Abstract
Purpose
Optimal integration of local therapy and systemic immune therapy for patients with mucosal melanoma (MM) is uncertain. We evaluated treatment patterns and outcomes following radiation therapy (RT) in combination with immune checkpoint inhibition (ICI) in MM.
Methods and Materials
Thirty-seven patients with localized (n = 32, 87%) or node-positive (n = 5, 14%) MM were treated across 4 institutions with RT to the primary tumor with or without oncologic resection (n = 28, 76%) and ICI from 2012 to 2020. Recurrence rates were estimated using cumulative incidence in the presence of the competing risk of death.
Results
Mucosal sites were head/neck (n = 29, 78%), vaginal (n = 7, 19%), and anorectal (n = 1, 3%). Patients received ICI prior to or concurrent with RT (n = 14, 38%), following RT (n = 5, 14%), or at recurrence (n = 18, 49%). The objective response rate for evaluable patients was 31% for ICI as initial treatment (95% CI, 11%-59%) and 19% for ICI at recurrence (95% CI, 4%-46%). Median follow-up was 26 months for living patients; median overall survival (OS) was 54 months (95% CI, 31 months-not reached). Two-year OS was 85%; distant metastasis-free survival 44%. The 2-year cumulative incidence of local recurrence (LR) was 26% (95% CI, 13%-41%). For 9 patients with unresectable disease, 2-year OS was 88% (95% CI, 35%-98%); LR was 25% (95% CI, 3%-58%). For 5 patients with positive nodes at diagnosis, 2-year OS was 100%; LR was 0%.
Conclusions
High rates of local control were achieved with RT with or without oncologic resection and ICI for localized and locally advanced MM. In particular, favorable local control was possible even for patients with unresectable or node-positive disease. Although risk of distant failure remains high, patients with MM may benefit from aggressive local therapy including RT in the setting of immunotherapy treatment.
Introduction
Mucosal melanoma (MM) is a rare cancer that arises from melanocytes in tissues that are not exposed to the sun and has lower mutation rates than cutaneous melanoma.1 Outcomes for MM are poor, with reported 5-year survival of 24% to 50%.2, 3, 4, 5, 6, 7, 8 Advanced tumor stage and node-positive disease are negative prognostic factors.2,9,10 Though immune checkpoint inhibitors (ICI) with programmed cell death protein 1 (PD-1) inhibitors have improved outcomes in cutaneous melanoma,11 the response rates for MM are lower. For example, the objective response rate (ORR) for nivolumab is 23% for MM versus 41% for cutaneous melanoma.12,13 Based on this reduced efficacy in the metastatic population, patients with MM have been generally excluded from clinical trials investigating the use of adjuvant ICI.
Given the modest response rates reported for ICI, optimizing the role of radiation in the treatment of localized MM remains important. Retrospective studies evaluating patients with head and neck MM demonstrate 5-year local control rates of 75% with adjuvant radiation therapy (RT) versus 43% for surgery alone.2 The benefit of RT is reflected in National Comprehensive Cancer Network guidelines, which recommend postoperative or definitive RT for T3-4 MM of the head and neck and note that data for adjuvant systemic therapy are limited compared to cutaneous melanoma.14 Given the challenge of attaining local control even with surgery and radiation, recent studies have explored the addition of ICI to local therapy for MM, particularly for vaginal and anorectal disease.7,8,15
In this study, using a competing risk analysis, we review recurrence and survival outcomes from a multi-institutional retrospective cohort of patients with nonmetastatic MM of the head and neck, gastrointestinal tract, and vagina treated with curative intent with RT to the primary site and ICI therapy.
