Abstract
Background
Frontal plane QRS‐T angle (fQRS‐T) and platelet‐to‐lymphocyte ratio (PLR) are highly important parameters that well‐predict unfavorable outcomes in patients with ST‐elevated myocardial infarction (STEMI).There are limited data on the predictive significance of ischemic cardiomyopathy (I‐CMP) from the combination of fQRS‐T and PLR in STEMI, compared to using fQRS‐T and PLR alone.
Aim
We aimed to evaluate the ability of the combination of fQRS‐T and PLR routinely obtained on admission to identify STEMI patients at risk of I‐CMP.
Method
Six hundred and thirty‐eight consecutive patients with STEMI who underwent primary percutaneous coronary intervention between 2018 and 2021 were included. The assessment of I‐CMP was conducted through two‐dimentional (2D)‐echocardiography 6 weeks post‐STEMI and I‐CMP was defined as a left ventricular ejection fraction (LVEF) of 50% or less. Multivariate logistic regression analysis and receiver operating curve (ROC) analysis were performed to predict the development of I‐CMP.
Results
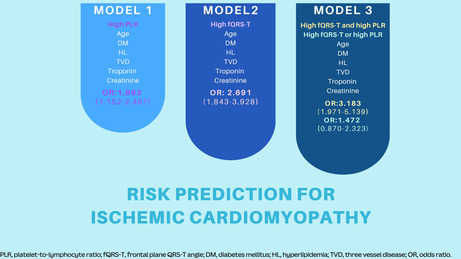
In ROC analysis, the cut‐off values of fQRS‐T and PLR for best predicting I‐CMP were 66.72° and 101.23, respectively. The model using the combination of two markers was the most powerful predictor of I‐CMP risk (OR: 3.183, 95% CI: 1.971–5.139, p = .001) when included in a single variable such as high fQRS‐T or high PLR (OR: 1.422, 95% CI: 0.870–0.232, p = .160). Additionally, the concomitant presence of high fQRS‐T and high PLR exhibited the highest specificity (77%) for I‐CMP relative to the individual presence of high fQRS‐T (66%) or PLR (49%).
Conclusion
The combination of fQRS‐T and PLR, which is a simple and cost‐effective risk assessment, may serve as a more reliable prognosticator for I‐CMP as opposed to the use of fQRS‐T and PLR alone for STEMI.
Keywords: frontal plane QRS‐T angle, ischemic cardiomyopathy, platelet‐to‐lymphocyte ratio, risk stratification
The concurrent presence of a high frontal plane QRS‐T angle (fQRS‐T) and a high platelet‐to‐lymphocyte ratio (PLR) poses the highest risk for the development of ischemic cardiomyopathy 6 weeks after ST‐elevation myocardial infarction, in comparison to solely high fQRS‐T or high PLR alone.
1. INTRODUCTION
Ischemic cardiomyocytes activate an intense thromboinflammatory response that primarily involves activated platelets engaging with various cells (Li et al., 2021; Schrottmaier et al., 2020; Sexton et al., 2015). The intricate interplay between various factors triggers the initiation of the coagulation cascade and the subsequent formation of a nascent clot (Banka et al., 2023). Another key component that contributes to the formation of blood clots is the reduction in lymphocyte count (Ommen et al., 1997). Lymphocytes are identified as cells that limit disease progression through the regulation of thromboinflammation in myocardial infarction (Li et al., 2021; Ommen et al., 1997; Tang et al., 2012). Consequently, an increased platelet‐to‐lymphocyte ratio (PLR) may serve as an innovative biomarker indicating the formation of a fresh thrombus in ST‐elevated myocardial infarction (STEMI).
The electrophysiological features of ischemic cardiomyocytes are mainly characterized by disturbances in the repolarization process and its interaction with depolarization (Dilaveris et al., 2017; Rodríguez et al., 2006). Since frontal plane QRS‐T angle (fQRS‐T) has been defined as a novel electrocardiographic marker of myocardial depolarization and repolarization heterogeneity and measured the absolute value of frontal plane T wave (fT) and frontal plane QRS axis (fQRS), electrophysiological changes in myocardium result in alterations in the myocardial repolarization (fT) and myocardial depolarization (fQRS), leading to an increased frontal plane QRS‐T angle (fQRS‐T) (Dilaveris et al., 2017; Kurisu et al., 2019; Michael et al., 2007; Oehler et al., 2014; Tanriverdi et al., 2020). If the angle exceeds 180°, fQRS‐T was calculated by subtracting from 360° (Colluoglu et al., 2018).
Ischemic cardiomyopathy (I‐CMP) may be one of the devastating consequences of STEMI and the recently published RECORD‐MI study demonstrated that the incidence of heart failure occurrence within a 1‐year period was found to be 30%. In this trial, patients diagnosed with newly developed heart failure exhibited poorer outcomes, including a higher likelihood of experiencing recurrent MI, cardiovascular hospitalization, and an increased risk of all‐cause mortality (Butler, 2023; Schuster et al., 2012; Wang et al., 2016). Timely identification of patients who are at an increased risk of the development of I‐CMP is crucial to avert such a catastrophic outcome. Various risk parameters have been employed to facilitate early identification of STEMI patients who are at a heightened risk of adverse outcomes (Granger et al., 2003; Morrow et al., 2000). The risk parameters for patients with STEMI may incorporate fQRS‐T and PLR as individual factors. Elevated PLR and widened fQRS‐T have been associated with a poorer prognosis in such patients (Acet et al., 2015; Azab et al., 2012; Lown et al., 2012). However, there has been a lack of scientific evidence regarding the combined use of PLR and fQRS‐T in risk stratification to better predict I‐CMP in STEMI patients. Therefore, we conducted this retrospective study of PLR and fQRS‐T in STEMI patients. We aimed to investigate the effect of their combined use on I‐CMP.
