Abstract
Purpose
Stereotactic body radiation therapy (SBRT) is considered the standard of care for medically inoperable early-stage non-small cell lung cancer. There is mixed evidence on the prognostic significance of tumor metabolic activity assessed by positron emission tomography combined with computed tomography (PET/CT) using F-18 fluorodeoxyglucose (FDG). The objectives of this study were to evaluate the maximum standardized uptake value (SUVmax) pretreatment and at 3 and 6 months after SBRT for prediction of tumor control and survival outcomes.
Methods and Materials
Consecutive patients from a single institution with T12N0M0 non-small cell lung cancer receiving primary treatment with SBRT with pretreatment FDG-PET/CT (n = 163) and follow-up FDG-PET/CT at 3 or 6 months (n = 71) were included. Receiver operator characteristic analysis was performed to dichotomize variables for Kaplan-Meier survival analysis. Multivariate analysis was performed with Cox proportional hazards regression.
Results
Median follow-up was 19 months. For the whole cohort, 1-year and 2-year local control, progression-free survival (PFS), and overall survival (OS) were 95.0% and 80.3%, 87.1% and 75.4%, and 67.0% and 49.6% respectively. The following pre-SBRT SUVmax cutoffs were significant: SUV > 4.0 for distant failure-free survival (adjusted hazard ratio [aHR], 3.33, P = .006), >12.3 for PFS (aHR, 2.80, P = .011), and >12.6 for OS (aHR, 3.00, P = .003). SUVmax decreases of at least 45% at 3 months (aHR, 0.15, P = .018), and 53% at 6 months (aHR, 0.12, P = .046) were associated with improved local failure-free survival.
Conclusions
Pre-SBRT SUVmax cutoffs can predict distant failure, PFS, and OS. At both 3 and 6 months after SBRT, cutoffs for percentage change in SUVmax can potentially stratify risk of local recurrence.
Introduction
Lung cancer is a leading cause of cancer-related death, and non-small cell lung cancer (NSCLC) accounts for a majority of cases.1,2 Stereotactic body radiation therapy (SBRT) has emerged as the standard of care for medically inoperable early-stage NSCLC (ES-NSCLC). Compared with conventional radiation therapy techniques, SBRT has demonstrated improved local control (LC) and overall survival (OS).3, 4, 5
Adjuvant chemotherapy for NSCLC has demonstrated improved OS and reduced distant relapse rates in patients treated with surgery; however, patients undergoing SBRT may not always have pathologic data to inform decision-making about risk stratification and adjuvant therapy.6 A recent NCDB (National Cancer Database) analysis found that adjuvant chemotherapy after SBRT was more likely to be offered to younger patients, patients with tumor size >4 cm, patients with fewer comorbidities, and patients treated at community cancer programs.7 Given the limited evidence on determining which patients are candidates for further therapy, the identification of prognostic data that stratifies risk of tumor failure and survival, both during pretreatment planning and in serial follow-up imaging, has significant clinical importance.
Positron emission tomography combined with computed tomography (PET/CT) using F-18 fluorodeoxyglucose (FDG) is a standard pretreatment imaging modality for NSCLC staging. However, evidence for the ability of FDG-PET/CT data to predict outcomes in ES-NSCLC are not well established. Multiple studies have noted that a higher pre-SBRT maximum standardized uptake value (SUVmax) is associated with poorer LC,8, 9, 10, 11, 12 although many negative results have also been reported.13, 14, 15, 16 Evidence for the association between pre-SBRT SUVmax and OS is similarly equivocal. Higher pre-SBRT SUVmax has predicted worse OS in meta-analysis,17 but multiple studies with large cohorts published since then have shown negative results.18,19 As well, there is a paucity of available evidence for the predictive value of change in SUVmax between pre-SBRT imaging to post-SBRT FDG-PET/CT. The literature has often used arbitrary cutoff values for pretreatment SUVmax, and institutional protocols have differed in their time intervals for follow-up PET scans when investigating change in SUVmax.
