Abstract
The macrophage is a major component of the inflammatory response induced by lymphatic tissue-dwelling filariae. Intraperitoneal (i.p.) infections with Brugia pahangi in Mongolian gerbils, or jirds (Meriones unguiculatus), induce a peritoneal inflammatory response characterized by accumulation of numerous macrophages and fewer eosinophils. This inflammatory response is associated with the release of microfilariae by female worms. The aim of this study was to investigate the activation state of the peritoneal macrophages during the course of i.p. infections with either male or female worms. Activation was determined by a toxoplasmacidal assay and assays which measured the production of tumor necrosis factor (TNF)-like activity and nitric oxide (NO) production. The development of these assays with jirds was initially conducted in parallel with the mouse system, which served as a positive control. Jird macrophages became activated to kill Toxoplasma gondii by in vivo immunization with Mycobacterium bovis BCG in a pattern similar to that of mouse macrophages. However, unlike the mouse system, supernatants from purified protein derivative- or concanavalin A-stimulated jird splenocytes plus lipopolysaccharide failed to activate jird macrophages in vitro or induce NO production. These results indicate that factors involved in jird macrophage activation may differ from those demonstrated in the mouse system and other systems. i.p. infections of 15 days in duration with either male or female worms induced macrophage activation as measured by Toxoplasma killing and TNF production. These responses decreased as the infection progressed to the chronic period on a time course that parallels the down regulation of experimental B. pahangi granulomas. There was no evidence of NO production by activated jird macrophages. These data indicate that macrophage function is down modulated during filarial infection and suggest that mechanisms involved in macrophage deactivation are related to those that induce down modulation of the systemic granulomatous inflammatory response in the jird. This response is not dependent on the microfilarial stage of the parasite and is also independent of mechanisms which induce peritoneal accumulations of macrophages.
Wuchereria bancrofti and Brugia malayi are lymphatic tissue-dwelling filarial nematodes that infect humans in tropical and subtropical regions of the world. The pathology caused by filarial parasites is primarily characterized by a granulomatous inflammation response to the parasite and parasite products that has been attributed to a state of specific filarial immune hyperresponsiveness (35, 46). Nevertheless, a larger group in the infected population is asymptomatic despite its members having microfilariae (MF) in their peripheral blood and a tendency to manifest a state of filaria-specific hyporesponsiveness (25, 45, 47, 49).
In Brugia pahangi-infected jirds, the immune response has been implicated in the development of lymphatic granulomas. Presensitization and protective immunity result in an increase of these lymphatic lesions (28, 48). Chronic microfilaremic gerbils manifest a state of hyporesponsiveness (32) accompanied by decreased lymphatic lesion numbers (27, 29) and down modulation of the granulomatous response to antigen-coated beads embolized in the lungs (PGRN) (27).
The macrophage is a major cellular component of the granuloma and is often present covering the surfaces of filariae (23, 42, 44) and other nematodes (24). The role of the macrophage in the immune response to metazoan parasites has been thoroughly investigated in murine schistosomiasis, in which it has been shown that macrophage activation is important in resistance (4, 20, 21, 37). Furthermore, several studies suggest that the macrophage is important in the immunomodulation observed during schistosoma infection (reviewed in reference 58).
The purpose of the experiments described in this paper was to determine the state of macrophage activation in B. pahangi-infected jirds at times when systemic granulomatous modulation occurs. The potentially different effects of female worms and MF and of male worms on macrophage function were also investigated.
MATERIALS AND METHODS
Animals.
Inbred, female, 6- to 8-week-old jirds (Meriones unguiculatus) were obtained from Tumblebrook Farms (West Brookfield, Mass.). Inbred, female, BALB/c mice were originally obtained from Jackson Laboratories (Bar Harbor, Maine). Animals were maintained on standard rodent chow and water ad libitum. All jirds and mice used in these experiments were infected at 3 to 4 months of age.
In vivo infection with Mycobacterium bovis BCG.
Briefly, one group of 24 jirds and one group of 24 mice were inoculated intradermally (i.d.) with 3 × 106 CFU of living M. bovis bacillus Calmette-Guérin (BCG). Twenty-four and forty-eight hours before euthanasia, BCG-immunized animals were inoculated intraperitoneally (i.p.) with 50 μg of purified protein derivative (PPD) of the tubercle bacillus (Parke-Davis, Rochester, Mich.) dissolved in 0.5 ml of phosphate-buffered saline (PBS). The BCG-PPD immunization protocol and kinetics of macrophage activation by this method have previously been described for mice (53). Control animals, 24 per group, were inoculated i.d. with 0.05 ml of PBS and i.p. with 0.5 ml of PBS. Necropsies were performed at 15, 28, and 42 days postinfection (dpi) with BCG. These time points were chosen to correspond to periods of macrophage activation in mice following BCG injection (53) and points of differential regulation of the granulomatous inflammatory response by i.p. infections of jirds with B. pahangi (41).
Brugia parasites.
The B. pahangi life cycle was maintained in Aedes aegypti and jirds as previously described (29). Male and female adult B. pahangi worms were aseptically collected from the peritoneal cavities of jirds with patent i.p. infections. The worms were washed in RPMI 1640 medium supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) prior to being transferred to 3-ml syringes. Single-sex implantations of 10 female or 10 male worms into the peritoneal cavities of jirds were done with 16-gauge needles. Control animals were inoculated i.p. with RPMI medium. Necropsies were performed at 15, 50 to 56, and 135 dpi. These time points were chosen to correspond to periods of maximal (14 dpi) and decreased (50 to 56 and 135 dpi) periods of granulomatous inflammation in response to B. pahangi antigen (41).
