Abstract
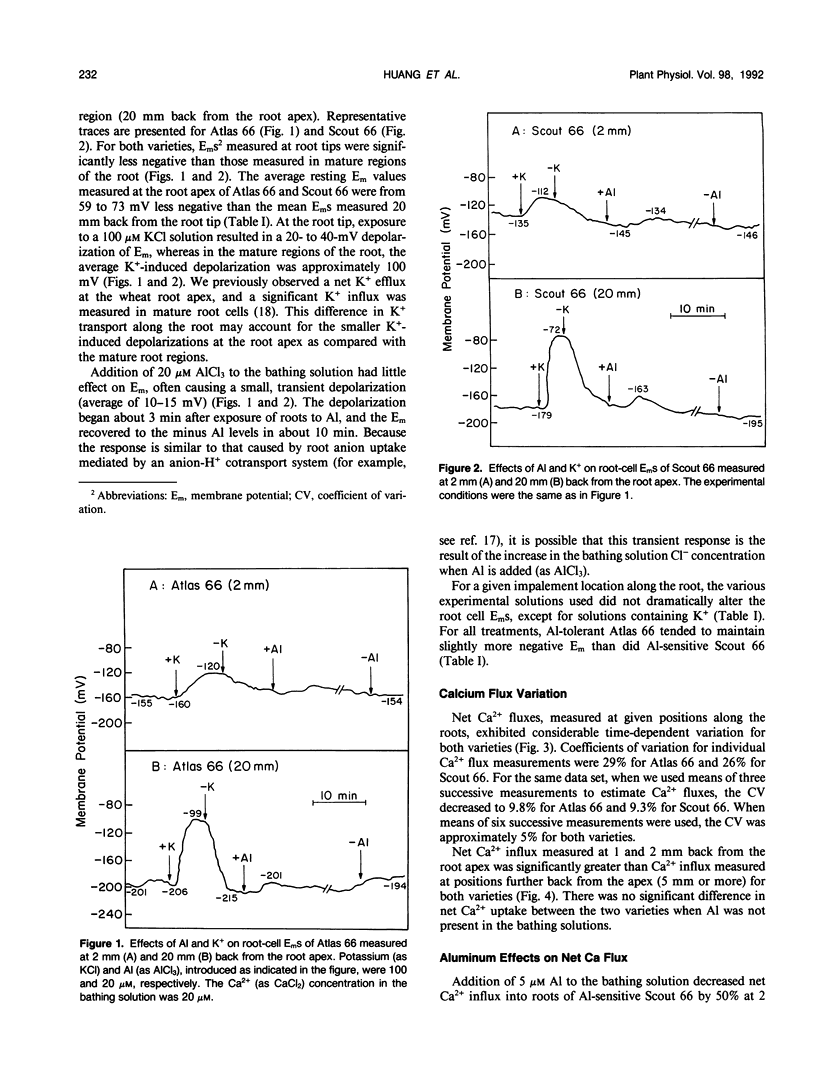
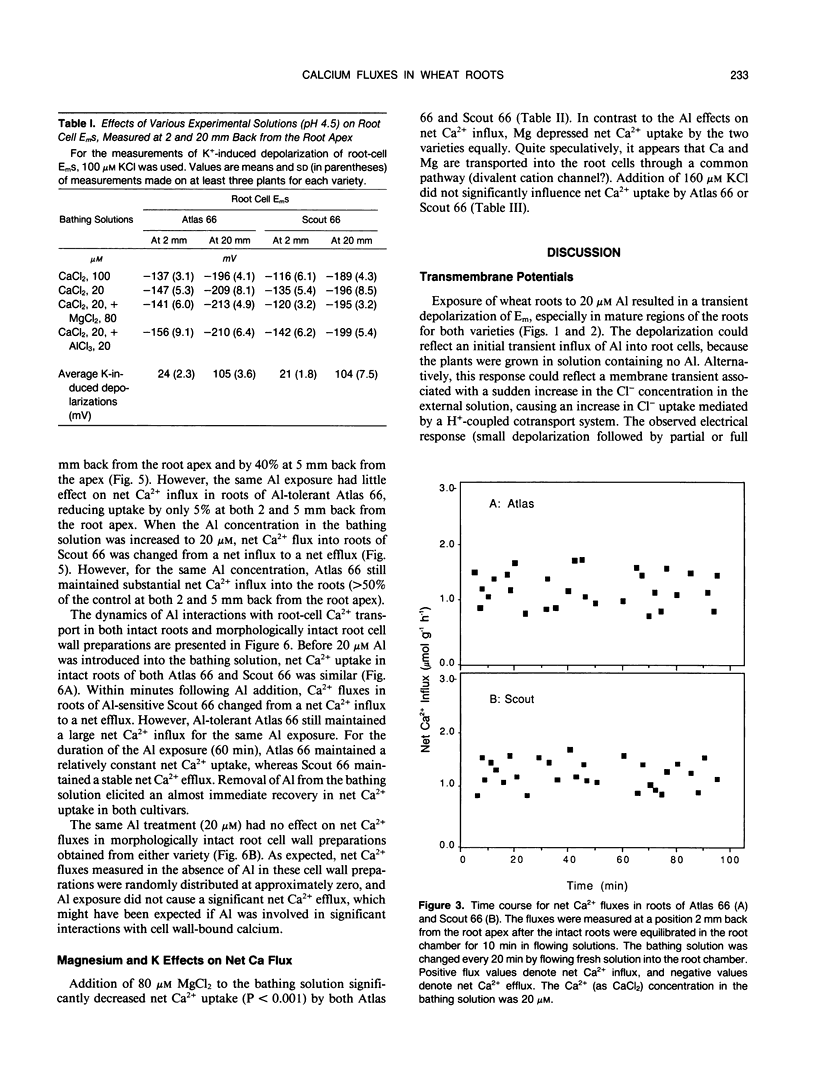
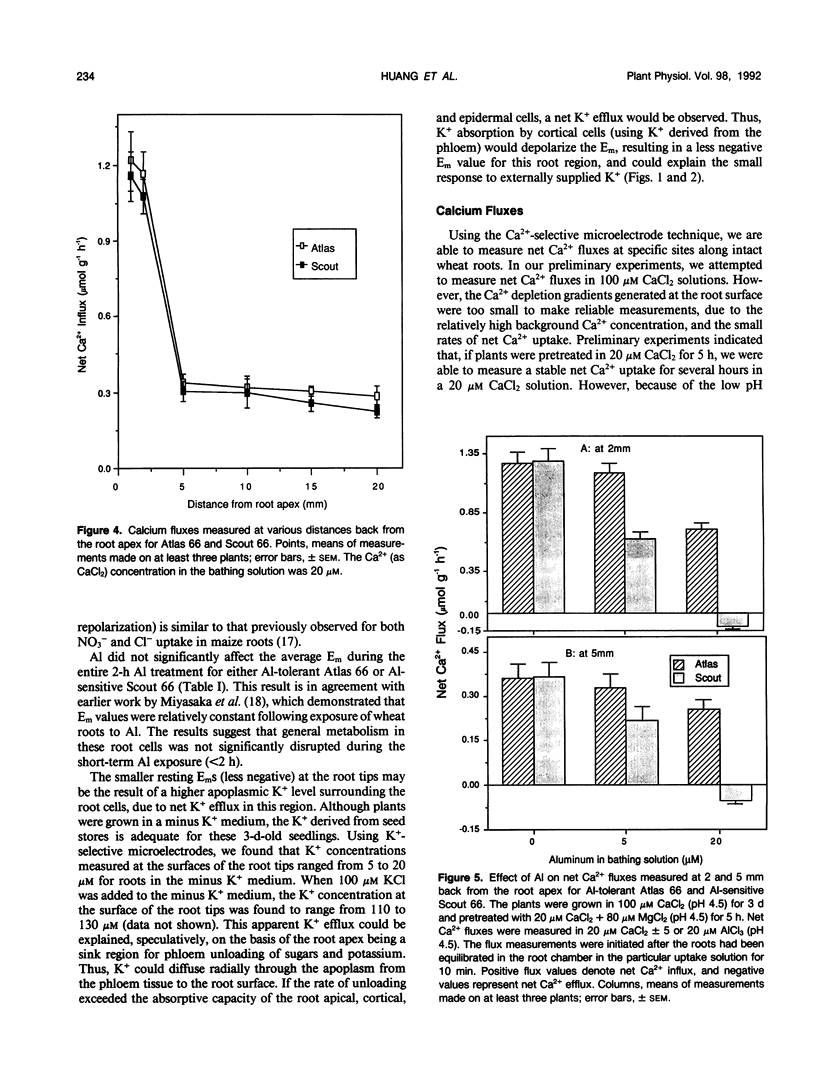
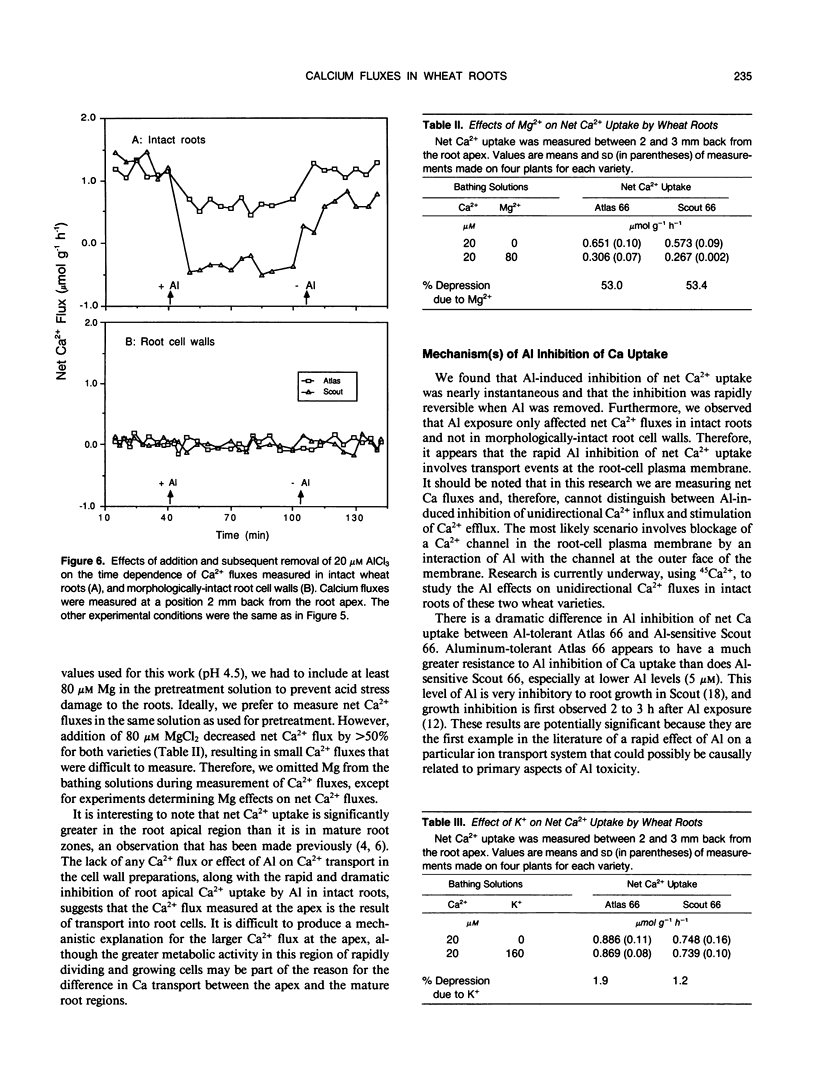
The role of Ca2+ transport in the mechanism of Al toxicity was investigated, using a Ca2+-selective microelectrode system to study Al effects on root apical Ca2+ fluxes in two wheat (Triticum aestivum L.) cultivars: Al-tolerant Atlas 66 and Al-sensitive Scout 66. Intact 3-day-old low-salt-grown (100 micromolar CaCl2, pH 4.5) wheat seedlings were used, and it was found that both cultivars maintained similar rates of net Ca2+ uptake in the absence of Al. Addition of Al concentrations that were toxic to Scout (5-20 micromolar AlCl3) immediately and dramatically inhibited Ca2+ uptake in Scout, whereas Ca2+ transport in Atlas was relatively unaffected. The Al-induced inhibition of Ca2+ uptake in Scout 66 was rapidly reversed following removal of Al from the solution bathing the roots. Similar studies with morphologically intact root cell wall preparations indicated that the Al effects did not involve