Methods and Materials
We performed a retrospective review at 4 National Cancer Institute Designated Comprehensive Cancer Centers after each site obtained local institutional review board approval. Eligible patients had MM with localized or locally advanced (node-positive) disease at presentation treated with RT to the primary tumor site from 2012 to 2020. All patients had ICI during their MM treatment course. ICI agents included pembrolizumab (200 mg every 3 weeks or 400 mg every 6 weeks), nivolumab (240 mg every 2 weeks or 480 mg every 4 weeks), ipilimumab and nivolumab (nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for 4 doses, followed by nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks), and atezolizumab (administered to 1 patient in a phase 1 trial, 1353 mg every 3 weeks). For patients who had measurable disease at the time of receiving ICI, best response was assessed based on review of the medical record including available radiology reports and measured according to Response Evaluation Criteria in Solid Tumors. Patients without measurable disease at the start of ICI were excluded from this analysis. Biologically effective dose calculations were performed using an α:β ratio of 2.5 Gy.16
Recurrence rates and survival outcomes were estimated using crude rates and Kaplan-Meier and are reported with associated 95% CIs. Survival and recurrence outcomes were measured from the date of pathologic diagnosis. Time to local, regional nodal, regional non-nodal, and distant recurrence were collected for each patient regardless of site of first failure. Cumulative incidence estimates are reported for these recurrence outcomes with death as the competing risk. Progression-free survival is defined as the time from diagnosis until the earliest local, regional nodal, or regional non-nodal recurrence, distant metastasis, or death. Cox proportional hazards models were used to estimate predictors of survival. The Fine and Gray method was used for univariate estimates of local and regional recurrences adjusting for competing risk of death.17 All tests were 2-sided. Data analyses were performed using R version 4.0.3 or SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Initial treatment
We identified 37 patients with localized (n = 32, 86%) or node-positive (n = 5, 14%) MM who received RT to the primary tumor site during their treatment course from 2012 to 2020 and ICI as part of initial management or at the time of recurrence (Table 1). The most common site was MM of the head and neck (n = 29, 78%), followed by vulvovaginal (n = 7, 19%) and anorectal (n = 1, 3%). Among 23 patients with available tumor sequencing, the most commonly mutated gene was NRAS (n = 8) followed by KIT (n = 2), CDK (n = 2), ATRX (n = 2), APC (n = 2), and TP53 (n = 2).
Table 1.
Clinical and treatment characteristics for 37 patients with mucosal melanoma
| Age at RT | Median (range)64 (36-90) |
|---|---|
| Sex | No (%) |
| Female | 19 (51) |
| Male | 18 (49) |
| ECOG performance status | |
| 0 | 20 (54) |
| 1 | 13 (35) |
| 2 | 3 (8) |
| 3 | 1 (3) |
| Mucosal site | |
| Sinonasal/orbital | 24 (65) |
| Sinus | 6 (25) |
| Nasal cavity | 11 (46) |
| Orbital/periorbital | 1 (4) |
| Overlapping sites | 6 (25) |
| Pharyngeal | 2 (5) |
| Nasopharynx | 1 (50) |
| Nasopharynx and oropharynx | 1 (50) |
| Oral cavity | 3 (8) |
| Vaginal | 7 (19) |
| Anorectal | 1 (3) |
| Node positive | 5 (14) |
| Previous therapy | |
| Surgical resection | |
| None or partial biopsy | 6 (16) |
| Excisional biopsy | 3 (8) |
| Conservative oncologic resection | 18 (49) |
| Radical resection | 10 (27) |
| Surgical nodal staging | |
| None | 28 (76) |
| SLN biopsy | 2 (5) |
| LN dissection | 7 (19) |
| Surgical margin status | |
| R0 | 17 (46) |
| R1 | 8 (22) |
| R2 or not resected | 12 (32) |
| Common tumor mutations identified* | |
| NRAS | 8 |
| KIT | 2 |
| CDK4 | 2 |
| ATRX | 2 |
| APC | 2 |
| TP53 | 2 |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; LN = lymph node; RT = radiation therapy; SLN = sentinel lymph node.
Additional mutations were identified in 1 patient each for KRAS, ALK, PIK3R1, DAXX, RHOA, TSC1, CTNNB1, HRAS, NF2, NF1, ATM, CHEK2, FAS, KDM6A, MDM2, PTE, TET2, and PDGFRA.
Twenty-eight patients (76%) underwent oncologic resection of the primary tumor, with 9 patients (24%) undergoing surgical nodal evaluation (7 patients underwent nodal dissection and 2 patients underwent sentinel node biopsy). Complete resection with negative (R0) margins was achieved in 17 of 28 patients (61%).