Macrophage culture.
Peritoneal cells were aseptically collected from the peritoneal cavities of jirds and mice in PBS containing 10 U of heparin (Sigma) per ml. Peritoneal cells were washed once at 250 × g for 10 min and transferred to RPMI 1640 (GIBCO, Grand Island, N.Y.) supplemented with antibiotics (described above), HEPES buffer (25 mM), 2-mercaptoethanol (2 × 10−5 M), l-glutamine (2 mM), and 10% heat-inactivated fetal bovine serum. Cells were prepared on LUX coverslips (Miles Scientific, Division of Miles Laboratories, Inc., Naperville, Ill.) in 24-well tissue culture plates (GIBCO) at 3 × 106 cells/well and were incubated at 37°C. After 4 to 6 h of culture, nonadherent cells were removed by washing the coverslips with PBS. Macrophage monolayers on the coverslips were used in the bioassays for Toxoplasma killing and for tumor necrosis factor (TNF) and nitric oxide (NO) production.
Spleen cell culture and collection of macrophage activating factors (MAF).
MAF were collected from cultures of spleen cells from BCG-PPD-inoculated animals or from untreated animals.
Single-cell suspensions were obtained from spleens of BCG-PPD-inoculated and untreated animals as described previously (29). Spleen cells were cultured in 5% fetal bovine serum–RPMI 1640 medium supplemented as indicated above. To obtain BCG-PPD MAF, spleen cells were incubated at a concentration of 4 × 106 cells/ml with 40 μg of PPD per ml in 25-cm2 plastic tissue culture flasks (Costar, Cambridge, Mass.) (40). After 48 and 72 h, culture supernatants were collected, centrifuged at 10,000 × g for 10 min, and stored at −20°C until use.
To obtain concanavalin A (ConA)-induced MAF, spleen cells were incubated at a concentration of 1.5 × 106 cells/ml with 1, 2, or 3 μg of ConA (Sigma) per ml in 25-cm2 plastic tissue culture flasks for 24, 48, 72, 96, or 120 h. Culture supernatants were collected, centrifuged at 10,000 × g for 10 min, and stored at −20°C until use. Previous work in our laboratory and by other investigators (32) has demonstrated that 3 μg of ConA per ml induces spleen cell proliferation as measured by tritiated thymidine incorporation.
Reagents.
Bacterial lipopolysaccharide (LPS) from Escherichia coli O111.B4 was used (Sigma). Recombinant murine gamma interferon (rIFN-γ) was obtained from Genentech, Inc. (South San Francisco, Calif.).
In vitro activation of macrophages.
Either BCG-PPD MAF, ConA MAF (1:1, 1:2, or 1:4 MAF/medium ratio), or murine rIFN-γ (500 or 1,000 U/ml) with 10 or 50 ng of LPS per ml was added to the macrophage monolayers. Macrophages were cultured undisturbed overnight. Attempts to activate jird and mouse macrophages in vitro were repeated at least three times for each treatment.
Toxoplasma killing assay.
Tachyzoites of Toxoplasma gondii RH were harvested from the peritoneal cavities of BALB/c mice 2 days after infection and purified by filtration through 3-μm-pore-size polycarbonate membranes (Nuclepore Corp., Pleasanton, Calif.) as described previously (54, 61). Macrophage monolayers from controls and from animals infected with BCG-PPD or B. pahangi were challenged with 1.5 × 106 freshly harvested T. gondii cells. One hour later, extracellular T. gondii cells were washed off and macrophage monolayers were reincubated. Microbicidal activity was assessed after 20 h of culture. Coverslips were fixed and stained with Hema 3 (Curtin Matheson Scientific, Inc., Houston, Tex.). The numbers of intracellular Toxoplasma cells were counted in 100 macrophages per coverslip. Triplicate samples were examined for each treatment.
TNF-like activity.
Levels of TNF-like activity were determined in supernatants of macrophage cultures by a modified L929 fibroblast cell lytic assay (2, 55). An antibody that specifically neutralizes jird TNF is not available. Thus, it can only be presumed that cytolysis of L929 cells by macrophage supernatants was due to TNF. However, for the purposes of this paper this factor will be referred to as TNF. Macrophage monolayers were stimulated with 50 μg of LPS (Sigma) per ml in medium alone. Supernatants were collected after 4 h, centrifuged at 10,000 × g for 10 min, and stored at −70°C until use. Duplicate samples were serially diluted threefold in 96-well, flat-bottomed tissue culture plates (Costar). L929 cells were added, and plates were incubated at 37°C. In the absence of a jird recombinant TNF standard, 100% cell lysis was accomplished with 3.0 M guanidine hydrochloride. Concentrations of TNF were calculated in units defined as the reciprocal dilutions of supernatants which yield 50% lysis of L929 cells.
Measurement of nitrite production.
Levels of nitrite (NO2−) in macrophage supernatants were determined spectrophotometrically at 540 nm following reaction with the Griess reagent (1, 10). NO2− is the stable end product of nonenzymatic degradation of NO. NO2− levels were measured in supernatants from macrophage monolayers treated in vitro with either IFN-γ or ConA MAF and LPS as second signal or with only LPS. NO2− production was also determined in cultured macrophages from BCG-PPD-immunized animals. NO2− concentration was calculated from a NaNO2 standard curve, and results are expressed as micromolar concentrations.
Statistical analysis.
When deemed necessary, results were analyzed statistically with a comparative analysis of variance with Tukey’s Studentized range test.
RESULTS
Characterization of jird macrophage activation by Toxoplasma killing.
