Abstract
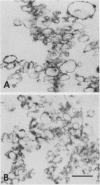
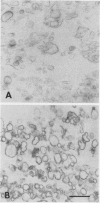
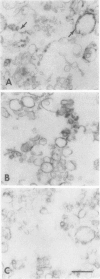
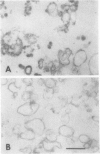
Right-side-out vesicles of plasma membrane from soybean (Glycine max Merr.) were isolated by aqueous two-phase partition. Inside-out vesicles were formed when these preparations were diluted or frozen and thawed. Sidedness (orientation) was determined by preparative free-flow electrophoresis, concanavalin A binding, and ATPase latency. Under usual conditions of aqueous two-phase partition, the bulk of the vesicles were strongly reactive with concanavalin A-peroxidase and showed a high level of structure-linked latency as expected of a right-side-out (cytoplasmic-side-in) orientation. The vesicles migrated as a single electrophoretic peak. When frozen and thawed, vesicle diameters were reduced and a second population of vesicles of increased electrophoretic mobility was obtained. This second population of vesicles was weakly reactive with concanavalin A-peroxidase and showed low latency as expected of an inside-out (cytoplasmic-side-out) orientation. If the plasma membrane vesicles were diluted with water, a mixture of right-side-out and inside-out vesicles again was obtained. However, some of the cytoplasmic-side-out vesicles that were concanavalin A-unreactive and had low ATPase latency migrated more slowly as a second, less electronegative peak, upon free-flow electrophoresis. The results suggest that right-side-out and inside-out plasma membrane vesicles differ in electrophoretic mobility but that both the orientation and the absolute electrophoretic mobility of the differently oriented vesicles may be influenced by the preparative conditions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canut H., Brightman A., Boudet A. M., Morré D. J. Plasma membrane vesicles of opposite sidedness from soybean hypocotyls by preparative free-flow electrophoresis. Plant Physiol. 1988 Feb;86(2):631–637. doi: 10.1104/pp.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner L. E., Kjellbom P., Larsson C., Møller I. M. Surface properties of right side-out plasma membrane vesicles isolated from barley roots and leaves. Plant Physiol. 1985 Sep;79(1):72–79. doi: 10.1104/pp.79.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. V., Steponkus P. L. Plasma Membrane Lipid Alterations Associated with Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1987 Apr;83(4):761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren M. G., Askerlund P., Fredrikson K., Widell S., Sommarin M., Larsson C. Sealed inside-out and right-side-out plasma membrane vesicles : optimal conditions for formation and separation. Plant Physiol. 1990 Apr;92(4):871–880. doi: 10.1104/pp.92.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelius A. S., Penel C., Auderset G., Brightman A., Millard M., Morré D. J. Isolation of highly purified fractions of plasma membrane and tonoplast from the same homogenate of soybean hypocotyls by free-flow electrophoresis. Plant Physiol. 1986 May;81(1):177–185. doi: 10.1104/pp.81.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom R. P., Deboer A. H., Lomax T. L., Cleland R. E. Latency of Plasma Membrane H-ATPase in Vesicles Isolated by Aqueous Phase Partitioning : Increased substrate Accessibility or Enzyme Activation. Plant Physiol. 1987 Nov;85(3):693–698. doi: 10.1104/pp.85.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Isolation and Identification of Plasma Membrane from Light-Grown Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1983 Nov;73(3):586–597. doi: 10.1104/pp.73.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widell S., Lundborg T., Larsson C. Plasma membranes from oats prepared by partition in an aqueous polymer two-phase system : on the use of light-induced cytochrome B reduction as a marker for the plasma membrane. Plant Physiol. 1982 Nov;70(5):1429–1435. doi: 10.1104/pp.70.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Properties of Plasma Membrane Isolated from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):152–160. doi: 10.1104/pp.80.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass (Dactylis glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984 May;75(1):31–37. doi: 10.1104/pp.75.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]