Nine patients (24%) with unresectable disease received definitive RT, while 28 patients received adjuvant RT (76%). The majority of patients were treated with conventionally fractionated intensity modulated RT (n = 23, 62%), while 3 patients were treated with stereotactic body RT, 7 with proton RT, 3 with brachytherapy, and 1 with 3-dimensional conformal RT. All 3 patients treated with stereotactic body RT had vaginal disease, and brachytherapy was used for 2 patients with vaginal disease and 1 with nasal cavity disease. The median biologically effective dose (α:β = 2.5) for this cohort was 115 Gy, with dose varying by treatment site as shown in Table 2. Eight patients (22%) received RT to draining lymph nodes in addition to the primary tumor: 6 for head and neck tumors, 1 for a vaginal tumor, and 1 for an anorectal tumor.
Table 2.
Details of radiation and immunotherapy
| RT site | No. (%) |
|---|---|
| Primary only | 29 (78) |
| Primary and regional LNs | 8 (22) |
| Median (range) | |
| BED2.5, Gy | 115 (77-168) |
| Adjuvant RT | 113 (90-135) |
| Definitive RT | 119 (77-168) |
| Dose delivered, Gy | Median (range) |
| Sinonasal/orbital | |
| EBRT alone | 66 (30-70) |
| EBRT + HDR brachy | 45 Gy EBRT, 3 Gy x 5 HDR |
| Pharyngeal | 65 (60-70) |
| Oral cavity | 60 (48-66) |
| Vaginal/vulvar | |
| EBRT alone | 38 (30-54) |
| EBRT + HDR brachy | 45 Gy EBRT, 3 Gy x 5 HDR |
| HDR brachy alone | 6 Gy x 5 HDR |
| Anorectal | 50 (50) |
| Number of fractions (EBRT) | 30 (5-35) |
| RT technique | No (%) |
| IMRT/VMAT | 23 (62) |
| 3D conformal | 1 (3) |
| SBRT | 3 (8) |
| Proton | 7 (19) |
| HDR brachytherapy | 1 (3) |
| Conformal and HDR brachytherapy | 2 (5) |
| Date of RT treatment initiation | |
| 2012-2015 | 12 (32) |
| 2016-2020 | 25 (68) |
| ICI type | |
| CTLA4 | 3 (8) |
| PD-1/PD-L1 | 19 (51) |
| Combination | 15 (41) |
| Timing of ICI therapy | |
| Prior to RT | 5 (14) |
| Concurrent with RT | 9 (24) |
| After surgery/RT | 5 (14) |
| At recurrence/metastasis | 18 (49) |
| Response to ICI among evaluable patients | |
| CR | 7 (22) |
| PR | 1 (3) |
| SD | 3 (9) |
| PD | 21 (66) |
Abbreviations: 3D = 3-dimensional; BED = biologically effective dose (α:β = 2.5); CR = complete response; CTLA4 = cytotoxic T-lymphocyte-associated protein 4; EBRT = external beam radiation therapy; HDR = high dose rate; ICI = immune checkpoint inhibition; IMRT = intensity modulated radiation therapy; LN = lymph node; PD = progressive disease; PD-1 = programmed cell death protein 1; PD-L1 = programmed cell death ligand; PR = partial response; RT = radiation therapy; SBRT = stereotactic body radiation therapy; SD = stable disease; VMAT = volumetric modulated arc therapy.
All 37 patients received ICI. The timing of first course of immunotherapy was prior to RT for 5 patients (14%), concurrent with RT for 9 patients (24%), after RT for 5 patients (14%), and at the time of recurrence or metastasis for 18 patients (49%). Of the 18 patients first receiving ICI at recurrence, 12 received RT prior to 2016 when PD-1 therapy was approved. For those first receiving ICI at recurrence, the median time from RT to ICI initiation was 14.0 months. The ORR when ICI was delivered with RT was 31% (5 of 16 response-evaluable; 95% CI, 11%-59%) for patients who received RT as part of initial management and 19% (3 of 16 evaluable; 95% CI, 4%-46%) for patients who first received ICI at recurrence with or without RT. Seven patients experienced a complete response, with 4 receiving concurrent ICI and RT and 3 receiving ICI after recurrence (1 received pembrolizumab, 1 received ipilimumab and nivolumab, and 1 received pembrolizumab and RT).