Toxoplasma killing is a sensitive, well-defined assay used to study macrophage activation (1, 54, 55). In order to validate the use of jird macrophages in this assay, parallel comparisons of murine and jird macrophage activity were conducted by standard in vitro methods of activation. Macrophages were activated in vivo by BCG inoculation demonstrated to be effective in mice (53).
Experiments with murine macrophages treated with mouse ConA MAF or murine rIFN-γ consistently showed activation (Table 1). However, in parallel, all attempts to activate jird macrophages in vitro with supernatants from ConA-stimulated spleen cultures or with murine rIFN-γ as a first signal, followed by LPS as a second signal, failed (Table 1).
TABLE 1.
Effects of ConA MAF and murine rIFN-γ on the toxoplasmacidal activities of jird and mouse macrophagesa
| Macrophage | % Infected macro- phagesd | No. of toxo- plasmas/ infected macro- phage | No. of toxo- plasmas/100 macro- phagesd |
|---|---|---|---|
| Jird | |||
| Controlb | 22.7 ± 7.13 | 3.18 | 72 ± 22.6 |
| Murine rIFN-γ plus LPS | 17.33 ± 0.94 | 3.35 | 58 ± 10.98 |
| Jird ConA MAF plus LPS | 22.67 ± 4.03 | 3.31 | 75 ± 12.35 |
| Mouse ConA MAF plus LPS | 21.5 ± 1.5 | 3.97 | 85.5 ± 15.5 |
| Mouse | |||
| Controlb | 15.57 ± 0.94 | 6.64 | 104 ± 15.77 |
| Murine rIFN-γ plus LPS | 3.67 ± 2.05c | 1.27c | 4.67 ± 2.86c |
| Jird ConA MAF plus LPS | 9 ± 1c | 4.22c | 38 ± 5c |
| Mouse ConA MAF plus LPS | 4 ± 0.82c | 1.15c | 4.6 ± 0.94c |
LPS was added as a second signal.
Peritoneal macrophages from control uninfected animals that had no in vitro treatment.
Significant difference (P < 0.05) from controls.
Results are expressed as means ± standard deviations.
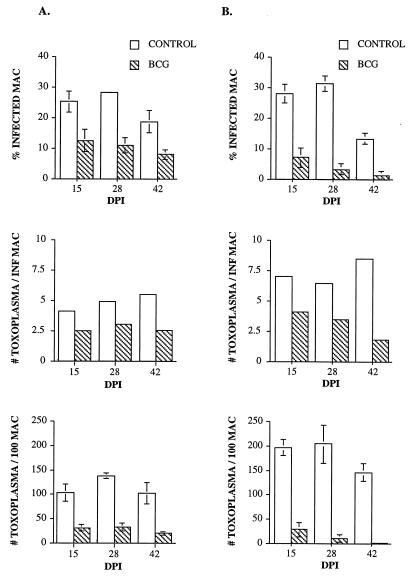
Alternatively, we chose to activate macrophages in vivo with BCG and test them ex vivo as previously demonstrated in the murine system (53). Results showed that macrophages recovered from BCG-PPD-treated mice and jirds restricted the intracellular growth of Toxoplasma cells compared to control macrophages at the three times tested (14, 27, and 42 dpi) (P < 0.05). Microbicidal activity of BCG-PPD-treated peritoneal macrophages was manifested by a decrease in the percentages of Toxoplasma-infected macrophages, a decrease in the numbers of Toxoplasma cells per infected macrophage, and a decrease in the numbers of Toxoplasma cells per 100 macrophages (Fig. 1). Toxoplasmacidal activity did not vary significantly in mice or jirds throughout the infection, although activated jird macrophages appeared to have a lower capacity to restrict Toxoplasma growth than did mouse macrophages.
FIG. 1.
Effect of BCG-PPD immunization on jird (A) and mouse (B) macrophage activation measured by the percentages of Toxoplasma-infected macrophages, numbers of Toxoplasma cells per infected macrophage, and total numbers of Toxoplasma cells in 100 macrophages. DPI indicates days after i.d. BCG inoculation. Bars represent standard deviations of the means.
Because of the success of in vivo activation of macrophages with BCG, attempts were made to activate macrophages in vitro with MAF obtained from these in vivo-activated macrophages. The addition of murine BCG-PPD MAF to macrophage monolayers from control mice resulted in a marked increase of microbicidal activity measured via Toxoplasma killing (Table 2). However, jird BCG-PPD MAF did not restrict the growth of Toxoplasma in macrophages from control jirds.
TABLE 2.
Effects of BCG-PPD MAF on the toxoplasmacidal activities of jird and mouse macrophagesa
| Macrophage | % Infected macrophagesb | No. of toxo- plasmas/infected macrophage | No. of toxo- plasmas/100 macrophagesb |
|---|---|---|---|
| Jird | |||
| Set at first time | |||
| Control | 28.3 ± 0.47 | 4.91 | 139 ± 5.43 |
| 15-dpi BCG MAF plus LPS | 21 ± 0.82 | 4.03 | 84.7 ± 14 |
| Set at second time | |||
| Control | 15.7 ± 1.25 | 4.29 | 67.3 ± 3.4 |
| 28-dpi BCG MAF plus LPS | 15.5 ± 0.5 | 4.45 | 69 ± 7 |
| 42-dpi BCG MAF plus LPS | 12.7 ± 1.69 | 3.87 | 49 ± 8.64 |
| Mouse | |||
| Set at first time | |||
| Control | 31.3 ± 2.49 | 6.5 | 204 ± 39 |
| 15-dpi BCG MAF plus LPS | 7 ± 5c | 1.28c | 9 ± 7c |
| Set at second time | |||
| Control | 13.3 ± 1.7 | 8.5 | 146.7 ± 18 |
| 28-dpi BCG MAF plus LPS | 1 ± 0c | 1c | 1 ± 0c |
| IFN-γ plus LPS | 1 ± 0c | 1c | 1 ± 0c |
LPS was added as a second signal. BCG-PPD MAF from animals with 15, 28, and 42 days of BCG infection were used.