2. METHOD
2.1. Study population
The retrospective evaluation of 696 consecutive patients admitted to Karabuk University Hospital, Cardiology Clinic, between January 2018 and December 2021 with STEMI who underwent primary percutaneous coronary intervention (pPCI). The determination of a STEMI diagnosis was established by the 2017 guideline for STEMI management by the European Society of Cardiology (Ibanez et al., 2018). The study employed several major exclusion criteria, including the presence of a complete or incomplete right or left bundle branch block (n = 14), the presence of a pathological Q wave (n = 3), pacemaker rhythm (n = 8), the receiving thrombolytic therapy (n = 5), a history of CAD (n = 18), and poor echocardiographic image quality (n = 10). Consequently, our study included a total of 638 patients who underwent revascularization with pPCI (Figure S1). The study was performed in accordance with the principles stated in the Declaration of Helsinki and this study was approved by the Karabuk University Ethics Committee of Non‐interventional Clinical Research (approval number: 2022/1036).
2.2. Electrocardiography
Standard 12‐lead ECGs were recorded on admission at a paper speed of 25 mm/s with an amplification of 10 mm/mV for all participants. All ECG data were transmitted to a computer for further analysis of fQRS, fT, and fQRS‐T by a single expert cardiologist. The cardiologist analyzed fQRS‐T at a magnification of 200% with dedicated software. fQRS‐T was defined as the absolute value of the difference between fQRS and fT, and was adjusted to an acute angle by (360°‐) for an angle >180°(Figure 1).
FIGURE 1.
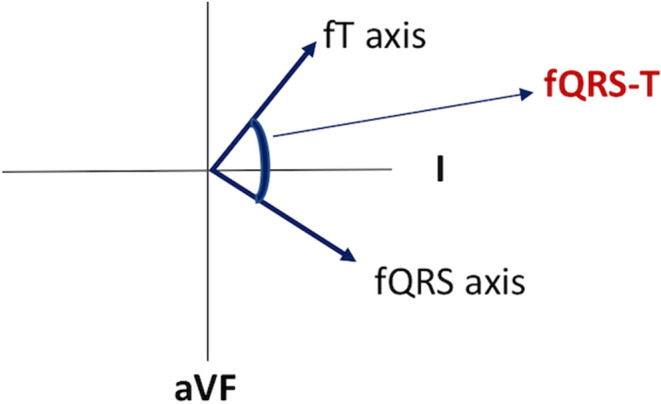
The calculation of frontal plane QRS‐T angle. fQRS axis, frontal plane QRS axis; fQRS‐T, frontal plane QRS‐T angle; fT axis, frontal plane T axis.
2.3. Laboratory measurements
Before the pPCI procedure, venous blood samples were collected in Vacusera‐K2 ethylenediaminetetraacetic acid (EDTA) tubes (Vacusera®) and VacuSel gel tubes with clot activator (Vacusera®) by sterile venipuncture in the emergency room. A total of 9 biomarkers were measured. These included biomarkers of thrombo‐inflammation (PLR, platelet, lymphocyte, and neutrophil count), biomarkers of myocardial ischemia (troponin), biomarkers of renal function (creatinine and estimated glomerular filtration rate (eGFR)), and biomarkers of dyslipidemia (LDL‐C and HDL‐C). The total numbers of platelets, lymphocytes, and neutrophils were measured by the Coulter LH series (Beckman Coulter, Inc., Florida). The PLR prior to the pPCI procedure was calculated by dividing the platelet count by the lymphocyte count using the same blood sample.
2.4. 2D‐echocardiography
All echocardiographic studies were performed on hospital admission and after 6 weeks of STEMI. The Philips Epic7C ultrasound system (Andover, USA) was used to perform transthoracic echocardiography. Left ventricular ejection fraction (LVEF was determined using the modified biplane Simpson method according to the recommendations of the American Society of Echocardiography) (Lang et al., 2015). The state of I‐CMP was I‐CMP as a LVEF of 50% or less (Schuster et al., 2012). I‐CMP was evaluated following 6 weeks of STEMI by 2D‐transthoracic echocardiography.
2.5. Coronary angiography
All pPCI procedures were performed with the standard femoral arterial approach. The infarct‐related artery was treated if it was totally occluded, and stenting of the culprit lesion was attempted in all patients. Angioplasty of non‐infarct‐related arteries was not performed unless the patient was in cardiogenic shock. Successful revascularization was defined as a final stenosis of <20% with thrombolysis in myocardial infarction (TIMI) flow grade 3 (Levine et al., 2011). Two experienced cardiologists independently interpreted all angiographic images. The interobserver and intraobserver variabilities were 3% and 2% for TIMI 0–1 grade, 1.6% and 1% for TIMI 2 grade, and 0% and 0.6% for TIMI 3 grade.
2.6. Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. The Student t‐test was used for continuous variables with a normal distribution (presented as mean ± standard deviation), and the Mann–Whitney‐U test was used for continuous variables without a normal distribution (presented as median [interquartile range]). Categorical variables were compared with the chi‐square test. A receiver operating characteristic (ROC) curve analysis was applied to assess the diagnostic performance and predictive capability of the PLR and fQRS‐T with an area under the curve (AUC); sensitivity and specificity were also calculated. The optimal cut‐off values of PLR and fQRS‐T were the thresholds yielding the maximal sum of sensitivity and specificity. Continuous variables were compared between the three groups using one‐way analysis of variance, or the Kruskal–Wallis test. The association between I‐CMP and high fQRS‐T (Hf), high PLR (HP), either high fQRS‐T and low PLR (HfLP) or low fQRS‐T and high PLR (LfHP), and high fQRS‐T and high PLR (HfHP) was estimated using multivariate logistic regression analysis using stepwise forward‐Wald. For these analyses, we created three different models. Model 1 was adjusted for HP, age, diabetes mellitus (DM), hyperlipidemia, three‐vessel disease (TVD), troponin and creatinine. Model 2 was adjusted for Hf, age, DM, hyperlipidemia, TVD, troponin, and creatinine. Model 3 was adjusted for HfHP, age, DM, hyperlipidemia, TVD, troponin and creatinine. A probability value <.05 was considered statistically significant. All analyses were performed using SPSS 25.0 (IBM Corporation).
