Abstract
Deployment of the tear gas agent 2-chlorobenzalmalononitrile (CS) for riot control has significantly increased in recent years. The effects of CS have been believed to be transient and benign. However, CS induces severe pain, blepharospasm, lachrymation, airway obstruction, and skin blisters. Frequent injuries and hospitalizations have been reported after exposure. We have identified the sensory neuronal ion channel, transient receptor potential ankyrin 1 (TRPA1), as a key CS target resulting in acute irritation and pain and also as a mediator of neurogenic inflammation. Here, we examined the effects of pharmacologic TRPA1 inhibition on CS-induced cutaneous injury. We modeled CS-induced cutaneous injury by applying 10 μl CS agent [200 mM in dimethyl sulfoxide (DMSO)] to each side of the right ears of 8- to 9-week-old C57BL/6 male mice, whereas left ears were applied with solvent only (DMSO). The TRPA1 inhibitor HC-030031 or A-967079 was administered after CS exposure. CS exposure induced strong tissue swelling, plasma extravasation, and a dramatic increase in inflammatory cytokine levels in the mouse ear skin. We also showed that the effects of CS were not transient but caused persistent skin injuries. These injury parameters were reduced with TRPA1 inhibitor treatment. Further, we tested the pharmacologic activity of advanced TRPA1 antagonists in vitro. Our findings showed that TRPA1 is a crucial mediator of CS-induced nociception and tissue injury and that TRPA1 inhibitors are effective countermeasures that reduce key injury parameters when administered after exposure. Additional therapeutic efficacy studies with advanced TRPA1 antagonists and decontamination strategies are warranted.
SIGNIFICANCE STATEMENT
2-Chlorobenzalmalononitrile (CS) tear gas agent has been deployed as a crowd dispersion chemical agent in recent times. Exposure to CS tear gas agents has been believed to cause transient acute toxic effects that are minimal at most. Here we found that CS tear gas exposure causes both acute and persistent skin injuries and that treatment with transient receptor potential ion channel ankyrin 1 (TRPA1) antagonists ameliorated skin injuries.
Introduction
Deployment of the tear gas agent 2-chlorobenzalmalononitrile (CS; C10H5ClN2, CAS: 2698-41-1) for riot control has significantly increased in recent years (Olajos and Salem, 2001; Blain, 2003; Bessac et al., 2009; Bessac and Jordt, 2010; Schep et al., 2015; Rothenberg et al., 2016; Torgrimson-Ojerio et al., 2021). CS tear gas has been commonly used by police forces as a riot control agent (RCA) to disperse crowds and subdue individuals. Although CS tear gas was banned as a chemical weapon in warfare, its use in domestic riot control is allowed but has been highly controversial. Some countries, including the United States, train their newly recruited military and armed forces with tear gas agents under different simulative conditions as part of their chemical, biologic, radiologic, nuclear, and high-yield explosives (CBRNE) training (Hout et al., 2011; https://www.cbsnews.com/news/navy-seal-tear-gas-video-investigation/). Some casualties have been reported during such CBRNE training sessions (Hout et al., 2011). Despite its description as a safe riot control agent, CS tear gas agent is not without harmful side effects. The effects of CS tear gas have been believed to be transient. Reported deaths are few, whereas injuries are many (Rappert, 2003; Watson and Rycroft, 2005; Agrawal et al., 2009; Lam et al., 2020). Several countries do not have proper regulations on the use of tear gas agents for incapacitating riots. In the last couple of years, the indiscriminate use of tear gas as a riot control agent in the United States, Middle Eastern countries, and many other countries around the world has led to worldwide criticism from several corners, including the Human Rights Watch (HRW) (CS gas: infringing the gas laws, 1970; Aktan, 2013). Some countries have banned the use of CS tear gas agents due to their perceived effects on the reproductive system (https://latindispatch.com/2011/05/19/chile-suspends-use-of-tear-gas-amid-concerns-over-miscarriages/). Of note, the Biologic and Chemical Weapons Convention, signed by almost all countries, had banned the use of tear gas on the war front, but its use in domestic riot control is allowed (https://www.armscontrol.org/blog/2020-06-05/tear-gas-banned-war-time-ban-its-domestic-use).