Results are expressed as means ± standard deviations.
Significantly different (P < 0.05) from the controls.
Nitrite production.
NO is a known important effector molecule produced by activated macrophages and has been demonstrated to be important in killing of filariae in mice (50). Thus, we chose to measure the production of NO by jird macrophages stimulated both in vitro and in vivo. As for the Toxoplasma killing assay, results obtained from jird cell culture were compared with those from similarly treated mouse cells. NO2− release was not detected in any of the supernatants from jird macrophage cultures treated with either ConA MAF or IFN-γ plus LPS or LPS alone or not treated. However, parallel experiments with mouse ConA MAF or IFN-γ on murine macrophages resulted in NO2− production as previously described (9). NO2− production increased over time in LPS-treated murine macrophages from 1.5 ± 2.1 μM at 4 h to 39.9 ± 2.3 μM at 24 h of culture. The greatest NO2− production was observed in murine macrophages treated with a combination of IFN-γ and LPS (52.5 ± 1.3 μM) after 20 h.
The production of NO by macrophages was examined further with cells recovered from BCG-sensitized jirds and mice. The greatest levels of NO2 were measured in cultures of BCG-stimulated murine macrophages at 12 h of culture when stimulated in vitro with LPS (Table 3). NO production above control levels was not found in macrophages from BCG-PPD-immunized jirds.
TABLE 3.
NO production by macrophages from jirds and mice immunized with BCG-PPDa
| dpib | TXc | ITXd | Production by macrophage
|
|||
|---|---|---|---|---|---|---|
| Mouse
|
Jird
|
|||||
| Controle | BCG-PPDf | Controle | BCG-PPDf | |||
| 15 | 0 | 4 | 1.29 ± 0.51 | 2 ± 0.8 | 2 ± 0.88 | 0.65 ± 0.7 |
| LPS | 4 | 1.5 ± 0.39 | 2.4 ± 0.3 | 1.7 ± 0.5 | 0.8 ± 0.5 | |
| 0 | 12 | NDg | 13.4 ± 14 | 1.3 ± 0 | 1.12 ± 1.2 | |
| LPS | 12 | 5.5 ± 0.8 | 22.7 ± 6.6 | 0 | 0.9 ± 1.28 | |
| 28 | 0 | 4 | 0.15 ± 0.22 | 1.16 ± 0.7 | 1.4 ± 0.6 | 1.4 ± 0.76 |
| LPS | 4 | ND | 3.33 ± 1.4 | 0.8 ± 0.5 | 1.46 ± 0.42 | |
| 0 | 12 | ND | 0.17 ± 0.19 | 0 | 0.4 ± 0.44 | |
| LPS | 12 | ND | 14.6 ± 4.4 | 0.3 ± 0.29 | 0.29 ± 0.38 | |
| 42 | 0 | 4 | 0.32 ± 0.45 | 4.6 ± 4.3 | 0.4 ± 0.5 | 0.54 ± 0.94 |
| LPS | 4 | 0.63 ± 0.6 | 4.7 ± 6.7 | 0.17 ± 0.3 | 0.8 ± 0.89 | |
| 0 | 12 | ND | 14.3 ± 13.9 | 0 | 0 | |
| LPS | 12 | ND | 34.8 ± 20.8 | 0 | 0 | |
Results are expressed as micromolar concentrations of NO2− (means ± standard deviations).
Number of dpi with BCG.
Macrophages were cultured with or without LPS.
Hours of culture with or without LPS.
Uninfected animals.
Animals were infected with BCG and boosted i.p. with PPD, 24 h and 48 h before euthanasia.
ND, not determined.
Macrophage activation after i.p. infection of jirds with adult female or male B. pahangi.
Previous studies have demonstrated that in vivo granulomatous inflammatory responses of jirds to B. pahangi antigen are stimulated by 15 days after i.p. infection with female or male worms. Conversely, infection with female worms but not with male worms induces a persistent peritoneal exudate of predominantly macrophages during this period (41). In order to characterize these changes in inflammatory events at the cellular level, the state of macrophage activation was determined by the toxoplasmacidal assay. Time points for peritoneal macrophage collection during the course of B. pahangi infection were chosen to coincide with the changes seen in filaria-induced inflammation.
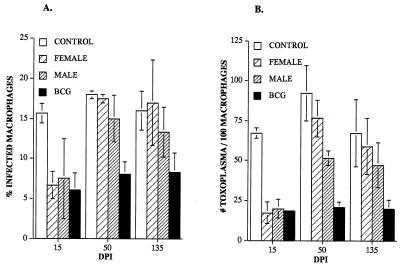
Toxoplasmacidal activity was measured in peritoneal macrophages from jirds with i.p. infections with either adult female or male B. pahangi. Macrophages from uninfected jirds served as negative controls, and macrophages from BCG-PPD-immunized jirds served as positive controls. Macrophages from jirds infected with adult female or male B. pahangi at 15 dpi restricted the growth of Toxoplasma cells in a manner similar to that of macrophages from BCG-PPD-immunized jirds. Percentages of infected macrophages, numbers of Toxoplasma cells per infected macrophage, and total numbers of Toxoplasma cells per 100 macrophages at 15 dpi were significantly decreased with respect to control macrophages (P < 0.05) (Fig. 2). In contrast, macrophages from female or male worm infections at 50 or 135 dpi showed percentages of infected macrophages, numbers of mean Toxoplasma cells per infected macrophage, and total numbers of Toxoplasma cells per 100 macrophages significantly higher than those of macrophages from BCG-PPD-immunized jirds and not significantly different from those of uninfected controls (P > 0.05) (Fig. 2).