3. RESULTS
3.1. Baseline characteristics and laboratory, echocardiographic and angiographic parameters
A total of 638 STEMI patients were included in this study. Baseline characteristics were seen in Table 1. The mean age of patients was 59.40 ± 12.50 years and 21.3% (136) were female. The median PLR was 110.47 (73.42–174.37) and fQRS‐T was 53.11° (28.24°–95.00°). I‐CMP was developed in 175 (27.4%) patients in the sixth week after STEMI. Patients diagnosed with I‐CMP exhibited a higher mean age and a greater prevalence of DM (30.9% vs. 21.2%, p = .010), TVD (26.9% vs. 14.0%, p = .001), and hyperlipidemia (26.9% vs. 18.8%, p = .026) in comparison to the cohort without I‐CMP (Table 1). While the patients who developed I‐CMP had higher median fQRS‐T (84.56° [51.24°–131.48°] vs. 45.00° [23.00°–81.00°], p = .001), PLR (138.59 [80.59–191.33] vs. 104.09 [69.23–164.54], p = .001), troponin (50.00 [49.75–80.00] vs. 35.32 [12.00–50.00], p = .001), and creatinine (0.93 [0.78–1.07] vs. 0.87 [0.74–1.01], p = .007) levels; median LVEF (35% [30%–40%] vs. 50% [45%–55%], p = .001) was significantly lower in the patients who developed I‐CMP. Regarding the results of the pPCI procedure, patients who developed I‐CMP had a lower TIMI flow grade compared to those without I‐CMP (2.55 ± 0.85 vs. 2.69 ± 0.72, p = .040) (Table 1).
TABLE 1.
Baseline clinical, laboratory and angiographic characteristics of the study population.
| STEMI patients (n = 638) | Patients with I‐CMP (n = 175) | Patients without I‐CMP (n = 463) | p | |
|---|---|---|---|---|
| Age (years) | 59.40 ± 12.50 | 62.41 ± 13.24 | 58.27 ± 12.03 | .001 |
| Sex (M/F) | 502/136 | 135/40 | 367/96 | .559 |
| Hypertension (%) | 304 (47.6) | 93 (53.1) | 211 (45.6) | .088 |
| Diabetes mellitus (%) | 152 (23.8) | 54 (30.9) | 98 (21.2) | .010 |
| Hyperlipidemia (%) | 134 (21.0) | 47 (26.9) | 87 (18.8) | .026 |
| PAD (%) | 18 (2.8) | 3 (1.7) | 15 (3.2) | .299 |
| LVEF (%) | 45.00 (40.00–54.00) | 35.00 (30.00–40.00) | 50.00 (45.00–55.00) | .001 |
| Troponin (ng/mL) | 43.40 (14.16–78.00) | 50.00 (49.75–80.00) | 35.32 (12.00–50.00) | .001 |
| Creatinine (mg/dL) | 0.88 (0.75–1.03) | 0.93 (0.78–1.07) | 0.87 (0.74–1.01) | .007 |
| eGFR (mL/min/1.73 m2) | 91.11 (75.00–106.00) | 86.10 (73.87–109.00) | 92.00 (75.00–105.75) | .433 |
| LDL‐C (mg/dL) | 113.00 (91.60–142.00) | 118.00 (92.50–146.00) | 112.00 (91.20–139.60) | .330 |
| HDL‐C (mg/dL) | 38.00 (33.00–46.00) | 38.00 (34.00–46.00) | 39.00 (33.00–46.00) | .895 |
| fQRS‐T (°) | 53.11 (28.24–95.00) | 84.56 (51.24–131.48) | 45.00 (23.00–81.00) | .001 |
| Hematological parameters | ||||
| Hemoglobin (g/dL) | 13.90 (12.50–15.00) | 14.10 (12.70–15.10) | 13.80 (12.50–15.00) | .608 |
| WBC (×10/μL) | 11.63 (9.30–14.40) | 11.80 (9.90–14.60) | 11.60 (9.10–14.40) | .039 |
| Neutrophil count (×103/μL) | 8.16 (6.40–11.20) | 8.70 (6.70–11.61) | 7.95 (6.10–11.00) | .032 |
| Lymp. count (×103/μL) | 1.98 (1.30–2.70) | 1.70 (1.20–2.40) | 2.00 (1.40–2.70) | .023 |
| Platelet count (×103/μL) | 236.00 (200.00–272.00) | 235.00 (205.00–279.00) | 237.00 (198.00–268.00) | .608 |
| PLR | 110.47 (73.42–174.37) | 138.59 (80.59–191.33) | 104.09 (69.23–164.54) | .001 |
| Angiographic parameters | ||||
| Proximal lesion (%) | 330 (51.7) | 101 (57.7) | 229 (49.5) | .063 |
| Three vessel disease (%) | 112 (17.6) | 47 (26.9) | 65 (14.0) | .001 |
| TIMI flow grade | 2.65 ± 0.76 | 2.55 ± 0.85 | 2.69 ± 0.72 | .040 |
Abbreviations: eGFR, estimated glomerular filtration rate; fQRS‐T, frontal plane QRS‐T angle; HDL‐C, high density lipoprotein cholesterol; I‐CMP, ischemic cardiomyopathy; LDL‐C, low density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; Lymp., lymphocyte; PAD, peripheral arterial disease; PLR, platelet‐to‐lymphocyte ratio; TIMI, thrombolysis in myocardial infarction; WBC, white blood counts.