Recurrence patterns and management
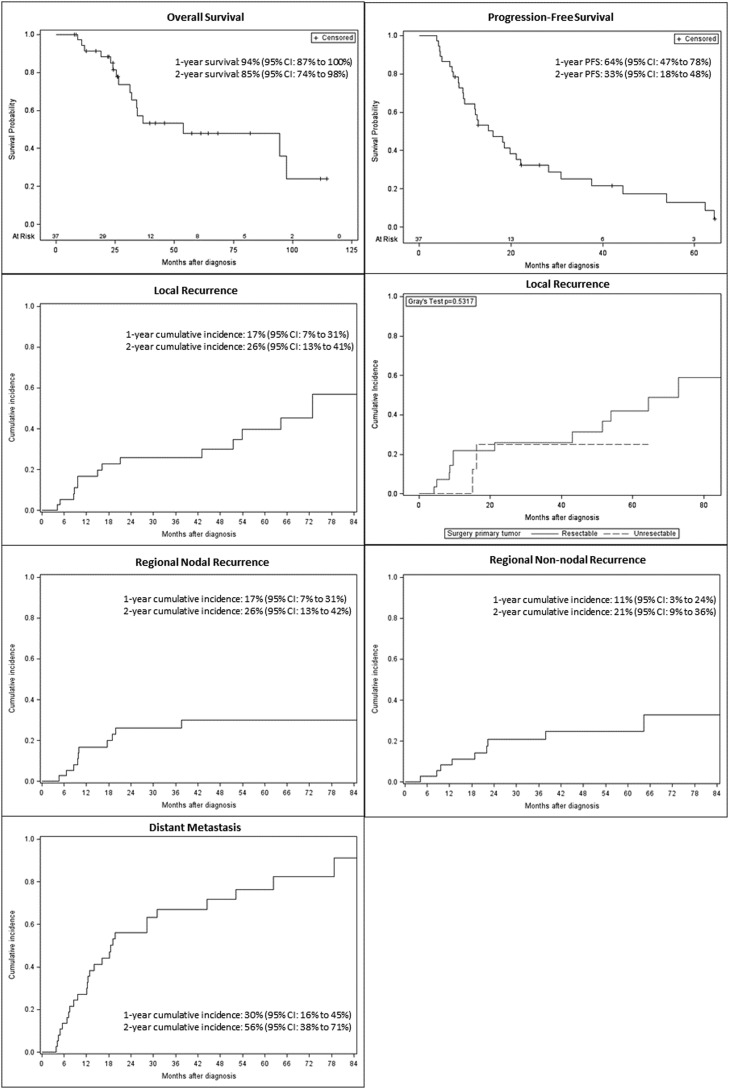
The median follow-up for living patients was 26 months, and the median overall survival for the entire cohort was 54 months (95% CI, 31 months-not reached) (Fig. 1). Two-, 3-, and 5-year survival estimates were 85% (95% CI, 74%-98%), 57% (95% CI, 41%-80%), and 48% (95% CI, 32%-73%). Of the 16 patients who died, all experienced distant metastasis and 6 experienced both locoregional and distant recurrence. Median progression-free survival for the entire cohort was 16 months (95% CI, 9.7-22.1 months); the 2-year progression-free survival estimate was 33% (95% CI, 18%-48%).
Figure 1.
Survival curves for overall survival and progression-free survival, cumulative incidence of local recurrence for the entire cohort and for resectable versus unresectable patients, regional nodal recurrence, regional non-nodal recurrence, and distant metastasis.
Fourteen patients developed local recurrence after treatment to the primary site, with an estimated 2-year cumulative incidence of 26% (95% CI, 13%-41%). Of the 14 patients with local recurrence, 7 were alive at last follow-up, 1 developed distant metastasis prior to local recurrence, 8 subsequently developed distant metastasis, and 5 did not develop distant metastasis. Eleven of the patients who subsequently developed local recurrence had a head and neck primary treated at first diagnosis with resection and adjuvant RT, and 3 received upfront ICI with ipilimumab/nivolumab. Three patients with local recurrence had a vaginal primary; initial therapy at diagnosis was resection and adjuvant RT for 2 patients and 1 received definitive RT. Three patients were receiving ICI at the time of local recurrence, 3 had previously completed or discontinued ICI, and 8 first received ICI after recurrence.