FIG. 2.
Macrophage activation in jirds inoculated i.p. with female or male B. pahangi measured by the percentages of Toxoplasma-infected macrophages (A) and the mean numbers of Toxoplasma cells per 100 macrophages (B). Bars represent standard deviations of the means.
TNF-like production by peritoneal macrophages from jirds infected with adult female or male B. pahangi.
In the murine system, TNF alpha (TNF-α) has been demonstrated to play an active role in macrophage activation and serves as an inducer of many other inflammatory events. The production of this important inflammatory cytokine during the induction and regulation of macrophage activation was estimated in B. pahangi-infected jirds by the L929 lytic assay. Spontaneous release of TNF above controls occurred erratically in macrophages from female and male worm-inoculated jirds throughout infection. Release of TNF from LPS-stimulated macrophages was markedly elevated with respect to uninfected controls at 15 and 56 dpi in the female and male worm infections, and this response was down modulated at 135 dpi (Table 4).
TABLE 4.
TNF-like production by peritoneal macrophages from jirds with female or male B. pahangi infectiona
| Infection | 15 dpi
|
56 dpi
|
135 dpi
|
|||
|---|---|---|---|---|---|---|
| No LPS | LPS | No LPS | LPS | No LPS | LPS | |
| Female worm | 12.3 ± 0.2 | 95 ± 73.8 | 0.9 ± 1.4 | 173 ± 83 | 7.7 ± 4.6 | 42 ± 9.5 |
| Male worm | 3.6 ± 0.8 | 24 ± 2.5 | 3.2 ± 0.6 | 138 ± 57 | 2.3 ± 2.3 | 5.5 ± 2.3 |
| Control | 2.4 ± 0.04 | 8.7 ± 0 | 1.2 ± 0.9 | 7.14 ± 4 | 3 ± 0.18 | 6 ± 3.7 |
Macrophage monolayers were untreated (no LPS) or stimulated with 50 ng of LPS for 4 h. TNF-like activity was quantitated with the L929 cell line and expressed as units, defined as the reciprocals of the supernatant dilutions which produced 50% lysis of L929 cells (means ± standard deviations). dpi, days postinfection with B. pahangi. Control, uninfected control.
DISCUSSION
Most macrophage activation systems consist of IFN-γ as a first signal, with a second signal represented either by the endogenous production of TNF-α (13, 33, 55) or by that of an exogenous factor (such as LPS) that acts independently of IFN-γ production (55) or induces TNF-α production (12). Macrophage activation in the murine system has been exhaustively studied. However, extrapolation to other species has not always been successful (9, 30, 52, 60). In the present studies, activation of jird macrophages was not accomplished by standard in vitro methods routinely employed in the murine and other systems. Parallel in vitro experiments in mice resulted in macrophage activation, indicating that these methods and reagents are effective in our laboratory. However, BCG-PPD immunization activated jird and murine macrophages to kill Toxoplasma cells when tested ex vivo.
The reasons why jird macrophages could not be activated in vitro to kill Toxoplasma via MAF are at this moment uncertain. If the murine model is the “gold standard” for analyzing NO-enhanced microbicidal effects in vitro, the jird is not the only species that does not conform with accepted dogma. Human macrophages treated with combinations of IFN-γ and TNF-α or LPS in vitro are unable to kill or inhibit Mycobacterium leprae (30), Mycobacterium tuberculosis, or other mycobacteria (3, 9, 52, 60), although similar treatment of human macrophages in vitro will activate them to kill other intracellular pathogens such as Leishmania (9, 18, 39) and Toxoplasma (30) cells. These differences in cell responses between and within animal species indicate that the signaling networks that lead to macrophage activation may be more complex than was first believed.
Furthermore, the fact that the NO pathway cannot be demonstrated consistently in human macrophages (review in reference 5) has also raised doubts about the concept of macrophage activation as defined in the murine system. The NO pathway has been implicated as the primary effector mechanism mediating cytotoxicity of activated macrophages (43). NO was not produced under any circumstance by jird macrophages. However, parallel experiments with murine macrophages demonstrated NO generation as previously reported by other investigators (1, 8, 14, 16, 19). In light of the recent observations on the importance of NO to Brugia killing (50) in murine models, it is interesting to speculate that the lack of NO production by jird macrophages is an important factor in the unique susceptibility of these rodents to filariae and other parasites.
Our results demonstrate that peritoneal macrophages from jirds infected with female or male B. pahangi were toxoplasmacidal at 15 dpi but not at 50 and 135 dpi. Macrophage activation in early B. pahangi infection coincided with the peak in PGRN to Brugia antigens observed in previous studies (41). The loss of toxoplasmacidal activity in peritoneal macrophages later in the course of infection corresponds to down regulation of the PGRN. At 135 dpi, no difference in macrophage microbicidal activity was demonstrated between female and male B. pahangi infections, indicating that the presence of MF, which are produced by the females, was not required for macrophage deactivation. Similarly, previous studies (41, 42) indicate that down regulation of the PGRN is not dependent on the presence of MF. A state of filarial immune hyporesponsiveness with an absence of marked inflammation has been associated with a shift to the Th2 cell phenotype in humans (26, 38). The lack of toxoplasmacidal activity of macrophages in chronically B. pahangi-infected jirds may be related to the presence of Th2 cytokines that deactivate macrophages, such as interleukin 4 (IL-4) (17, 34) and IL-10 (6, 7, 56). These cytokines exert a negative effect on proinflammatory molecules, which would explain the depressed PGRN found previously. The temporal expression of cytokines in the jird during the course of B. pahangi infection and their potential regulatory effects on inflammation are yet to be determined.