Therefore, the primary objectives of the present study were to assess the tumor failure patterns and survival outcomes of early-stage NSCLC based on pretreatment SUVmax and percent change in SUVmax at the specific time intervals of 3 and 6 months after SBRT. To our knowledge, this study is the first to assess change in SUVmax at multiple time points after SBRT to predict clinical outcomes.
Methods and Materials
Cohort
We queried an institutional review board approved institutional database for patients receiving primary treatment for AJCC (American Joint Committee on Cancer) seventh edition T12N0M0 NSCLC with SBRT between 2012 and 2018 (study #20200251). Diagnosis was based on biopsy for a majority of patients; however, diagnosis by serial CT growth of FDG-PET/CT was permitted for patients unable or declining to undergo biopsy. Absence of regional or distant metastases was determined by initial FDG-PET/CT at diagnosis. Patients were either deemed medically inoperable by cardiothoracic surgery or pulmonology or had declined surgery. Patients were excluded if they had a history of prior primary lung cancer, other concurrent or active malignancy, or prior thoracic radiation therapy.
The technique used for SBRT has been previously described.20,21 In this study, 50 to 60 Gy was delivered in 3 to 5 fractions per institutional protocol. Dose-fractionation schedules are displayed in Table 1. After SBRT, patients were scheduled for repeat FDG-PET/CT imaging in 3 or 6 months based on provider preference. Time to failure was determined from the date of the last SBRT fraction. Change in SUVmax was included if the follow-up FDG-PET/CT scan was performed within 14 days of date corresponding to 3 or 6 months after the date of the last fraction of SBRT. In general, patients were followed 1 month after completion of SBRT, then in 2- to 4-month intervals for the next 2 years, and then semiannually for 5 years. Response was assessed according to RECIST (Response Evaluation Criteria in Solid Tumors) criteria. PET-CT or biopsy were required to determine disease progression.
Table 1.
Baseline cohort characteristics
| Characteristic | Type | n | % |
|---|---|---|---|
| Pre-SBRT FDG-PET/CT, no. | 163 | ||
| Pre- and post-SBRT FDG-PET/CT, no. | 71 | 43.6% | |
| Median age (range), y | 75 (52-91) | ||
| Female | 78 | 48.9% | |
| Histology | Squamous cell carcinoma | 57 | 35.0% |
| Adenocarcinoma | 76 | 46.6% | |
| NOS | 30 | 18.4% | |
| Stage | T1a | 72 | 44.2% |
| T1b | 58 | 35.6% | |
| T2a | 29 | 17.8% | |
| T2b | 4 | 2.5% | |
| Dose-fractionation schedule | 50 Gy/4 fx | 56 | 34.4% |
| 50 Gy/5 fx | 77 | 47.2% | |
| 54 Gy/3 fx | 27 | 16.6% | |
| 55 Gy/5 fx | 1 | 0.6% | |
| 57.5 Gy/5 fx | 1 | 0.6% | |
| 60 Gy/5 fx | 1 | 0.6% |
Abbreviations: FDG-PET/CT = 18F-fluoro-deoxyglucose positron emission tomography computed tomography; fx = fractions; NOS = not otherwise specified; SBRT = stereotactic body radiation therapy.