3.2. Optimal cut‐off values of frontal plane QRS‐T angle and platelet‐to‐lymphocyte ratio for ischemic cardiomyopathy
ROC curve analysis of PLR and fQRS‐T individually showed the best cut‐off values of 101.23 (AUC = 0.591, CI95 = 0.551–0.640, p = .001) and 66.72° (AUC = 0.702, CI95 = 0.658–0.746, p = .001), respectively, for predicting the development of I‐CMP (Figure 2a, Table S1). The criterion for HP was established as an PLR ≥101.23, whereas LP was defined as an PLR <101.23. The criterion for Hf was established as an fQRS‐T ≥ 66.72°, whereas Lf was defined as an fQRS‐T < 66.72°. The combination of Hf and HP exhibited the greatest level of specificity in predicting I‐CMP with a sensitivity of 46% and specificity of 77% (AUC = 0.615, CI95 = 0.564–0.665, p = .001) (Figure 2b, Table S1).
FIGURE 2.
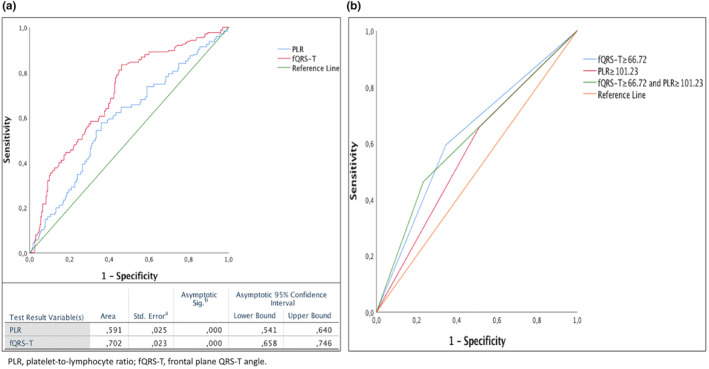
(a) ROC curve analysis of frontal plane QRS‐T angle (fQRS‐T) and platelet‐to‐lymphocyte ratio (PLR) for predicting ischemic cardiomyopathy, (b) ROC curve analysis of fQRS‐T, PLR, and the combination of fQRS‐T and PLR for predicting ischemic cardiomyopathy. fQRS‐T, frontal plane QRS‐T angle; PLR, platelet‐to‐lymphocyte ratio.
3.3. Findings based on cut‐off values of fQRS‐T angle and PLR
Following the cut‐off values of fQRS‐T and PLR, patients were divided into the following three groups: Group I: HfHP; Group II: HfLP or LfHP; Group III: LfLP. The presence of Group I was identified in 189 (29%) patients, of whom 81 (42.9%) experienced I‐CMP. Patients in Group I exhibited advanced age and a greater incidence of I‐CMP as compared to those in Group II and Group III. LVEF, hemoglobin, and lymphocyte count were lower in subjects belonging to Group I. The median of fQRS‐T, PLR, troponin, creatinine, and LDL‐C were higher in patients belonging to Group I in comparison to those in Group II and Group III (Table 2).
TABLE 2.
Clinical characteristics, electrocardiographic data, echocardiographic data, and coronary angiographic data according to cut‐off values of fQRS‐T and PLR.
| Group I (HfHP) (n = 189) | Group II (HfLP or LfHP) (n = 237) | Group III (LfLP) (n = 212) | p | |
|---|---|---|---|---|
| Age (years) | 62.71 ± 11.90 | 58.83 ± 13.24 | 57.08 ± 11.57 | .030 |
| Sex (M/F) | 147/42 | 187/50 | 168/44 | .933 |
| Hypertension (%) | 94 (49.7) | 116 (48.9) | 94 (44.3) | .492 |
| Diabetes mellitus (%) | 52 (27.5) | 48 (20.3) | 52 (24.5) | .208 |
| Hyperlipidemia (%) | 40 (21.2) | 52 (21.9) | 42 (19.8) | .856 |
| PAD (%) | 2 (1.1) | 6 (2.5) | 10 (4.7) | .082 |
| I‐CMP (%) | 81 (42.9) | 56 (23.6) | 38 (17.9) | .001 |
| LVEF (%) | 45.00 (35.00–50.00) | 45.00 (40.00–54.00) | 45.00 (40.00–55.00) | .016 |
| Troponin (ng/mL) | 50.00 (30.00–68.00) | 39.79 (15.56–64.15) | 37.40 (9.25–50.00) | .005 |
| Creatinine (mg/dL) | 0.94 (0.77–1.09) | 0.87 (0.73–0.99) | 0.88 (0.77–1.01) | .036 |
| eGFR (mL/min/1.73 m2) | 86.10 (72.50–108.00) | 91.00 (75.00–105.00) | 94.00 (77.00–109.00) | .293 |
| LDL‐C (mg/dL) | 117.00 (96.75–149.50) | 113.00 (97.80–145.00) | 110.00 (84.40–134.00) | .001 |
| HDL‐C (mg/dL) | 36.00 (32.00–47.00) | 39.00 (34.00–45.70) | 38.00 (32.85–46.00) | .123 |
| fQRS‐T (°) | 118.00 (87.49–147.00) | 47.00 (25.44–77.04) | 31.93 (17.30–47.19) | .001 |
| Hematological parameters | ||||
| Hemoglobin (g/dL) | 13.20 (12.10–14.60) | 14.00 (12.70–14.80) | 14.10 (12.90–15.10) | .004 |
| WBC (×10/μL) | 11.61 (9.00–15.00) | 11.90 (9.59–14.38) | 11.66 (9.20–14.30) | .548 |
| Neutrophil count (×103/μL) | 9.20 (6.72–12.55) | 8.78 (6.80–10.95) | 6.87 (5.50–10.10) | .001 |
| Lymp. count (×103/μL) | 1.50 (1.00–1.90) | 2.00 (1.30–2.60) | 2.52 (1.80–3.70) | .001 |
| Platelet count (×103/μL) | 243.00 (195.00–277.00) | 241.00 (213.00–284.00) | 225.00 (190.00–265.00) | .004 |
| PLR | 166.87 (132.10–266.66) | 124.58 (94.58–182.30) | 63.48 (40.25–83.24) | .001 |
| Angiographic parameters | ||||
| Proximal lesion (%) | 94 (49.7) | 134 (56.5) | 102 (48.1) | .165 |
| Three vessel disease (%) | 39 (20.6) | 39 (16.5) | 34 (16.0) | .412 |
| TIMI flow grade | 2.49 ± 0.92 | 2.71 ± 0.67 | 2.72 ± 0.68 | .001 |
Abbreviations: eGFR, estimated glomerular filtration rate; fQRS‐T, frontal plane QRS‐T angle; HDL‐C, high density lipoprotein cholesterol; HfHP, high fQRS‐T and high platelet‐to‐lymphocyte ratio; HfLP, high fQRS‐T and low platelet‐to‐lymphocyte ratio; I‐CMP, ischemic cardiomyopathy; LDL‐C, low density lipoprotein cholesterol; LfHP, low fQRS‐T and high platelet‐to‐lymphocyte ratio; LfLP, low fQRS‐T and low platelet‐to‐lymphocyte ratio; LVEF, left ventricular ejection fraction; Lymp., lymphocyte; PAD, peripheral arterial disease; PLR, platelet‐to‐lymphocyte ratio; TIMI, thrombolysis in myocardial infarction; WBC, white blood counts.