Tear gas agents are well known to cause several effects, including severe pain, blepharospasm, lachrymation, and airway obstruction (Olajos and Salem, 2001; Varma and Holt, 2001; Smith and Greaves, 2002; Karaman et al., 2009; Shambhu and Kurtis, 2011; Schep et al., 2015). Eyes, respiratory system, and skin are commonly exposed (Agrawal et al., 2009). Ocular and respiratory irritation occurs within a few seconds. The toxic effects of CS tear gas agents on the cutaneous system vary. Common cutaneous outcomes are irritation, bulla formation, burns, subcutaneous edema, and contact dermatitis (Southward, 2001; Watson and Rycroft, 2005; Arbak et al., 2014; Schep et al., 2015). Some studies have reported contact dermatitis with repeated exposure to CS tear gas agent (Kain et al., 2010; Shambhu and Kurtis, 2011; Bhargava et al., 2012; Lam et al., 2020). Several short-term and long-term effects of CS tear gas agents have been reported (Karagama et al., 2003; Karaman et al., 2009; Kain et al., 2010; Arbak et al., 2014). Some suggestions in the public domain for decontamination and treatment of tear gas agents include water, milk, petroleum jelly, vinegar, lemon juice, or antacids. However, these remedies and decontamination procedures are not scientifically proven. Decontamination of contaminated surfaces is also difficult, as the CS tear gas agent is almost insoluble in water and only slightly soluble in ethyl alcohol and carbon tetrachloride. The actual concentrations of incapacitating agents to which civilians and law enforcement authorities are exposed vary considerably, which complicates the assessment of consequences and further treatment. Although some therapeutics have been tested for CS tear agent–induced injuries, symptomatic treatment remains the most common approach to treatment (Jones, 1996; Viala et al., 2005; Luka et al., 2007; Carron and Yersin, 2009; Schep et al., 2015). Thus, to the best of our knowledge, no specific antidote for tear gas–induced cutaneous injuries is available (Schep et al., 2015). Indeed, the best prophylactic option for preventing injuries from CS tear gas exposure is running away or removing the individual from the source.
Although several studies have been performed to elucidate the mechanism of action of tear gas agents and their treatment, there remains a knowledge gap in the standards of care for tear gas agent–induced casualties based on mechanistic studies. The seminal work of Bessac et al. (2008) and other studies showed that transient receptor potential ion channel ankyrin repeat 1 (TRPA1) mediates CS tear gas–induced inflammation in in vitro studies (Bessac and Jordt, 2008, 2010; Brône et al., 2008). TRPA1 is a chemoreceptor for environmental irritants and inflammatory agents (Bautista et al., 2013; Julius, 2013; Achanta and Jordt, 2017). TRPA1 channels are activated by a variety of stimuli (either chemical or environmental), including but not limited to aldehydes [acrolein and allyl isothiocyanate (AITC)], terpenes (thymol and carvacrol), irritants, voltage, cold, stretch, and flufenamic acid. Endogenous products of reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as 4-hydroxynonal, activate TRPA1 channels (Bautista et al., 2013; Julius, 2013; Achanta and Jordt, 2020). However, no in vivo studies have demonstrated therapeutic effects of TRPA1 antagonists on CS tear gas–induced cutaneous inflammation.
The objective of this study is to confirm that TRPA1 is the mediator of CS tear gas agent–induced inflammation, using in vivo studies, and to test the therapeutic potential of TRPA1 antagonists such as HC-030031 and A-967079 in mouse ear models of CS tear gas agent–induced cutaneous injury.
Materials and Methods
Chemicals, Animals, and Drugs.
C57BL/6 male 8- to 9-week-old mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were given at least 1 week to acclimate before initiating studies and access to mouse chow and water ad libitum. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Yale University (New Haven, CT) and Duke University (Durham, NC).
The TRPA1 antagonist HC-030031 ((2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)-N-(4-isopropylphenyl)acetamide) was a generous gift from Hydra Biosciences (Cambridge, MA), and the TRPA1 antagonist A-967079 ((1E,3E)-1-(4-fluorophenyl)-2-methyl-1-pentene-3-one oxime) was custom synthesized by Medchem101 (Conshohocken, PA). AMG0902 (1-[[3-[2-(4-chlorophenyl)ethyl]-1,2,4-oxadiazol-5-yl]methyl]-7-methylpurin-6-one) was purchased from R&D Systems (Minneapolis, MN). GDC-0334 ((2S,4R,5S)-4-fluoro-1-((4-fluorophenyl)sulfonyl)-5-methyl-N-((5-(trifluoromethyl)-2-(2-(trifluoromethyl)pyrimidin-5-yl)pyridin-4-yl)methyl)pyrrolidine-2-carboxamide) was a generous gift from Genentech (San Francisco, CA). Methylcellulose (METHOCEL MC, medium viscosity, 1200–1800 mPa-s) was purchased from Fluka (Buchs, Switzerland). CS tear gas was purchased from Combi-Blocks (San Diego, CA). All other chemicals and reagents used were obtained from Fisher Scientific, Sigma-Aldrich, or other scientific suppliers.