FDG-PET/CT imaging
Each patient underwent FDG-PET/CT for staging before SBRT after a standard clinical procedure. Patients were asked to fast for 4 to 6 hours before imaging. The blood glucose was obtained before FDG injection to ensure appropriate fasting. The activity for F-18 FDG ranged between 10.0 and 15.0 mCi on a weight-based protocol. According to the clinical imaging protocol, patients underwent PET imaging approximately 60 minutes after the FDG injection. PET/CT imaging was performed on a state-of the art scanner from Philips (Philips Healthcare, Cleveland, OH) and Siemens Biograph20 mCT PET/CT (Siemens Healthcare, Chicago, IL). Scanners were checked to performance at regularly indicated intervals. The acquisition time ranged between 2 and 3 minutes per bed position based on patient body weight. PET imaging included the torso with the arms up and a dedicated imaging of the head and neck with the arms down. A low-dose CT was used for attenuation correction. The PET images were iteratively reconstructed based on the ordered subset expectation maximization method. Image analysis was performed on a MIM workstation (MIM software, Beachwood, OH). Standardized uptake values based on the highest voxel activity (SUVmax) were obtained by placing a region of interest on target tumor lesions.
Statistical analysis
Patient information, imaging results, and tumor characteristics were obtained by retrospective chart review. Clinical endpoints included local (LFFS), regional, and distant failure-free survival (DFFS), progression-free survival (PFS), and OS.
Receiver operator characteristic (ROC) analysis was used to determine optimal thresholds to dichotomize variables for survival analysis similar to prior studies in this field.22,23 Balanced error rates were calculated to determine optimal thresholds. The Kaplan-Meier method with log-rank testing was used to conduct survival analyses. Multivariate analysis with a Cox proportional hazards model including age, sex, T stage, histology, performance status, and SUVmax or change in SUVmax estimated hazard ratios (HRs) with a 95% CI. P values were 2-sided and considered statistically significant if less than .05. A Bonferroni correction for multiple comparisons was completed. All statistical analyses were conducted using R version 3.6.1 software.
Results
In the study, 227 cases of T1-2N0M0 NSCLC were identified, of which 171 underwent pretreatment FDG-PET/CT. Pretreatment FDG-PET/CT occurred a median of 36.5 days before the first fraction of SBRT (range, 1-250 days). In addition, 163 of these patients had a reported SUVmax, and 71 (43.6%) had both a pretreatment scan and a posttreatment FDG-PET/CT at 3 (n = 32) or 6 (n = 39) months. Baseline patient, tumor, and treatment characteristics are shown in Table 1. Median follow-up was 18.9 months (range, 0.2-78.3). For the whole cohort, 1-year and 2-year LFFS, PFS, and OS were 95.0% and 80.3%, 87.1% and 75.4%, and 67.0% and 49.6%, respectively.
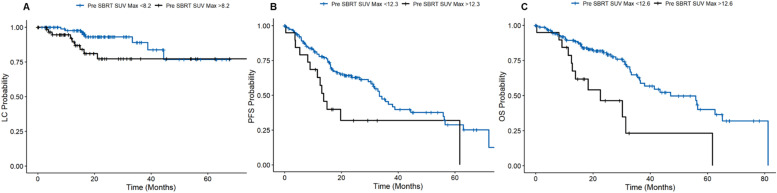
Table 2 displays results of the multivariate analysis for SUVmax-related variables. Pretreatment SUVmax cut-off points were significantly associated with DFFS (SUVmax >4.0; adjusted hazard ratio [aHR], 3.33; 95% CI, 1.42-7.84; P = .006), PFS (SUVmax >12.3; aHR, 2.8; 95% CI, 1.3-6.2; P = .011), and OS (SUVmax >12.6; aHR, 3.00; 95% CI, 1.6-5.8; P = .003). Patients with a SUVmax <12.3 had a median PFS of 16.9 months compared with 13.0 months for a SUVmax over 12.3. A SUVmax over 12.6 yielded a 3.5-month shorter median OS. Pre-SBRT SUVmax did not significantly predict local or regional tumor control (Fig. 1).
Table 2.
