gromeier M., Alexander L., Wimmer E. Internal Ribosomal Entry Site Substitution Eliminates Neurovirulence in Intergeneric Poliovirus Recombinants. Proc. Natl. Acad. Sci. USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gromeier M., Bossert B., Arita M., Nomoto A., Wimmer E. Dual Stem Loops within the Poliovirus Internal Ribosomal Entry Site Control Neurovirulence. J. Virol. 1999;73:958–964. doi: 10.1128/JVI.73.2.958-964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.PVSRIPO in Recurrent Malignant Glioma—Full Text View—ClinicalTrials.Gov. [(accessed on 9 November 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02986178.
- 179.Hamad A., Soboleva A.V., Vorobyev P.O., Mahmoud M., Vasilenko K.V., Chumakov P.M., Lipatova A.V. Development of a recombinant oncolytic poliovirus type 3 strain with altered cell tropism. Bull. Russ. State Med. Univ. 2022;4:5–10. doi: 10.24075/brsmu.2022.023. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.