Mouse Models of CS Tear Gas Agent–Induced Skin Injury.
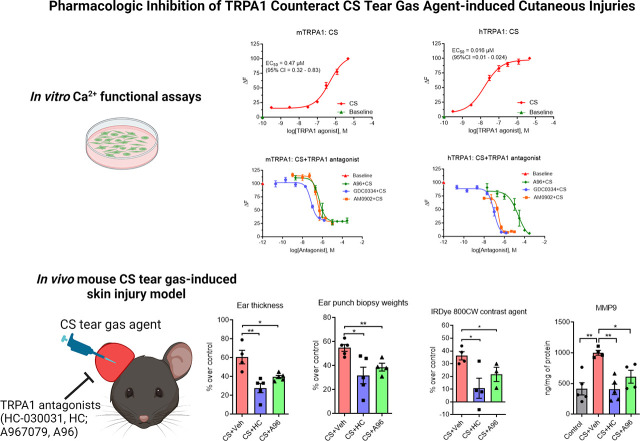
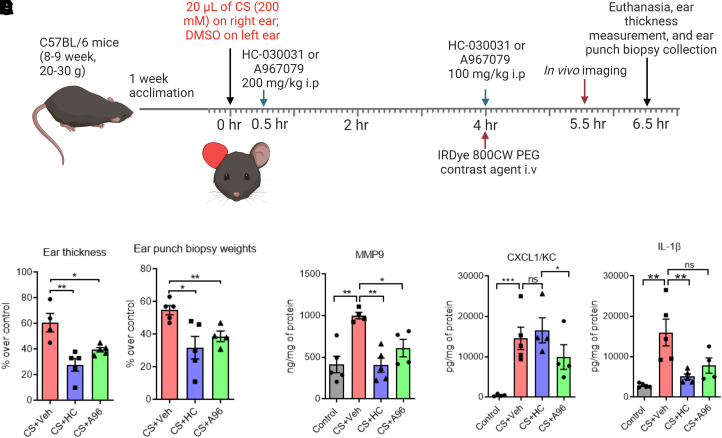
In the acute mouse model, the right ears of mice were exposed to 20 μl of 200 mM CS tear gas agent (10 μl on each surface of the ear), whereas the left ears were exposed to an equal volume of dimethyl sulfoxide (DMSO, a solvent for CS tear gas agent). At 0.5 and 4 hours after CS tear gas exposure, mice were administered a TRPA1 antagonist, HC-030031 (HC) or A-967079 (A96), or 0.5% methylcellulose (MC; vehicle for TRPA1 antagonists) intraperitoneally (i.p.) at a dose of 200 mg/kg and 100 mg/kg body weight, respectively. Figure 1A shows the study paradigm.
Fig. 1.
Effects of TRPA1 antagonists on CS tear gas agent–induced cutaneous inflammation. (A) CS tear gas exposure and treatment paradigm. The right ears of C57BL/6 male mice were exposed to CS (200 mM, 20 μl) and the left ears to DMSO (solvent for CS, 20 μl). At 0.5 and 4 hours after CS exposure, mice were treated with vehicle (0.5% methylcellulose), HC-030031 (HC), or A967079 (A96) intraperitoneally (i.p.). At 4 hours after CS exposure, mice were injected with IRDye 800CW contrast agent intravenously (i.v.), and in vivo imaging was performed at 5.5 hours after CS exposure. At 6.5 hours after CS exposure, mice were euthanized, ear thickness was measured, and ear punch biopsies were collected. (B–F) Ear thickness, ear punch biopsy weights, and proinflammatory cytokine markers in mice exposed to CS tear gas agent that received either vehicle (0.5% methylcellulose) or TRPA1 antagonist (HC or A96). Data were analyzed by one-way ANOVA with Tukey’s post hoc multiple comparison test. Data are presented as mean ± S.E.M., n = 5 per group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ns = nonsignificant.