Ten patients developed regional nodal recurrence and 9 developed regional non-nodal recurrence, with a 2-year cumulative incidence of 26% (95% CI, 13%-42%) for nodal recurrence and 21% (95% CI, 9%-26%) for non-nodal recurrence. Of 14 patients who developed regional nodal and/or non-nodal recurrence, 2 had nodal disease at diagnosis, both of whom were treated with a neck dissection and 1 received adjuvant nodal RT. Only 2 patients with regional recurrence had received elective nodal RT. Of the 29 patients who received RT to the primary without including the regional nodes, 9 (31%) developed nodal recurrence. Twenty-seven patients developed distant metastasis at a median of 19 months (95% CI, 12-31 months) with a 2-year cumulative incidence of 56% (95% CI, 38%-71%). The most common isolated site of recurrence was lung (n = 5, 16%) (Table 3).
Table 3.
Initial site of recurrence for 31 patients who developed recurrence
| Site of first recurrence | No. (%) |
|---|---|
| Multiple sites | 11 (35) |
| Lung | 5 (16) |
| Sinus | 3 (10) |
| Liver | 2 (6) |
| Other intrabdominal | 3 (10) |
| Nasal cavity | 2 (6) |
| Skin | 1 (3) |
| Bone | 1 (3) |
| Brain | 1 (3) |
| Neck | 1 (3) |
| Vagina | 1 (3) |
On univariate analysis, there were no significant predictors of local control, overall survival, progression-free survival, or distant metastasis (Table E2). For 9 patients with unresectable disease, 2-year overall survival was 88% (95% CI, 35%-98%). The estimated cumulative incidence of local recurrence was 25% (95% CI, 3%-58%), regional nodal recurrence 13% (95% CI, 0%-44%), and regional non-nodal recurrence 13% (95% CI, 0%-44%). Five of 9 patients developed recurrence, 1 local, 2 distant, 1 nodal and distant, and 1 local, regional, and distant. Two of these patients died after surviving for over 1 year with distant metastasis while maintaining local control. For 5 patients with positive nodes prior to RT, 2-year overall survival was 100%. Estimated cumulative incidence of local recurrence was 0%, regional nodal recurrence was 20% (95% CI, 0%-62%), and regional non-nodal recurrence was 0%. Three of these 5 patients (60%) developed recurrence, 1 local and regional non-nodal, 1 nodal and distant, and 1 distant.
Eight of the 21 patients alive at last follow-up were receiving ongoing ICI therapy. Subsequent systemic therapies for patients with recurrence included palbociclib, trametinib, alectinib, cabozantinib with MDM2 inhibitor, carboplatin/paclitaxel, temozolomide/cisplatin, carboplatin/paclitaxel/temozolomide, temozolomide monotherapy, paclitaxel monotherapy, dacarbazine, talimogene laherparepvec, and cell therapy KITE-718. Patients received RT to sites of recurrence including the brain, skull base, head and neck, spine, bone, skin, adrenal, abdomen, and vagina.
Safety and tolerability
Seventeen patients (46%) developed an immune-related adverse event (irAE) that required immunosuppression or discontinuation of ICI (Table E3). All 17 patients received both a cytotoxic T-lymphocyte-associated protein 4-directed and PD-1/programmed cell death ligand 1-directed agent at some point in their course. Of the 9 patients who received concurrent ICI and RT, 4 developed irAEs requiring immunosuppression or discontinuation of ICI at some point in their course, and none of the irAEs occurred within the RT field. Reported grade 2 or higher RT toxicities included central vision loss, glaucoma, epiphora, brain radionecrosis, dermatitis, mucositis, dysphagia, weight loss, xerostomia, and osteomyelitis.