Multivariate analysis of pretreatment SUVmax and change in SUVmax on tumor control and survival outcomes
| Outcome | LFFS |
RFFS |
DFFS |
PFS |
OS |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| cut-off | aHR (CI) | cut-off | aHR (CI) | cut-off | aHR (CI) | cut-off | aHR (CI) | cut-off | aHR (CI) | |
| Pre-SBRT SUVmax | >8.2 | 2.26 (0.87-5.87) | >4.7 | 1.06 (0.41-2.67) | >4 | 3.33* (1.42-7.84) | >12.3 | 2.80† (1.26-6.19) | >12.6 | 3.00* (1.56-5.76) |
| 3-mo SUVmax percentage change | <–45.0% | 0.15† (0.02-0.91) | <–44.6% | 0.34 (0.02-6.71) | <–65.3% | 1.07 (0.18-6.47) | <–50.0% | 0.96 (0.33-2.75) | <–45.0% | 0.63 (0.28-1.39) |
| 6-mo SUVmax percentage change | <–53.0% | 0.24† (0.06-0.94) | <–10.8% | 0.12† (0.02-0.96) | <–3.7% | 0.29 (0.03-2.65) | <–77.8% | 0.50 (0.13-1.95) | <–39.8% | 0.32 (0.08-1.28) |
Abbreviations: aHR = adjusted hazard ratio; LFFS/RFFS/DFFS = local/regional/distant failure-free survival; OS = overall survival; PFS = progression-free survival; SBRT = stereotactic body radiation therapy; SUVmax = maximum standardized uptake value.
P < .01.
P < .05.
Figure 1.
(A) Pre-SBRT SUVmax cutoffs for local control, (B) progression-free survival, (C) and overall survival. Abbreviations: LC = local control; OS = overall survival; PFS = progression free survival; SBRT = stereotactic body radiation therapy; SUVmax = maximum standardized uptake value.
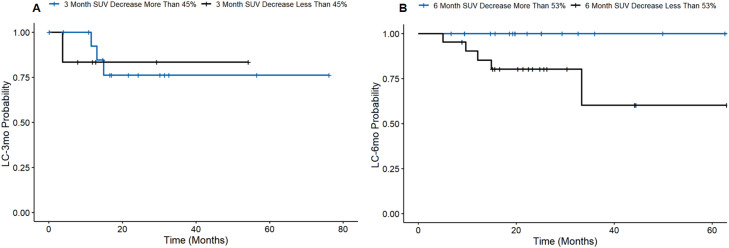
At 3 months after SBRT, a 45% decrease in SUVmax was associated with a longer LFFS (aHR, 0.15; 95% CI, 0.02-0.91; P = .018). A 6-month decrease in SUVmax >53% was also associated with improved LFFS (aHR, 0.24; 95% CI, 0.06-0.94; P = .038), and a decrease >11% demonstrated improved regional failure-free survival (aHR, 0.12; 95% CI, 0.02-0.96; P = .046). Figure 2 presents the LFFS between groups at 3 and 6 months.
Figure 2.
Cutoffs for percent change in SUVmax at (A) 3 months and (B) 6 months on local control. Abbreviations: LC = local control; SUVmax = maximum standardized uptake value.
Among other factors included in the multivariate analysis, performance status was significantly associated with PFS (aHR, 1.32; 95% CI, 1.04-1.67; P = .022) and OS (aHR, 1.47; 95% CI, 1.11-1.95; P = .007), and T stage was significantly associated with PFS (aHR, 4.34; 95% CI, 1.20-15.64; P = .025), but not with OS (aHR, 2.79; 95% CI, 0.40-19.55; P = .30).
Discussion
In the present study, we assessed SUVmax as a predictor of tumor control and survival outcomes. Our principal findings were (1) pre-SBRT SUVmax cutoff points can significantly predict DFFS, PFS, and OS, and (2) cutoff points in percentage change in SUVmax at both 3 and 6 months can predict for LC but are not associated with PFS or OS. Our study offers one of the larger cohorts among studies on a similar subject. Our study is also the first, to our knowledge, to report change in SUVmax at multiple time points after SBRT.