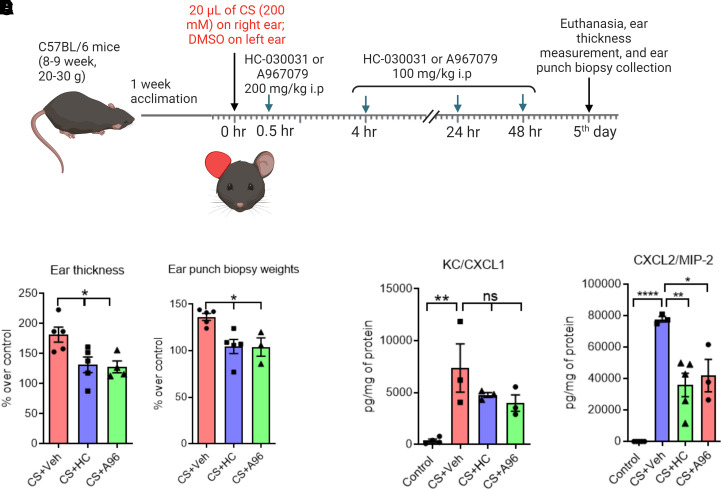
In chronic (extended observation) studies for up to 5 days after CS tear gas agent exposure, right ears were exposed to CS tear gas agent and left ears were exposed to DMSO, similar to the acute mouse model. Mice were administered HC (200 mg/kg, i.p.), A96 (200 mg/kg i.p.), or vehicle at 30 minutes after CS exposure. Additional doses of HC (100 mg/kg, i.p.), A96 (100 mg/kg, i.p.), or vehicle were given at 4 hours, 24 hours, and 48 hours after CS exposure. Figure 4A shows the study paradigm.
Fig. 4.
Persistent skin injuries after CS tear gas agent exposure and effects of TRPA1 antagonists on skin inflammation in an extended mouse observation model. (A) CS tear gas exposure and treatment. Right ears of C57BL/6 male mice were exposed to CS (200 mM, 20 μl) and left ears to DMSO (solvent for CS, 20 μl). At 0.5, 4, 24, and 48 hours after CS exposure, mice were treated with vehicle (0.5% methylcellulose), HC-030031 (HC), or A967079 (A96) intraperitoneally (i.p.). (B–E) Ear thickness, ear punch biopsy weights, and cytokine markers in mice receiving vehicle or TRPA1 antagonist after exposure to CS tear gas agent. Data were analyzed by one-way ANOVA with Tukey’s post hoc multiple comparison test. Data are presented as mean ± S.E.M., n = 5 per group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ns = nonsignificant.
Visualizing Extravasation of Inflammatory Exudate.
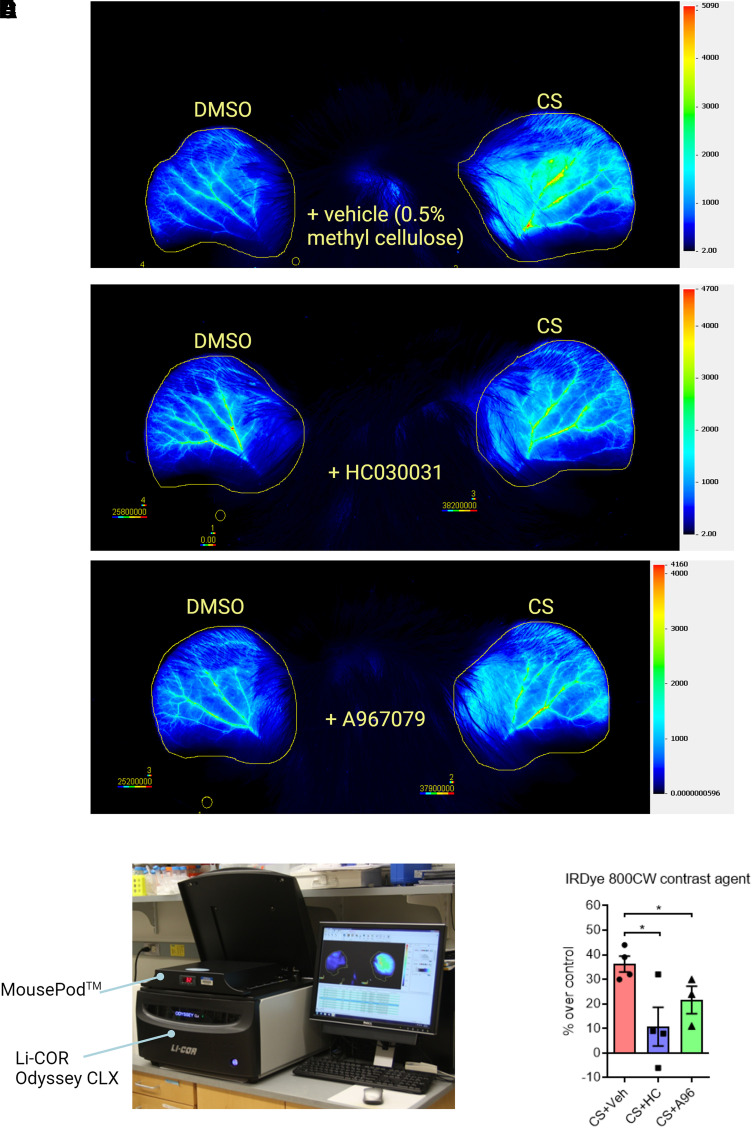
To visualize the extravasation of inflammatory exudate into surrounding tissues and monitor the healing process in CS tear gas–exposed ears, we injected IRDye 800CW intravenously (i.v.) at 4 hours after CS exposure. Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) cocktail injected (i.p.). Anesthetized mice were placed in supine positioning and scanned at 5.5 hours after CS tear gas exposure using Li-Cor Odyssey CLX fitted with Mouse-Pod (Li-Cor, Nebraska) set at 37°C temperature. We used the following settings for scanning of mice: channels – 700/800; scan resolution – 42 μm; intensities – 1 for both channels; data analysis – small animal; scan quality – medium (increasing the scan quality increases the time of scanning); focal offset – 1 mm. The manufacturer’s Small Animal Image Analysis Suite was used to evaluate the data.
Ear Punch Biopsy Sample Collection, Proinflammatory Cytokines, and Histopathology Analysis.
Mice were euthanized humanely at 6.5 hours after CS tear gas exposure using American Veterinary Medical Association (AVMA)- and IACUC-approved carbon dioxide inhalation method. Tissue edema was estimated by measuring ear thicknesses with electronic calipers (Mitutoyo QUICKmini, Japan) and ear punch biopsy weights (4-mm biopsy punch needle, Japan), as described previously (Achanta et al., 2018).