Discussion
In this heterogeneous cohort of localized and patients with locally advanced MM who received RT and ICI therapy with or without surgery, the 5-year overall survival of 48% is comparable to rates of 24% to 50% in studies prior to the use of ICI (Table 4).2, 3, 4, 5 A recent study of patients with MM of the head and neck treated with curative intent surgery and/or RT with ICI for disease recurrence reported a 3-year survival of 33%.18 Another group reported 49% 3-year survival for sinonasal MM and found a survival benefit for adjuvant RT compared to surgery alone.19 These findings compare with 57% 3-year survival in our study where approximately half of patients received ICI in initial management. Another study of resectable MM treated with neoadjuvant ICI demonstrated a 55% 3-year survival.15
Table 4.
Local control and overall survival outcomes of published studies of nonmetastatic mucosal melanoma
| Study (ref) | Number of patients | Disease site | Treatment | 1 yLC | 2 yLC | 2 yOS | 3 yLC | 3 yOS | 4 yLC | 4 yOS | 5 yLC | 5 yOS | Median OS(mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moreno et al4 | 58 | HN | S, RT, C, IO | 58% | 39% | ||||||||
| Benlyazid et al5 | 160 | HN | S, RT | 57% | 38% | ||||||||
| Gal et al3 | 304 | HN | S, RT | 24% | 18 | ||||||||
| Sun et al2 | 161 | HN | S S + RT RT |
43% 75% 56% |
50% 43% 28% |
||||||||
| Sinasac et al6 | 68 | Vulvar vaginal |
S, RT, C, Ifn | 45 10 |
|||||||||
| Sahovaler et al18 | 60* | HN | S, RT, C, IO | 69%† | 33% | ||||||||
| Mitra et al7 | 124 | Vulvar, vaginal, cervical | S, RT, C, IO | 46% | 48% | ||||||||
| Mitra et al8 | 108 | AR | S, RT, C, IO | 84% | 51%‡ | ||||||||
| Ho et al15 | 36 | AR, GU, HN, E | S, RT, C, IO | 64% | 55% | Not reached | |||||||
| Lechner et al19 | 505 | Sinonasal | S, RT, C, IO | 49% | 38% | ||||||||
| Smart et al (current study) | 37 | HN, AR, vaginal | S, RT, IO | 74% | 85% | 74% | 57% | 60% | 48% | 54 | |||
| Unresected | |||||||||||||
| Sun et al2 | 14 | HN | 56% | 28% | |||||||||
| Teterycz et al20 | 14§ | HN, GI, GU | RT, IO | 75% | |||||||||
| Sahovaler et al18 | 7 | HN | 86%† | 14% | |||||||||
| Mitra et al7 | 20 | Vulvar, vaginal, cervical | 74% | 36% | |||||||||
| Smart et al (current study) | 9 | HN, vaginal | 75% | 88% | |||||||||
| Node positive | |||||||||||||
| Sun et al2 | 36 | HN | 77% | 32% | |||||||||
| Mitra et al7 | 30 | Vulvar, vaginal, cervical | 53% | 31% | |||||||||
| Garg et al28 | 9 | AR | 30 | ||||||||||
| Smart et al (current study) | 5 | HN | 100% | 100% |
Abbreviations: AR = anorectal; C = chemotherapy; E = esophageal; GI = gastrointestinal; GU = genitourinary; HN = head and neck; Ifn = interferon; IO = immunotherapy; LC = local control; OS = overall survival; RT = radiation therapy; S = surgery.
Curative intent cohort.
Locoregional control.
Melanoma-specific survival.
Locally advanced unresectable subset.
While long-term survival remains low, local control of 74% at 2 years was achieved with postoperative or definitive RT, which is in line with prior studies.2,18,20,21 Case reports of RT and ICI for vaginal22, 23, 24, 25 and anorectal26,27 melanoma have also demonstrated durable responses. We found that favorable local control was possible even in patients with node-positive disease, with 2-year overall survival 100% and local control 100%. In patients with unresectable disease, 2-year overall survival was 88% and local control was 75%. Five-year overall survival of 28% and local control of 56% have been reported for head and neck MMs treated with definitive RT without ICI.2 A recent series demonstrated 75% local control at 1 year for unresectable MM treated with RT and ICI.20
All 8 patients with a complete or partial response to ICI with or without RT survived 2 years from diagnosis, compared to 65% 2-year survival for patients with progressive disease. In this cohort, the ORR to ICI was 31% for ICI as initial treatment when delivered in a multimodality approach and 19% for ICI at recurrence, which is in line with other reports,28 including a recent large retrospective cohort12 and a pooled analysis of clinical trials.13 Meanwhile, an ORR of 47% has been reported for neoadjuvant ICI monotherapy.15 Kim et al21 report an infield ORR of 53% for MM treated with ICI and RT. However, in our series, attribution of response was limited for 5 patients who responded to initial treatment with ICI and RT, and response at the primary site was attributed to both modalities.