3.4. Findings based on models to predict ischemic cardiomyopathy
According to Model 1, Model 2 and Model 3, both Hf and HP were independently associated with I‐CMP (Tables S2 and S3). Furthermore, the combination of these two variables exhibited a greater predictive capacity for I‐CMP compared to their individual use. The combined use of two variables had a higher hazard ratio of 3.183 (1.971–5.139) compared to the hazard ratio of either Hf or HP (1.422 (0.870–2.323)) (Table 3, Table S4).
TABLE 3.
Multivariate logistic regression analysis for left ischemic cardiomyopathy using cut‐off values of fQRS‐T and PLR on admission.
| Multivariate analysis a | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| HR (95%CI) | p Value | HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Ischemic cardiomyopathy | ||||||
| High fQRS‐T (°) | 2.691 (1.843–3.928) | .001 | ||||
| High PLR | 1.693 (1.152–2.487) | .007 | ||||
|
Low fQRS‐T (°) and low PLR (group I) |
Reference | |||||
| Either high fQRS‐T (°) or high PLR (group II) |
1.422 (0.870–2.323) |
.160 | ||||
| High fQRS‐T (°) and high PLR (group III) | 3.183 (1.971–5.139) | .001 | ||||
Note: Group I: high fQRS‐T and high PLR; Group II: Either high fQRS‐T or high PLR; Group III: low fQRS‐T and low PLR.
Abbreviations: CI, confidence interval; fQRS‐T, frontal plane QRS‐T angle; HR, hazard ratio; OR, odds ratio; PLR, platelet‐to‐lymphocyte ratio.
Entered variables: age, diabetes mellitus, hyperlipidemia, three‐vessel disease, troponin, creatinine. High fQRS‐T, fQRS‐T ≥66.72°; low fQRS‐T, fQRS‐T <66.72°; high PLR, PLR ≥101.23; low PLR, PLR<101.23. Model 1 included high fQRS‐T; Model 2, high PLR; Model 3, the groups stratified by fQRS‐T and PLR values.
4. DISCUSSION
The present study focused on evaluating the clinical efficacy of using both fQRS‐T and PLR in combination for the purpose of risk stratification for predicting I‐CMP in patients who underwent pPCI following 6 weeks of STEMI. Patients treated with thrombolytic therapy were not included in our study, as this form of treatment is typically inadequate for achieving optimal coronary flow. This situation may have resulted in the continuation of exacerbated ischemia and heterogeneous results in the assessment of risk.
PLR is a cost‐effective and easily accessible marker that offers an added layer of risk assessment beyond traditional risk parameters in predicting mortality rates during hospitalization and over an extended period (Dong et al., 2021; Serra et al., 2021). Furthermore, increased PLR may be a sign of suboptimal coronary flow following the pPCI procedure. Indeed, Kurtul et al. demonstrated that elevated PLR on admission was associated with no or slow flow after the pPCI procedure. Suboptimal coronary flow after pPCI was strongly linked to larger infarct size, worse LVEF recovery, and a higher incidence of mortality and morbidity (Bekler et al., 2015; Kurtul et al., 2014). Similarly, we found that HP exhibited independent predictability for I‐CMP in multivariate logistic regression analysis. HP demonstrated the greatest sensitivity for predicting I‐CMP following 6 weeks of STEMI, surpassing both Hf and HfHP. However, this finding suggests that false positives should be taken into account and highlights that the combined use of HP and Hf may be more reliable for predicting I‐CMP after 6 weeks of STEMI. Additionally, in Model 1, the presence of hyperlipidemia (β = 1.658) carried almost equal risk for the development of I‐CMP compared to HP (β = 1.693). As we know, hyperlipidemia is a potent risk factor for coronary heart disease and may further promote I‐CMP through low‐grade inflammation (Varbo et al., 2013). In fact, this finding may prove that increased PLR is somehow also a marker of thromboinflammation.
Research has demonstrated that patients with STEMI who exhibit widened fQRS‐T are at an increased risk of suffering from larger infarcts, impaired LVEF, longer stays in the hospital, and increased susceptibility to ventricular arrhythmias and cardiovascular mortality (Na Yang et al., 2022). Consistently, the results of our study indicated that patients with widened fQRS‐T exhibited a higher incidence of I‐CMP. fQRS‐T was also found to be an independent predictor for I‐CMP, with an estimated predicted critical value of 66.72°.