Ear punch biopsy samples were homogenized in 50 mM Tris-base and 150 mM NaCl, with EDTA-free protease inhibitor and 0.5% Triton-X, using a bullet blender and zirconium oxide beads (Next Advance, Troy, NY). Homogenization was performed for at least 20 minutes at 10-speed setting on the Next Advance bullet blender (Troy, NY). After that, samples were centrifuged at 10,000 g for 10 minutes at 4°C. Homogenized supernatant samples were used to measure inflammatory cytokine markers, including interleukin-1 beta (IL-1β), keratinocyte chemoattractant/chemokine (C-K-X motif) ligand 1 (KC/CXCL1), chemokine (CXC) ligand 2/macrophage inflammatory protein 2 (CXCL2/MIP-2), and matrix metalloproteinase 9 (MMP9). R&D Systems cytokine kits (Minneapolis, MN) or a high throughput multiplex cytokine assay system (MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel, Millipore, MO) was used for cytokine quantification. All samples were evaluated at least in duplicate on the Infinite M200 Pro (Tecan, Germany) or Bio-Plex 200 system (Bio-Rad, Hercules, CA) systems. The concentrations of cytokines were measured using a 5-parameter logistic regression analysis or a standard curve, in accordance with the manufacturer’s instructions. Cytokine concentrations that fell outside of the standard curve range were not included. The concentrations of protein in homogenate samples were determined using Pierce BCA protein assay (Thermo Scientific, Rockford, IL).
Histopathology.
For histopathology evaluation, 4-mm circular ear punch biopsy samples were fixed in 10% formaldehyde, embedded in paraffin, sectioned at a 5-μm thickness, and stained with hematoxylin and eosin (H&E) as per standard protocols. AxioVision Rel. 4.7 software (Zeiss, Munich, Germany) was used to examine the images, which were captured using a Zeiss Axio Imager Z1 microscope. The evaluation of cutaneous histopathology was conducted in accordance with the standards provided by Silny et al. (2005). Primarily, we assessed histopathological features such as edematous swelling, integrity of the epidermis, epidermal thickening, and infiltration of leukocytes.
Decontamination of CS Tear Gas Skin Exposure with Water Washing.
Currently, neither antidotes nor decontamination strategies are available to protect against injury from CS tear gas exposure. To assess the decontamination efficacy of water washing after skin exposure to CS tear gas agent 30 minutes after CS exposure, we washed both surfaces of ears three times with fresh cotton applicators moistened with water (Supplemental Fig. 3).
In Vitro Cell Culture and Pharmacologic Screening of Advanced TRPA1 Antagonists.