ICI therapy represents a major advance in the management of melanoma. However, given the lower response rate, patients with MM may benefit from aggressive local therapies including oncologic resection and RT. Limitations of our study include the small size and heterogeneity in primary tumor site and treatment approach, while strengths include its multi-institutional scope and use of competing risks analysis. These data support a role for surgery and RT in management of localized and locally advanced MM.
Conclusion
The use of definitive or adjuvant RT with or without oncologic resection and ICI therapy for patients with locally advanced MM demonstrated promising 2-year local control in this multi-institutional retrospective cohort, consistent with other retrospective series. Durable local control of the primary was possible with aggressive local therapy for patients with node-positive or unresectable disease. As in other studies, response to ICI was lower compared to cutaneous melanoma. While patients experience a high incidence of distant metastasis, further studies should investigate the role of aggressive local control, including RT for MM in the immunotherapy era.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Sources of support: This work had no specific funding.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2023.101310.
Appendix. Supplementary materials
References
- 1.Lazarev S, Gupta V, Hu K, Harrison LB, Bakst R. Mucosal melanoma of the head and neck: A systematic review of the literature. Int J Radiat Oncol Biol Phys. 2014;90:1108–1118. doi: 10.1016/j.ijrobp.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Sun S, Gao L, Huang X, et al. Long-term treatment outcomes and prognosis of mucosal melanoma of the head and neck: 161 cases from a single institution. Oral Oncol. 2017;74:115–122. doi: 10.1016/j.oraloncology.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Gal TJ, Silver N, Huang B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope. 2011;121:2026–2033. doi: 10.1002/lary.21925. [DOI] [PubMed] [Google Scholar]
- 4.Moreno MA, Roberts DB, Kupferman ME, et al. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer. 2010;116:2215–2223. doi: 10.1002/cncr.24976. [DOI] [PubMed] [Google Scholar]
- 5.Benlyazid A, Thariat J, Temam S, et al. Postoperative radiotherapy in head and neck mucosal melanoma: A GETTEC study. Arch Otolaryngol Head Neck Surg. 2010;136:1219–1225. doi: 10.1001/archoto.2010.217. [DOI] [PubMed] [Google Scholar]
- 6.Sinasac SE, Petrella TM, Rouzbahman M, Sade S, Ghazarian D, Vicus D. Melanoma of the vulva and vagina: Surgical management and outcomes based on a clinicopathologic review of 68 cases. J Obstet Gynaecol Can. 2019;41:762–771. doi: 10.1016/j.jogc.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Mitra D, Farr M, Nagarajan P, et al. Gynecologic tract melanoma in the contemporary therapeutic era: High rates of local and distant disease progression. Gynecol Oncol. 2022;167:483–489. doi: 10.1016/j.ygyno.2022.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra D, Rao PK, Nagarajan P, et al. Outcomes after sphincter-sparing local therapy for anorectal melanoma: 1989 to 2020. Pract Radiat Oncol. 2022;12:437–445. doi: 10.1016/j.prro.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Jethanamest D, Vila PM, Sikora AG, Morris LGT. Predictors of survival in mucosal melanoma of the head and neck. Ann Surg Oncol. 2011;18:2748–2756. doi: 10.1245/s10434-011-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuman AG, Light E, Olsen SH, et al. Mucosal melanoma of the head and neck: Predictors of prognosis. Arch Otolaryngol Head Neck Surg. 2011;137:331–337. doi: 10.1001/archoto.2011.46. [DOI] [PubMed] [Google Scholar]
- 11.Dimitriou F, Long GV, Menzies AM. Novel adjuvant options for cutaneous melanoma. Ann Oncol. 2021;32:854–865. doi: 10.1016/j.annonc.2021.03.198. [DOI] [PubMed] [Google Scholar]
- 12.Dimitriou F, Namikawa K, Reijers ILM, et al. Single-agent anti-PD-1 or combined with ipilimumab in patients with mucosal melanoma: An international, retrospective, cohort study. Ann Oncol. 2022;33:968–980. doi: 10.1016/j.annonc.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 13.D'Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients withmucosal melanoma: A pooled analysis. J Clin Oncol. 2017;35:226–235. doi: 10.1200/JCO.2016.67.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddad RI, Hicks WL, Hitchcock YJ, et al. NCCN Guidelines Version 2.2022 Head and neck cancers Available at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed July 27, 2023.