Female worm infection resulted in a high accumulation of peritoneal macrophages, presumably due to the continuous release of MF, which act as a potent inflammatory stimulus (41). Interestingly, these macrophages were not activated to kill Toxoplasma and rarely formed granulomas. As has been shown in diverse macrophage-parasite interactions (reviewed in reference 51), defects in macrophage effector functions may result in suppressive effects on other immune cells. For instance, macrophages can be induced to secrete IL-10, transforming growth factor β, and prostaglandin E2, which down regulate cell-mediated immunity and may drive the immune response to a Th2 phenotype. Molecules from filarial nematodes that may exert a direct effect on macrophage function have not been well studied. However, MF have been shown to release prostaglandin E2 (36). This inflammatory molecule has been demonstrated to be a potent immune modulator suppressing macrophage (56, 57, 59) and lymphocyte (11) functions.
Previous studies demonstrated that macrophages from B. pahangi-infected jirds with chronic infections were activated to kill Staphylococcus aureus (22). These results may differ from the current data because the stage initiating infection was L3, which in the chronic phase resulted in the greatest macrophage accumulation with characteristic granuloma formation (23). On the other hand, the immunological requirements to kill the obligate intracellular protozoan Toxoplasma clearly vary from those normally required to kill the extracellular bacterium S. aureus. Microbicidal activity to kill S. aureus by macrophages from L3 B. pahangi-infected jirds was similar to that of thioglycolate-elicited control macrophages. However, attempts to obtain toxoplasmacidal activity in thioglycolate-elicited macrophages failed (data not shown). Killing of Toxoplasma organisms probably requires different immune system-mediated signals than does killing of facultative organisms such as S. aureus. Other investigators have demonstrated variations in the ability of macrophages to cope with organisms of more similar background (18, 31, 39).
An increase in spontaneous and LPS-induced TNF production above that of controls occurred at 15 dpi in both female and male worm infections, corresponding to macrophage activation. LPS-induced TNF production peaked at 56 dpi and decreased markedly at 135 dpi in both female and male infections. The peak in TNF production occurred at the moment macrophages were accumulating in large numbers, especially in the female infection, and may be related to the potent chemotactic function of this cytokine. The subsequent decrease could have been induced by similar factors that caused macrophage deactivation. It has been demonstrated that deactivating cytokines such as IL-10 and IL-4 inhibit production of TNF-α (6, 15). These cytokines may be more abundant or may exert a more intense down-regulatory effect as the infection progresses to the chronic time period.
We have demonstrated that the parasite-specific hyporesponsive state defined for jirds infected with B. pahangi may be associated with a defect in macrophage function that is manifested as an incapacity to kill Toxoplasma and to produce TNF-like molecules. The macrophage is recruited locally in large numbers in response to MF and may prove to be a key effector cell implicated in the immunoregulatory mechanisms that determine disease outcome in filariasis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-19199 from the National Institutes of Health.
We thank J. P. Pasqua for technical assistance and Michael Kearney for help with statistical analysis.
REFERENCES
- 1.Adams L B, Hibbs J B J, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from l-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Agarrwal B B, Kohr W J, Hass P E, Moffat B, Spencer S A, Henzel W J, Bringman T S, Nedwin G E, Goeddel D V, Harkins R N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985;260:2345–2354. [PubMed] [Google Scholar]
- 3.Alfes C, Steele J, Stanford J, Rook G. The effect of lymphokines on the ability of macrophages to protect mycobacteria from a bactericidal antibiotic. Tubercle. 1985;1:1–7. doi: 10.1016/0041-3879(85)90063-7. [DOI] [PubMed] [Google Scholar]
- 4.Civil R H, Warren K S, Mahmoud A A F. Conditions for Bacille Calmette-Guerin-induced resistance to infection with Schistosoma mansoni in mice. J Infect Dis. 1978;137:550–555. doi: 10.1093/infdis/137.5.550. [DOI] [PubMed] [Google Scholar]
- 5.Denis M. Human monocytes/macrophages: NO or no NO? J Leukocyte Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 6.De Waal-Malefyt R, Abrams J, Bennett B, Figdor C G, De Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Waal-Malefyt R, Haanen J, Spits H, Roncarolo M G, Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, De Vries J. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 9.Douvas G S, Looker D L, Vatter A E, Crowle A F. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1986;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drapier J C, Hibbs J B J. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in l-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cell. J Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- 11.Ellner J J, Spagnuold P J. Suppression of antigen and mitogen induced human T-lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979;123:2689–2695. [PubMed] [Google Scholar]