There have been several prior studies on the use of FDG-PET/CT in patients with NSCLC. Multiple meta-analyses and systematic reviews have noted that a high pretreatment SUVmax was a poor prognostic factor in cohorts of stage I to IV NSCLC, surgically resected stage I NSCLC, and NSCLC receiving any type of radiotherapy.24, 25, 26
SUVmax as prognostic data, especially during serial follow-up imaging, may be used for determination of adjuvant therapy in future studies which is not standard of care at present. Although the 3- and 6-month follow-up scans may be too late for the clinical determination of adjuvant therapy need, treatment intensification, or an increased frequency of serial imaging could still be considered, and randomized series may be warranted to study this. Tumor size has been shown to be a predictor of adjuvant chemotherapy in NSCLC after surgical resection, but our study showed that SUVmax was a stronger predictor of OS than T stage.7 There are many potential mechanisms by which a higher SUVmax may lead to a poorer prognosis. Hypoxic conditions increase expression of hypoxia inducible factor 1α (HIF-1α), resulting in increased cell membrane glucose transporters that can increase FDG uptake.27 Furthermore, hypoxia may decrease tumor radiosensitivity and response to other antitumor therapies, and multiple trials are ongoing to target intermediates in the hypoxia pathway in NSCLC.28 A meta-analysis has demonstrated that HIF-1α expression of tumor tissues was negatively associated with OS.29
Cellular proliferation may also serve as a link between SUVmax and prognosis. FDG uptake is positively correlated with Ki-67, a DNA-binding protein expressed during cell proliferation.30 A high Ki-67 proliferation index is associated with worse survival.31 Although poor differentiation status was not studied here, it may have been partially accounted for given that squamous cell carcinomas tend to have a higher SUVmax than adenocarcinomas and histology was included in the multivariate analysis.30
SUVmax on staging FDG-PET/CT has previously demonstrated predictive ability in patients with NSCLC receiving primary treatment with SBRT; however, there are relevant methodological limitations to the available evidence. For example, one study demonstrated that a 3-month post-SBRT SUVmax reduction of <2.55 was associated with increased risk of distant failure, but the 3-month time point included post-SBRT PET scans done from 2.1 to 4 months (65-123 days) after SBRT.8 It is unclear whether the earlier or later timing of the scan may have affected the results. Our study used stricter criteria for including scans at the 3- and 6-month post-SBRT time points. As well, the staging FDG-PET/CT may precede SBRT by a variable duration of time, raising the question of whether the SUVmax on the staging FDG-PET/CT represents the metabolic activity of the tumor at the time of SBRT. FDG uptake also varies with NSCLC histologic subtype, tumor differentiation, and tumor volume, which are not adjusted for in many analyses.12,30
Although a meta-analysis showed that there is a significant signal toward pre-SBRT SUVmax being a small negative predictor of OS (HR, 1.10; 95% CI, 1.01-1.15) and distant metastases (HR, 1.09; 95% CI, 1.03-1.16), the included studies demonstrated significant heterogeneity in both OS (I2 = 70%, Pheterogeneity = .003) and distant metastases (I2 = 74%, Pheterogeneity = .020), suggesting that publication bias against negative results, limited follow-up time, and unadjusted analyses may have played a role in their conclusions.17 Studies have often dichotomized results into “high SUV” and “low SUV” groups, as was done in the present study, but the studies in the meta-analysis had SUVmax cut-offs ranging from 2.47 to 8, which affects the ability to group them into high and low SUVmax groups.17
The main limitation of our study was the small number of total events due to our stricter criteria for 3- and 6-month post-SBRT PET scans. This may have affected our estimation of differences in regional (3 events in 3-month group, 6 in 6-month group) and distant (5 events in 3-month group, 5 in 6-month group) failure. There may also be selection bias because all patients were recommended to receive PET scans. Those who completed serial imaging may have slower-progressing tumors or better health care access. Of note, the post-SBRT time intervals of 3 and 6 months for follow-up imaging were selected based on institutional protocol. Additional research may seek to determine the posttreatment time point at which change in SUVmax is most strongly associated with outcomes of interest in place of serial PET-CT scans. It may also be difficult to compare SUVmax across institutions as scan protocol and reconstruction methods are not standardized. As this study was retrospective in nature, our results should be considered hypothesis-generating and require further study. The present study attempted to account for some of these potential limitations by using a standardized FDG-PET/CT protocol, using only images obtained at our institution, and including results obtained by the same group of nuclear medicine physicians. Other measures of tumor metabolic activity such as peak standardized uptake value and metabolic tumor volume were unable to be included in this study as they were not frequently reported on radiology reports.