- 15.Ho J, Mattei J, Tetzlaff M, et al. Neoadjuvant checkpoint inhibitor immunotherapy for resectable mucosal melanoma. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overgaard J. The role of radiotherapy in recurrent and metastatic malignant melanoma: A clinical radiobiological study. Int J Radiat Oncol Biol Phys. 1986;12:867–872. doi: 10.1016/0360-3016(86)90378-0. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Sahovaler A, Ziai H, Cardemil F, et al. Importance of margins, radiotherapy, and systemic therapy in mucosal melanoma of the head and neck. Laryngoscope. 2021;131:2269–2276. doi: 10.1002/lary.29555. [DOI] [PubMed] [Google Scholar]
- 19.Lechner M, Takahashi Y, Turri-Zanoni M, et al. International multicenter study of clinical outcomes of sinonasal melanoma shows survival benefit for patients treated with immune checkpoint inhibitors and potential improvements to the current TNM staging system. J Neurol Surg B Skull Base. 2022;84:307–319. doi: 10.1055/s-0042-1750178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teterycz P, Czarnecka AM, Indini A, et al. Multimodal treatment of advanced mucosal melanoma in the era of modern immunotherapy. Cancers (Basel) 2020;12:1–15. doi: 10.3390/cancers12113131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HJ, Chang JS, Roh MR, et al. Effect of radiotherapy combined with pembrolizumab on local tumor control in mucosal melanoma patients. Front Oncol. 2019;9:835. doi: 10.3389/fonc.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiavone MB, Broach V, Shoushtari AN, et al. Combined immunotherapy and radiation for treatment of mucosal melanomas of the lower genital tract. Gynecol Oncol Rep. 2016;16:42–46. doi: 10.1016/j.gore.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parisi S, Lillo S, Cacciola A, et al. Vaginal mucosal melanoma: A complete remission after immunotherapy and “0-7-21” radiotherapy regimen (24 Gy/3 fractions/21 days) Folia Med (Plovdiv) 2020;62:605–609. doi: 10.3897/folmed.62.e49926. [DOI] [PubMed] [Google Scholar]
- 24.Sezen D, Patel RR, Tang C, et al. Immunotherapy combined with high- and low-dose radiation to all sites leads to complete clearance of disease in a patient with metastatic vaginal melanoma. Gynecol Oncol. 2021;161:645–652. doi: 10.1016/j.ygyno.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Ishiguro A, Ogata D, Okuma K, et al. Malignant melanoma treatment using brachytherapy: Two case reports and 15 case series. J Dermatol. 2023;50:94–97. doi: 10.1111/1346-8138.16599. [DOI] [PubMed] [Google Scholar]
- 26.Wallington DG, Rashid AS, Buchwald ZS, Sudmeier LJ, Khan MK. Complete and durable response after radiation therapy to primary tumor site of a patient with metastatic anorectal mucosal melanoma with oligoprogression on nivolumab. Adv Radiat Oncol. 2020;5:503–510. doi: 10.1016/j.adro.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai H, Takeda M, Sakai K, Nishio K, Nakagawa K. Long-term response to ipilimumab after nivolumab failure in a case of anorectal melanoma with an intermediate tumor mutation burden and negative for PD-L1 expression. Mol Clin Oncol. 2020;13:175–178. doi: 10.3892/mco.2020.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg V, Rastogi S, Aswar H, et al. Clinicopathological profile and outcomes of anorectal melanoma from a tertiary care center in India. Future Sci OA. 2022;8 doi: 10.2144/fsoa-2021-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.