Al-Ca interactions in the cell wall. These results suggest that Al inhibits Ca2+ influx across the root plasmalemma, possibly via blockage of calcium channels. The differential effect of Al on Ca2+ transport in Al-sensitive Scout and Al-tolerant Atlas suggests that Al blockage of Ca2+ channels could play a role in the cellular mechanism of Al toxicity in higher plants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kochian L. V., Shaff J. E., Lucas W. J. High affinity k uptake in maize roots: a lack of coupling with h efflux. Plant Physiol. 1989 Nov;91(3):1202–1211. doi: 10.1104/pp.91.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas E. V. Calcium uptake by excised maize roots and interactions with alkali cations. Plant Physiol. 1969 Jul;44(7):985–989. doi: 10.1104/pp.44.7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Kochian L. V., Spanswick R. M., Shaff J. E. Evidence for cotransport of nitrate and protons in maize roots : I. Effects of nitrate on the membrane potential. Plant Physiol. 1990 May;93(1):281–289. doi: 10.1104/pp.93.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka S. C., Kochian L. V., Shaff J. E., Foy C. D. Mechanisms of Aluminum Tolerance in Wheat : An Investigation of Genotypic Differences in Rhizosphere pH, K, and H Transport, and Root-Cell Membrane Potentials. Plant Physiol. 1989 Nov;91(3):1188–1196. doi: 10.1104/pp.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman I. A., Kochian L. V., Grusak M. A., Lucas W. J. Fluxes of h and k in corn roots : characterization and stoichiometries using ion-selective microelectrodes. Plant Physiol. 1987 Aug;84(4):1177–1184. doi: 10.1104/pp.84.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra R., Haug A. The effect of A13+ on the physical properties of membrane lipids in Thermoplasma acidophilum. Biochem Biophys Res Commun. 1978 Sep 14;84(1):138–143. doi: 10.1016/0006-291x(78)90274-7. [DOI] [PubMed] [Google Scholar]
- Zhao X. J., Sucoff E., Stadelmann E. J. Al and Ca Alteration of Membrane Permeability of Quercus rubra Root Cortex Cells. Plant Physiol. 1987 Jan;83(1):159–162. doi: 10.1104/pp.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]