The observation that an elevated PLR and widened fQRS‐T upon admission were linked to increased I‐CMP could be attributed to the greater extent of myocardial ischemia upon admission in STEMI. The present findings show a similarity to those of prior studies that have reported an association between increased PLR and widened fQRS‐T and a higher rate of adverse outcomes, including reduced LVEF, recurrent hospitalization for heart failure, and mortality. However, the aforesaid investigations have solely assessed either fQRS‐T or PLR in patients with STEMI (Azab et al., 2012; Colluoglu et al., 2018; Ozcan Cetin et al., 2016; Raposeiras‐Roubín et al., 2014; Sun et al., 2017). We also evaluated both factors on I‐CMP separately and in combination. In the three different models, the prognostic discriminatory capacity of HP (Model 1), Hf (Model 2), and HfHP (Model 3) remained significant after adjusting multiple covariates. The use of both variables in the process of risk stratification showed that the risk of I‐CMP is more accurately assessed compared to relying on either variable individually. Model 1 and Model 2 had a single variable, such as Hf or HP, whereas Model 3 included both variables, which had higher cut‐off values according to the ROC analysis. In Model 1 or Model 2, only one individual parameter was an independent risk factor for the development of I‐CMP, while in Model 3, HfHP leads to a 3.1 fold increase in the risk of I‐CMP development, and the presence of either Hf or HP alone is linked to a 1.4 fold increase in the risk of I‐CMP, respectively. Therefore, using only single parameters may overlook the development of I‐CMP, and drugs that provide reverse remodeling may not be prescribed to appropriate patients at the appropriate time. We may have an unfavorable effect on the progression of I‐CMP and heart failure, which are already at high risk following STEMI. Moreover, our predictive model exhibited a notable enhancement in discerning I‐CMP due to its impact on specificity. The increased specificity is a promising advancement, as it substantially reduces the likelihood of misclassifying patients with I‐CMP. However, it is noteworthy that this improvement in specificity comes at the expense of sensitivity, particularly in accurately identifying all patients with I‐CMP. While the inclusion of these predictors may enhance our ability to correctly identify patients without I‐CMP, there may be a corresponding decrease in the sensitivity to detect patients developing I‐CMP. This trade‐off necessitates careful consideration of the clinical context and the objectives of diagnostic accuracy.
5. LIMITATIONS
The limited number of patients from a single center and the retrospective design of the study are the main limitations. We only assessed fQRS‐T and PLR on admission. Also, we lacked detailed data on the biomarker profile of the study patients, and an investigation on the association and comparison of prognostic values among PLR and other biomarkers of myocyte stress or fibrosis was not performed. The viability of myocardium (hibernating or stunning myocardium) was not evaluated. Our results cannot be generalized to all ACS patients, as the main focus of this study was STEMI patients.
6. CONCLUSION
The combination of fQRS‐T and PLR is more useful in comparison to the individual use of fQRS‐T or PLR for the prediction of postdischarge I‐CMP in STEMI. Thus, this basic and economical risk stratification approach may aid in identifying patients who need conventional medical treatment for I‐CMP during the initial event, with the aim of avoiding the advancement of severe I‐CMP.
Author Contribution
Tugce Colluoglu: Study design, data collection, writing.Melahat Hicran Aksu: Data collection.Yeşim Akın: Resources, writing, study designOrhan Önalan: Resources
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Supporting information
Appendix S1
Colluoglu, T. , Aksu, M. H. , Akın, Y. , & Onalan, O. (2024). Combined use of frontal plane QRS‐T angle and platelet‐to‐lymphocyte ratio in the risk prediction of ischemic cardiomyopathy in STEMI. Annals of Noninvasive Electrocardiology, 29, e13106. 10.1111/anec.13106
All authors have confirmed that this manuscript is original.
DATA AVAILABILITY STATEMENT
The data that support the findings will be available in Karabuk University at 1234 following an embargo from the date of publication to allow for commercialization of research findings.
REFERENCES
- Acet, H. , Ertaş, F. , Bilik, M. Z. , Akıl, M. A. , Özyurtlu, F. , Aydın, M. , Oylumlu, M. , Polat, N. , Yüksel, M. , Yıldız, A. , Kaya, H. , Akyüz, A. , Ayçiçek, H. , Özbek, M. , & Toprak, N. (2015). The relationship between neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and thrombolysis in myocardial infarction risk score in patients with ST elevation acute myocardial infarction before primary coronary intervention. Postepy w Kardiologii Interwencyjnej = Advances in Interventional Cardiology, 11(2), 126–135. 10.5114/pwki.2015.52286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab, B. , Shah, N. , Akerman, M. , & McGinn, J. T., Jr. (2012). Value of platelet/lymphocyte ratio as a predictor of all‐cause mortality after non‐ST‐elevation myocardial infarction. Journal of Thrombosis and Thrombolysis, 34(3), 326–334. 10.1007/s11239-012-0718-6 [DOI] [PubMed] [Google Scholar]