A previous pilot trial on serial FDG-PET/CT scans did not support their routine use over serial CT imaging after SBRT. This trial had a small population (n = 14) without any local recurrences, but it did note that a substantial proportion had a persistently elevated SUVmax at 12 months without evidence of local failure.32 Other studies have shown FDG-PET/CT to be specific but insensitive for determination of recurrence and suggested that it was better reserved for evaluation of new CT findings.22,33 Based on this study and the Clarke et al8 report, there appears to be a potential prognostic utility of follow-up FDG-PET/CT data that may have gone unmeasured in the prior literature. The ideal role for serial FDG-PET/CT deserves additional study.
Finally, there are several ongoing clinical trials adding immunotherapy to SBRT, such as KEYNOTE-867 (NCT03924869) and PACIFIC-4 (NCT03833154) for which the present study has relevance. In particular, SWOG/NRG S1914 (NCT03775265) is a randomized phase 3 clinical trial assessing the addition of atezolizumab to SBRT in the neoadjuvant, concurrent, and adjuvant setting for high-risk, ES- NSCLC.34,35 Notably, this trial uses an SUVmax greater than or equal to 6.2 as one of the risk factors that meets inclusion criteria. In our study, we found that an SUVmax >12.6 was a significant cutoff value for OS and would consider post hoc analysis of the patients over this cutoff to be prudent to see whether they derive more benefit. The S1914 differs from ours in that it includes T1-3 NSCLC while our study only included T1-2.34,35
Conclusion
Our study revealed that the cutoff values using ROCanalysis that demonstrated that pre-SBRT SUVmax can predict PFS and OS. Additionally, the percent change in SUVmax on both 3- and 6-month follow-up FDG-PET/CT after SBRT can predict risk of local failure. A future study is required to investigate value of posttreatment PET at fixed time point in conjunction with serial CT scan to establish its role in SBRT treatment and evaluation.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Sources of support: This work had no specific funding.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20:494–503. doi: 10.1016/S1470-2045(18)30896-9. [DOI] [PubMed] [Google Scholar]
- 4.von Reibnitz D, Shaikh F, Wu AJ, et al. Stereotactic body radiation therapy (SBRT) improves local control and overall survival compared to conventionally fractionated radiation for stage I non-small cell lung cancer (NSCLC) Acta Oncologica. 2018;57:1567–1573. doi: 10.1080/0284186X.2018.1481292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grutters JP, Kessels AG, Pijls-Johannesma M, et al. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: A meta-analysis. Radiother Oncol. 2010;95:32–40. doi: 10.1016/j.radonc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Bria E, Gralla RJ, Raftopoulos H, et al. Magnitude of benefit of adjuvant chemotherapy for non-small cell lung cancer: Meta-analysis of randomized clinical trials. Lung Cancer. 2009;63:50–57. doi: 10.1016/j.lungcan.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Grinnell M, Appiah AK, Baine M, et al. Adjuvant chemotherapy following SBRT for early stage non-small cell lung cancer (NSCLC) in older patients. J Geriatr Oncol. 2020;11:1145–1153. doi: 10.1016/j.jgo.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Clarke K, Taremi M, Dahele M, et al. Stereotactic body radiotherapy (SBRT) for non-small cell lung cancer (NSCLC): Is FDG-PET a predictor of outcome? Radiother Oncol. 2012;104:62–66. doi: 10.1016/j.radonc.