- 12.Green S J, Chen T-Y, Crawford R M, Nacy C N, Morrison D C, Meltzer M S. Cytotoxic activity and production of toxic nitrogen oxides by macrophages treated with IFN-gamma and monoclonal antibodies against the 73kD LPS receptor. J Immunol. 1992;149:2069–2075. [PubMed] [Google Scholar]
- 13.Green S J, Crawford R M, Meltzer M S, Hibbs J B J, Nacy C A. Leishmania provide a second signal for nitric oxide production by interferon-gamma treated macrophages by stimulation of TNF-alpha. J Immunol. 1990;145:4290–4297. [Google Scholar]
- 14.Green S J, Meltzer M S, Hibbs J B, Nacy C A. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–284. [PubMed] [Google Scholar]
- 15.Hart P H, Vitti G F, Burgess D R, Whitty G A, Piccoli D S, Hamilton J A. Potential anti-inflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, prostaglandin E2. Proc Natl Acad Sci USA. 1989;86:3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hibbs J B, Taintor R R, Vavrin Z. Macrophage cytotoxicity: role for l-arginine deiminase and imino nitrogen oxidation of nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 17.Ho J L, He S H, Rios M J, Wick E A. Interleukin-4 inhibits human macrophage activation by tumor necrosis factor, granulocyte-monocyte colony-stimulating factor, and interleukin-3 for antileishmanial activity and oxidative burst capacity. J Infect Dis. 1992;165:344–351. doi: 10.1093/infdis/165.2.344. [DOI] [PubMed] [Google Scholar]
- 18.Hoover D L, Nacy D A, Meltzer M S. Human monocyte activation for cytotoxicity against intracellular Leishmania donovani amastigotes: induction of microbicidal activity by interferon-gamma. Cell Immunol. 1985;94:500–511. doi: 10.1016/0008-8749(85)90274-6. [DOI] [PubMed] [Google Scholar]
- 19.James S L, Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–4212. [PubMed] [Google Scholar]
- 20.James S L, Lazdins J K, Meltzer M S, Sher A. Macrophages as effector cells of protective immunity in murine schistosomiasis. I. Activation of peritoneal macrophages during natural infection. Cell Immunol. 1982;67:255–266. doi: 10.1016/0008-8749(82)90218-0. [DOI] [PubMed] [Google Scholar]
- 21.James S L, Sher A, Lazdins J K, Meltzer M S. Macrophages as effector cells of protective immunity in murine schistosomiasis. II. Killing of newly transformed schistosomula in vitro by macrophages activated as a consequence of Schistosoma mansoni infection. J Immunol. 1982;128:1535–1540. [PubMed] [Google Scholar]
- 22.Jeffers G W, Klei T R, Enright F M. Activation of gerbil (Meriones unguiculatus) macrophages by the filarial parasite Brugia pahangi. Infect Immun. 1984;43:43–48. doi: 10.1128/iai.43.1.43-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffers G W, Klei T R, Enright F M, Henk G W. The granulomatous inflammatory response in gerbils, Meriones unguiculatus, to Brugia pahangi: an ultrastructural and histochemical comparison of reaction in the lymphatics and peritoneal cavity. J Parasitol. 1987;73:1220–1233. [PubMed] [Google Scholar]
- 24.Jeska E L. Mouse peritoneal exudate reactions to parasitic worms. I. Cell adhesion reactions. Immunology. 1969;16:761–771. [PMC free article] [PubMed] [Google Scholar]
- 25.King C L, Kumaraswami V, Poindexter R W, Kumari S, Jayaraman K, Alling D W, Ottesen E A, Nutman T B. Immunological tolerance in lymphatic filariasis: diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J Clin Invest. 1992;89:1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King C L, Mahanty S, Kumaraswami V, Abrams J S, Raganathan J, Jayaraman K, Ottesen E A, Nutman T B. Cytokine control of parasite specific anergy in human lymphatic filariasis: preferential induction of a regulatory Th2 lymphocyte subset. J Clin Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klei T R, Enright F M, Blanchard D P, Uhl S A. Specific hypo-responsive granulomatous tissue reactions in Brugia pahangi-infected jirds. Acta Trop. 1981;38:267–276. [PubMed] [Google Scholar]
- 28.Klei T R, Enright F M, Blanchard D P, Uhl S A. Effects of presensitization on the development of lymphatic lesions in Brugia pahangi-infected jirds. Am J Trop Med Hyg. 1982;31:280–291. doi: 10.4269/ajtmh.1982.31.280. [DOI] [PubMed] [Google Scholar]
- 29.Klei T R, McVay C S, Dennis V A, Coleman S U, Enright F M, Casey H A. Brugia pahangi: effects of duration of infection and parasite burden on lymphatic lesion severity, granulomatous hypersensitivity, and immune responses in jirds (Meriones unguiculatus) Exp Parasitol. 1990;71:393–405. doi: 10.1016/0014-4894(90)90065-k. [DOI] [PubMed] [Google Scholar]
- 30.Krahenbuhl J L, Adams L B. The role of the macrophage in resistance to the leprosy bacillus. Immunol Ser. 1994;60:281–302. [PubMed] [Google Scholar]
- 31.Lainson R. Toxoplasmosis in England. II. Variation factors in the pathogenesis of Toxoplasma infections: the sudden increase in virulence of a strain after passage in multimammate rats and canaries. Ann Trop Med Parasitol. 1955;49:397–416. [PubMed] [Google Scholar]
- 32.Lammie P J, Katz S P. Immunoregulation in experimental filariasis. II. Responses to parasite and nonparasite antigens in jirds infected with Brugia pahangi. J Immunol. 1983;130:1386–1389. [PubMed] [Google Scholar]
- 33.Langermans J A, Van der Hulst M E, Nibberling P H, Hiemstra P S, Fransen L, Van Furth R. IFN-gamma-induced l-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992;148:568–574. [PubMed] [Google Scholar]
- 34.Lehn M, Weiser W Y, Engelhorn S, Gillis S, Remold H G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989;143:3020–3024. [PubMed] [Google Scholar]