- Banka, A. L. , Guevara, M. V. , Brannon, E. R. , Nguyen, N. Q. , Song, S. , Cady, G. , Pinsky, D. J. , Uhrich, K. E. , Adili, R. , Holinstat, M. , & Eniola‐Adefeso, O. (2023). Cargo‐free particles divert neutrophil‐platelet aggregates to reduce thromboinflammation. Nature Communications, 14(1), 2462. 10.1038/s41467-023-37990-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekler, A. , Gazi, E. , Yılmaz, M. , Temiz, A. , Altun, B. , Barutçu, A. , & Peker, T. (2015). Could elevated platelet‐lymphocyte ratio predict left ventricular systolic dysfunction in patients with non‐ST elevated acute coronary syndrome? Anatolian Journal of Cardiology, 15(5), 385–390. 10.5152/akd.2014.5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, J. (2023). RECORD‐MI: Heart failure complicating acute myocardial infarction. ESC Congress Amsterdam. [Google Scholar]
- Colluoglu, T. , Tanriverdi, Z. , Unal, B. , Ozcan, E. E. , Dursun, H. , & Kaya, D. (2018). The role of baseline and post‐procedural frontal plane QRS‐T angles for cardiac risk assessment in patients with acute STEMI. Annals of Noninvasive Electrocardiology: The Official Journal of the International Society for Holter and Noninvasive Electrocardiology, Inc, 23(5), e12558. 10.1111/anec.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilaveris, P. , Antoniou, C. K. , Gatzoulis, K. , & Tousoulis, D. (2017). T wave axis deviation and QRS‐T angle – Controversial indicators of incident coronary heart events. Journal of Electrocardiology, 50(4), 466–475. 10.1016/j.jelectrocard.2017.02.008 [DOI] [PubMed] [Google Scholar]
- Dong, G. , Huang, A. , & Liu, L. (2021). Platelet‐to‐lymphocyte ratio and prognosis in STEMI: A meta‐analysis. European Journal of Clinical Investigation, 51(3), e13386. 10.1111/eci.13386 [DOI] [PubMed] [Google Scholar]
- Granger, C. B. , Goldberg, R. J. , Dabbous, O. , Pieper, K. S. , Eagle, K. A. , Cannon, C. P. , Van De Werf, F. , Avezum, A. , Goodman, S. G. , Flather, M. D. , Fox, K. A. , & Global Registry of Acute Coronary Events Investigators . (2003). Predictors of hospital mortality in the global registry of acute coronary events. Archives of Internal Medicine, 163(19), 2345–2353. 10.1001/archinte.163.19.2345 [DOI] [PubMed] [Google Scholar]
- Ibanez, B. , James, S. , Agewall, S. , Antunes, M. J. , Bucciarelli‐Ducci, C. , Bueno, H. , Caforio, A. L. P. , Crea, F. , Goudevenos, J. A. , Halvorsen, S. , Hindricks, G. , Kastrati, A. , Lenzen, M. J. , Prescott, E. , Roffi, M. , Valgimigli, M. , Varenhorst, C. , Vranckx, P. , Widimský, P. , & ESC Scientific Document Group . (2018). 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). European Heart Journal, 39(2), 119–177. 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- Kurisu, S. , Nitta, K. , Sumimoto, Y. , Ikenaga, H. , Ishibashi, K. , Fukuda, Y. , & Kihara, Y. (2019). Myocardial perfusion defect assessed by single‐photon emission computed tomography and frontal QRS‐T angle in patients with prior anterior myocardial infarction. Heart and Vessels, 34(6), 971–975. 10.1007/s00380-018-01330-9 [DOI] [PubMed] [Google Scholar]
- Kurtul, A. , Yarlioglues, M. , Murat, S. N. , Ergun, G. , Duran, M. , Kasapkara, H. A. , Demircelik, M. B. , Cetin, M. , & Ocek, A. H. (2014). Usefulness of the platelet‐to‐lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST‐segment elevation myocardial infarction. The American Journal of Cardiology, 114(3), 342–347. 10.1016/j.amjcard.2014.04.045 [DOI] [PubMed] [Google Scholar]
- Lang, R. M. , Badano, L. P. , Mor‐Avi, V. , Afilalo, J. , Armstrong, A. , Ernande, L. , Flachskampf, F. A. , Foster, E. , Goldstein, S. A. , Kuznetsova, T. , Lancellotti, P. , Muraru, D. , Picard, M. H. , Rietzschel, E. R. , Rudski, L. , Spencer, K. T. , Tsang, W. , & Voigt, J. U. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography, 28(1), 1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Levine, G. N. , Bates, E. R. , Blankenship, J. C. , Bailey, S. R. , Bittl, J. A. , Cercek, B. , Chambers, C. E. , Ellis, S. G. , Guyton, R. A. , Hollenberg, S. M. , Khot, U. N. , Lange, R. A. , Mauri, L. , Mehran, R. , Moussa, I. D. , Mukherjee, D. , Nallamothu, B. K. , & Ting, H. H. (2011). 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Journal of the American College of Cardiology, 58(24), e44–e122. 10.1016/j.jacc.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Li, L. , Ma, Y. , Geng, X. B. , Tan, Z. , Wang, J. H. , Cui, C. , Wang, H. L. , & Shang, X. M. (2021). Platelet‐to‐lymphocyte ratio relates to poor prognosis in elderly patients with acute myocardial infarction. Aging Clinical and Experimental Research, 33(3), 619–624. 10.1007/s40520-020-01555-7 [DOI] [PubMed] [Google Scholar]
- Lown, M. T. , Munyombwe, T. , Harrison, W. , West, R. M. , Hall, C. A. , Morrell, C. , Jackson, B. M. , Sapsford, R. J. , Kilcullen, N. , Pepper, C. B. , Batin, P. D. , Hall, A. S. , Gale, C. P. , & Evaluation of Methods and Management of Acute Coronary Events (EMMACE) Investigators . (2012). Association of frontal QRS‐T angle – Age risk score on admission electrocardiogram with mortality in patients admitted with an acute coronary syndrome. The American Journal of Cardiology, 109(3), 307–313. 10.1016/j.amjcard.2011.09.014 [DOI] [PubMed] [Google Scholar]