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Takeda A, Yokosuka N, Ohashi T, et al. The maximum standardized uptake value (SUVmax) on FDG-PET is a strong predictor of local recurrence for localized non-small-cell lung cancer after stereotactic body radiotherapy (SBRT) Radiother Oncol. 2011;101:291–297. doi: 10.1016/j.radonc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Horne ZD, Clump DA, Vargo JA, et al. Pretreatment SUV max predicts progression-free survival in early-stage non-small cell lung cancer treated with stereotactic body radiation therapy. Radiat Oncol. 2014;9:1–6. doi: 10.1186/1748-717X-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohutek ZA, Wu AJ, Zhang Z, et al. FDG-PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non-small cell lung cancer. Lung Cancer. 2015;89:115–120. doi: 10.1016/j.lungcan.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T, Kadoya N, Shirata Y, et al. Formula corrected maximal standardized uptake value in FDG-PET for partial volume effect and motion artifact is not a prognostic factor in stage I non-small cell lung cancer treated with stereotactic body radiotherapy. Ann Nucl Med. 2015;29:666–673. doi: 10.1007/s12149-015-0991-5. [DOI] [PubMed] [Google Scholar]
- 13.Burdick MJ, Stephans KL, Reddy CA, Djemil T, Srinivas SM, Videtic GM. Maximum standardized uptake value from staging FDG-PET/CT does not predict treatment outcome for early-stage non–small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1033–1039. doi: 10.1016/j.ijrobp.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 14.Vu CC, Matthews R, Kim B, Franceschi D, Bilfinger TV, Moore WH. Prognostic value of metabolic tumor volume and total lesion glycolysis from 18F-FDG PET/CT in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Nucl Med Commu. 2013;34:959–963. doi: 10.1097/MNM.0b013e32836491a9. [DOI] [PubMed] [Google Scholar]
- 15.Lee DS, Kim YS, Yoo IR, et al. Long-term clinical experience of high-dose ablative lung radiotherapy: High pre-treatment [18F] fluorodeoxyglucose-positron emission tomography maximal standardized uptake value of the primary tumor adversely affects treatment outcome. Lung Cancer. 2013;80:172–178. doi: 10.1016/j.lungcan.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Yaprak G, Ozen A, Tugrul F, Ozugur S, Isik N. Is there any prognostic significance of the level of change in SUVmax after SBRT in patients with early stage NSCLC? Ann Oncol. 2019;30:ii28. [Google Scholar]
- 17.Dong M, Liu J, Sun X, Xing L. Prognostic significance of SUV max on pretreatment 18F-FDG PET/CT in early-stage non-small cell lung cancer treated with stereotactic body radiotherapy: A meta-analysis. J Med Imaging Radiat Oncol. 2017;61:652–659. doi: 10.1111/1754-9485.12599. [DOI] [PubMed] [Google Scholar]
- 18.Hobbs CJ, Ko SJ, Paryani NN, et al. Stereotactic body radiotherapy for medically inoperable stage I-II non–small cell lung cancer: The Mayo Clinic Experience. Mayo Clin Proc Innov Qual Outcomes. 2017;2:40–48. doi: 10.1016/j.mayocpiqo.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oikonomou A, Khalvati F, Tyrrell PN, et al. Radiomics analysis at PET/CT contributes to prognosis of recurrence and survival in lung cancer treated with stereotactic body radiotherapy. Sci Rep. 2018;8 doi: 10.1038/s41598-018-22357-y. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podder T, Biswas T, Yao M, et al. Chest wall and rib irradiation and toxicities of early-stage lung cancer patients treated with CyberKnife stereotactic body radiotherapy. Future Oncol. 2014;10:2311–2317. doi: 10.2217/fon.14.158. [DOI] [PubMed] [Google Scholar]