- 35.Lichtenberg F. The early phase of endemic bancroftian filariasis in the male. Pathological study. J Mt Sinai Hosp. 1957;26:983–1000. [PubMed] [Google Scholar]
- 36.Liu L X, Buhlmann J E, Weller P F. Release of prostaglandin E2 by microfilariae of Wuchereria bancrofti and Brugia malayi. Am J Trop Med Hyg. 1992;46:520–523. doi: 10.4269/ajtmh.1992.46.520. [DOI] [PubMed] [Google Scholar]
- 37.Mahmoud A A F, Peters A S, Civil R H, Remington J S. In vitro killing of schistosomula of Schistosoma mansoni by BCG and C. parvum activated macrophages. J Immunol. 1979;122:1655–1662. doi: 10.2196/41502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maizels R M, Sartono E, Kurniawan A, Partono F, Selkirk M E, Yazdanbakhsh M. T-cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today. 1995;11:50–56. doi: 10.1016/0169-4758(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 39.Murray H W, Cartelli D M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. J Clin Invest. 1983;72:32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nacy C A, Fortier A H, Meltzer M S, Buchmeier N A, Schreiber R D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon gamma and non-interferon lymphokines. J Immunol. 1985;135:3505–3511. [PubMed] [Google Scholar]
- 41.Nasarre C, Coleman S U, Rao U R, Klei T R. Differential induction and regulation of inflammatory responses by life cycle stages of Brugia pahangi. Exp Parasitol. 1997;87:20–29. doi: 10.1006/expr.1997.4179. [DOI] [PubMed] [Google Scholar]
- 42.Nasarre C, Rao U R, Coleman S U, Klei T R. Effect of gamma radiation on Brugia L3 development in vivo and the kinetics of granulomatous inflammation induced by these parasites. J Parasitol. 1997;83:1119–1123. [PubMed] [Google Scholar]
- 43.Nathan C, Hibbs J B J. Role of nitric oxide synthesis in macrophage antimicrobial immunity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 44.Nelson D S, Subrahmanyam D, Rao Y V B C, Mehta K. Cellular morphology in pleural exudate of albino rats infected with Litomosoides carinii. Trans R Soc Trop Med Hyg. 1976;70:254–255. doi: 10.1016/0035-9203(76)90051-1. [DOI] [PubMed] [Google Scholar]
- 45.Nutman T B, Kumaraswami V, Ottesen E A. Parasite-specific anergy in human filariasis. Insights after analysis of parasite antigen-driven lymphokine production. J Clin Invest. 1987;79:1516–1523. doi: 10.1172/JCI112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ottesen E A. Immunopathology of lymphatic filariasis in man. Springer Semin Immunopathol. 1980;2:373–385. [Google Scholar]
- 47.Ottesen E A, Weller P F, Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977;33:413–421. [PMC free article] [PubMed] [Google Scholar]
- 48.Petit T A, Coleman S U, Jones K L, Enright F M, Klei T R. Brugia pahangi: effects of protective resistance on lymphatic lesions and granulomatous inflammation in infected gerbils (Meriones unguiculatus) Exp Parasitol. 1993;77:395–404. doi: 10.1006/expr.1993.1099. [DOI] [PubMed] [Google Scholar]
- 49.Piessens W F, McGreevy P B, Piessens P W, McGreevy M, Koiman J, Sarsoso H S, Dennis D T. Immune responses in human infections with Brugia malayi. Specific cellular unresponsiveness to filarial antigens. J Clin Invest. 1980;65:172–179. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajan T V, Porte P, Yates J A, Keefer L, Schultz L D. Role of nitric oxide in host defense against an extracellular, metazoan parasite, Brugia malayi. Infect Immun. 1996;64:3351–3353. doi: 10.1128/iai.64.8.3351-3353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiner N E. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today. 1994;15:374–381. doi: 10.1016/0167-5699(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 52.Rook G A W, Steel J, Ainsworth M, Champion B R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986;19:333–338. [PMC free article] [PubMed] [Google Scholar]
- 53.Ruco L P, Meltzer M S. Macrophage activation for tumor cytotoxicity: induction of tumoricidal macrophages by PPD in BCG-immune mice. Cell Immunol. 1977;32:203–215. [PubMed] [Google Scholar]
- 54.Sibley D L, Krahenbuhl J L. Defective activation of granuloma macrophages from Mycobacterium leprae-infected nude mice. J Leukocyte Biol. 1988;43:60–66. doi: 10.1002/jlb.43.1.60. [DOI] [PubMed] [Google Scholar]
- 55.Sibley L D, Adams L B, Fukutomi Y, Krahenbuhl J L. Tumor necrosis factor-alpha triggers antitoxoplasma activity of IFN-gamma-primed macrophages. J Immunol. 1991;147:2340–2345. [PubMed] [Google Scholar]
- 56.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1991;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snyder D S, Beller D I, Unanue E R. Prostaglandin-modulated macrophage Ia expression. Nature. 1982;299:163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- 58.Stadecker M J. The shrinking schistosomal egg granuloma: how accessory cells control T cell-mediated pathology. Exp Parasitol. 1994;79:198–201. doi: 10.1006/expr.1994.1080. [DOI] [PubMed] [Google Scholar]
- 59.Taffet S M, Russell S W. Macrophage-mediated tumor cell killing: regulation of expression of cytolytic activity by prostaglandin E. J Immunol. 1981;126:424–427. [PubMed] [Google Scholar]
- 60.Toba H, Crawford J, Ellner J. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophage-activating factor activity of human gamma interferon. Infect Immun. 1989;57:239–244. doi: 10.1128/iai.57.1.239-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson C B, Tsai V, Remington J S. Failure to trigger the oxidative metabolic burst by normal macrophages. J Exp Med. 1980;151:328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]