- Michael, M. A. , El Masry, H. , Khan, B. R. , & Das, M. K. (2007). Electrocardiographic signs of remote myocardial infarction. Progress in Cardiovascular Diseases, 50(3), 198–208. 10.1016/j.pcad.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Morrow, D. A. , Antman, E. M. , Charlesworth, A. , Cairns, R. , Murphy, S. A. , de Lemos, J. A. , Giugliano, R. P. , McCabe, C. H. , & Braunwald, E. (2000). TIMI risk score for ST‐elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation, 102(17), 2031–2037. 10.1161/01.cir.102.17.2031 [DOI] [PubMed] [Google Scholar]
- Na Yang, X. H. , Zhang, J. , Zhang, S. , & Sun, J. (2022). What can we find in QRS in patients with ST‐segment‐elevation myocardial infarction? Journal of Electrocardiology, 75, 52–59. [Google Scholar]
- Oehler, A. , Feldman, T. , Henrikson, C. A. , & Tereshchenko, L. G. (2014). QRS‐T angle: A review. Annals of Noninvasive Electrocardiology: The Official Journal of the International Society for Holter and Noninvasive Electrocardiology, Inc, 19(6), 534–542. 10.1111/anec.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ommen, S. R. , Gibbons, R. J. , Hodge, D. O. , & Thomson, S. P. (1997). Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. The American Journal of Cardiology, 79(6), 812–814. 10.1016/s0002-9149(96)00878-8 [DOI] [PubMed] [Google Scholar]
- Ozcan Cetin, E. H. , Cetin, M. S. , Aras, D. , Topaloglu, S. , Temizhan, A. , Kisacik, H. L. , & Aydogdu, S. (2016). Platelet to lymphocyte ratio as a prognostic marker of in‐hospital and long‐term major adverse cardiovascular events in ST‐segment elevation myocardial infarction. Angiology, 67(4), 336–345. 10.1177/0003319715591751 [DOI] [PubMed] [Google Scholar]
- Raposeiras‐Roubín, S. , Virgós‐Lamela, A. , Bouzas‐Cruz, N. , Bouzas‐Cruz, N. , López‐López, A. , Castiñeira‐Busto, M. , Fernández‐Garda, R. , García‐Castelo, A. , Rodríguez‐Mañero, M. , García‐Acuña, J. M. , Abu‐Assi, E. , & González‐Juanatey, J. R. (2014). Usefulness of the QRS‐T angle to improve long‐term risk stratification of patients with acute myocardial infarction and depressed left ventricular ejection fraction. The American Journal of Cardiology, 113(8), 1312–1319. 10.1016/j.amjcard.2014.01.406 [DOI] [PubMed] [Google Scholar]
- Rodríguez, B. , Trayanova, N. , & Noble, D. (2006). Modeling cardiac ischemia. Annals of the New York Academy of Sciences, 1080, 395–414. 10.1196/annals.1380.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrottmaier, W. C. , Mussbacher, M. , Salzmann, M. , & Assinger, A. (2020). Platelet‐leukocyte interplay during vascular disease. Atherosclerosis, 307, 109–120. 10.1016/j.atherosclerosis.2020.04.018 [DOI] [PubMed] [Google Scholar]
- Schuster, A. , Morton, G. , Chiribiri, A. , Perera, D. , Vanoverschelde, J. L. , & Nagel, E. (2012). Imaging in the management of ischemic cardiomyopathy: Special focus on magnetic resonance. Journal of the American College of Cardiology, 59(4), 359–370. 10.1016/j.jacc.2011.08.076 [DOI] [PubMed] [Google Scholar]
- Serra, R. , Ielapi, N. , Licastro, N. , Provenzano, M. , Andreucci, M. , Bracale, U. M. , Jiritano, F. , de Franciscis, S. , Mastroroberto, P. , & Serraino, G. F. (2021). Neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio as biomarkers for cardiovascular surgery procedures: A literature review. Reviews on Recent Clinical Trials, 16(2), 173–179. 10.2174/1574887115999201027145406 [DOI] [PubMed] [Google Scholar]
- Sexton, T. R. , Wallace, E. L. , Macaulay, T. E. , Charnigo, R. J. , Evangelista, V. , Campbell, C. L. , Bailey, A. L. , & Smyth, S. S. (2015). The effect of rosuvastatin on thromboinflammation in the setting of acute coronary syndrome. Journal of Thrombosis and Thrombolysis, 39(2), 186–195. 10.1007/s11239-014-1142-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. P. , Li, J. , Zhu, W. W. , Li, D. B. , Chen, H. , Li, H. W. , Chen, W. M. , & Hua, Q. (2017). Impact of platelet‐to‐lymphocyte ratio on clinical outcomes in patients with ST‐segment elevation myocardial infarction. Angiology, 68(4), 346–353. 10.1177/0003319716657258 [DOI] [PubMed] [Google Scholar]
- Tang, T. T. , Yuan, J. , Zhu, Z. F. , Zhang, W. C. , Xiao, H. , Xia, N. , Yan, X. X. , Nie, S. F. , Liu, J. , Zhou, S. F. , Li, J. J. , Yao, R. , Liao, M. Y. , Tu, X. , Liao, Y. H. , & Cheng, X. (2012). Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Research in Cardiology, 107(1), 232. 10.1007/s00395-011-0232-6 [DOI] [PubMed] [Google Scholar]
- Tanriverdi, Z. , Besli, F. , Gungoren, F. , & Tascanov, M. B. (2020). What is the normal range of the frontal QRS‐T angle? Diabetes Research and Clinical Practice, 160, 107645. 10.1016/j.diabres.2019.02.028 [DOI] [PubMed] [Google Scholar]
- Varbo, A. , Benn, M. , Tybjærg‐Hansen, A. , & Nordestgaard, B. G. (2013). Elevated remnant cholesterol causes both low‐grade inflammation and ischemic heart disease, whereas elevated low‐density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation, 128(12), 1298–1309. 10.1161/circulationaha.113.003008 [DOI] [PubMed] [Google Scholar]
- Wang, N. , Hung, C. L. , Shin, S. H. , Claggett, B. , Skali, H. , Thune, J. J. , Køber, L. , Shah, A. , McMurray, J. J. , Pfeffer, M. A. , Solomon, S. D. , & VALIANT Investigators . (2016). Regional cardiac dysfunction and outcome in patients with left ventricular dysfunction, heart failure, or both after myocardial infarction. European Heart Journal, 37(5), 466–472. 10.1093/eurheartj/ehv558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings will be available in Karabuk University at 1234 following an embargo from the date of publication to allow for commercialization of research findings.