- 21.Deshpande SR, Grubb W, Kharouta M, et al. A comparative study of patients with early-stage non-small cell lung cancer treated with stereotactic body radiation therapy using CyberKnife and linear accelerator–based volumetric modulated arc therapy. Pract Radiat Oncol. 2022;12:200–209. doi: 10.1016/j.prro.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Takeda A, Kunieda E, Fujii H, et al. Evaluation for local failure by 18F-FDG PET/CT in comparison with CT findings after stereotactic body radiotherapy (SBRT) for localized non-small-cell lung cancer. Lung Cancer. 2013;79:248–253. doi: 10.1016/j.lungcan.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Pyka T, Bundschuh RA, Andratschke N, et al. Textural features in pre-treatment [F18]-FDG-PET/CT are correlated with risk of local recurrence and disease-specific survival in early stage NSCLC patients receiving primary stereotactic radiation therapy. Radiat Oncol. 2015;10:1–9. doi: 10.1186/s13014-015-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): A systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 25.Nair VS, Krupitskaya Y, Gould MK. Positron emission tomography 18F-fluorodeoxyglucose uptake and prognosis in patients with surgically treated, stage I non-small cell lung cancer: A systematic review. J Thorac Oncol. 2009;4:1473–1479. doi: 10.1097/JTO.0b013e3181bccbc6. [DOI] [PubMed] [Google Scholar]
- 26.Na F, Wang J, Li C, Deng L, Xue J, Lu Y. Primary tumor standardized uptake value measured on F18-Fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non–small-cell lung cancer receiving radiotherapy: Meta-analysis. J Thorac Oncol. 2014;9:834–842. doi: 10.1097/JTO.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–7998. [PubMed] [Google Scholar]
- 28.Salem A, Asselin MC, Reymen B, et al. Targeting hypoxia to improve non–small cell lung cancer outcome. J Natl Cancer Inst. 2018;110:14–30. doi: 10.1093/jnci/djx160. [DOI] [PubMed] [Google Scholar]
- 29.Ren W, Mi D, Yang K, Cao N, Tian J, Li Z, Ma B. The expression of hypoxia-inducible factor-1α and its clinical significance in lung cancer: A systematic review and meta-analysis. Swiss Med Wkly. 2013;143:w13855. doi: 10.4414/smw.2013.13855. [DOI] [PubMed] [Google Scholar]
- 30.Vesselle H, Salskov A, Turcotte E, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971–978. doi: 10.1097/JTO.0b013e31818307a7. [DOI] [PubMed] [Google Scholar]
- 31.Martin B, Paesmans M, Mascaux C, et al. Ki-67 expression and patients survival in lung cancer: Systematic review of the literature with meta-analysis. Br J Cancer. 2004;91:2018–2025. doi: 10.1038/sj.bjc.6602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson MA, Hoopes DJ, Fletcher JW, et al. A pilot trial of serial 18F-fluorodeoxyglucose positron emission tomography in patients with medically inoperable stage I non–small-cell lung cancer treated with hypofractionated stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:789–795. doi: 10.1016/j.ijrobp.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastis NJ, Jr, Greer TJ, Tanner NT, et al. Assessing the usefulness of 18F-fluorodeoxyglucose PET-CT scan after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Chest. 2014;146:406–411. doi: 10.1378/chest.13-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly K, Daly ME, Mirhadi A, et al. Atezolizumab plus stereotactic ablative therapy for medically inoperable patients with early-stage non-small cell lung cancer. 2020;38(15 Suppl):9011. [DOI] [PMC free article] [PubMed]
- 35.Daly ME, Redman M, Simone CB, II, et al. SWOG/NRG S1914: A randomized phase III trial of induction/consolidation atezolizumab+ SBRT versus SBRT alone in high risk, early-stage NSCLC (NCT# 04214262) Int J Radiat Oncol Biol Phys. 2023;114:e414. [Google Scholar]