Cell culture and in vitro pharmacologic screening of advanced TRPA1 antagonists for inhibition of CS tear gas–induced calcium (Ca2+) influx was performed as described previously (Caceres et al., 2017). Briefly, human embryonic kidney 293T (HEK293T) cells were cultured and plated on poly-D-lysine–coated 100-mm tissue culture plastic dishes in Dulbecco’s modified Eagle medium (DMEM) and supplemented with 10% FBS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. At 60%–70% confluency, using Eugene 6 transfection reagent and Opti-MEM, cells were transiently transfected with mouse or human TRPA1 plasmid DNA according to the manufacturer’s protocols for 16–24 hours. Cells were then resuspended and seeded at a density of 50,000 cells per well (100 μl per well) onto poly-D-lysine–coated 96-well plates. After 24 hours, the intracellular influx of calcium ([Ca2+]i) was determined by loading Calcium 6, a no-wash fluorescent indicator, for ∼1.5 hours (Molecular Devices, San Jose, CA). Fluorescence was measured in the FlexStation at 37°C (excitation 485 nm, emission 525 nm at every 1.8 seconds) (FlexStation III; Molecular Devices). After recording baseline fluorescence, fluorescence was monitored for response to agonist (CS tear gas agent) for a total of 60 seconds. To determine the pharmacologic effects of TRPA1 antagonists, TRPA1-transfected HEK293T cells were pretreated with advanced TRPA1 antagonists such as A967079, AMG0902 (AM0902), or GDC0334 and then exposed to CS tear gas agent (agonist, a concentration near EC50). The change in fluorescence was determined (ΔF= Fmax − F0, where Fmax is the maximum fluorescence and F0 is the baseline fluorescence measured in each well). The EC50 and IC50 values were determined by nonlinear regression analysis with a four-parameter logistic equation (GraphPad Prism version 10.0.3 for Windows, San Diego, CA).
Data Analysis and Statistics.
GraphPad Prism version 10.0.3 for Windows was used to analyze the data. One-way ANOVA with Tukey’s post hoc multiple comparison test or the two- or one-tailed Student’s t test was used to assess for statistical differences. Outliers were removed by Dixon’s Q-test. Statistical significance was denoted by *P < 0.05, **P < 0.01, ***P < 0.001, or ns = nonsignificant. Some of the illustrations have been created using BioRender.com.
Results
Significant Acute Edema and Inflammation Were Observed after Cutaneous CS Tear Gas Agent Exposure.
A CS tear gas agent–induced mouse ear model of inflammation was established (Fig. 1A; Supplemental Figs. 1 and 2). After cutaneous exposure to the CS tear gas agent, skin edema [determined by ear thickness (61% increase over control) and ear punch biopsy weights (55% increase over control)] was observed (Fig. 1, B and C) and levels of proinflammatory cytokines such as MMP9 (59% increase over control), KC/CXCL1 (97% increase over control), and IL-1β (82% increase over control) were elevated (Fig. 1, B–F). Using in vivo imaging, we found significant vascular leakage (36% increase in the intensity of IRDye 800 CW dye fluorescence over control) into the subcutaneous tissue after CS tear gas agent exposure (Fig. 2A). Cutaneous edema was the most prominent feature in histopathology profiles after CS tear gas exposure (Fig. 3B).
Fig. 2.
Effect of TRPA1 antagonists on decreasing CS tear gas agent–induced cutaneous vascular leakage. The right ears of C57BL/6 male mice were exposed to CS (200 mM, 20 μl) and the left ears to DMSO (solvent for CS, 20 μl). At 0.5 and 4 hours after CS exposure, mice were treated with vehicle (0.5% methylcellulose), HC-030031 (HC), or A967079 (A96) intraperitoneally (i.p.). At 4 hours after CS exposure, mice were injected with IRDye 800CW contrast agent intravenously (i.v.), and in vivo imaging was performed at 5.5 hours after CS exposure using MousePOD fitted on Li-COR Odyssey CLX. Representative scan profiles of mice showing profound vascular leakage in the control group (A) and decreased vascular leakage in the treated groups (B and C). Photograph showing MousePOD fitted with Li-COR Odyssey CLX (D). Bar graph showing the quantification of IRDye 800CW contrast agent that leaked into the ear subcutaneous tissue (E). Data were analyzed by one-way ANOVA with Tukey’s post hoc multiple comparison test. Data are presented as mean ± S.E.M., n = 3 to 4 per group. *P ≤ 0.05.
Fig. 3.
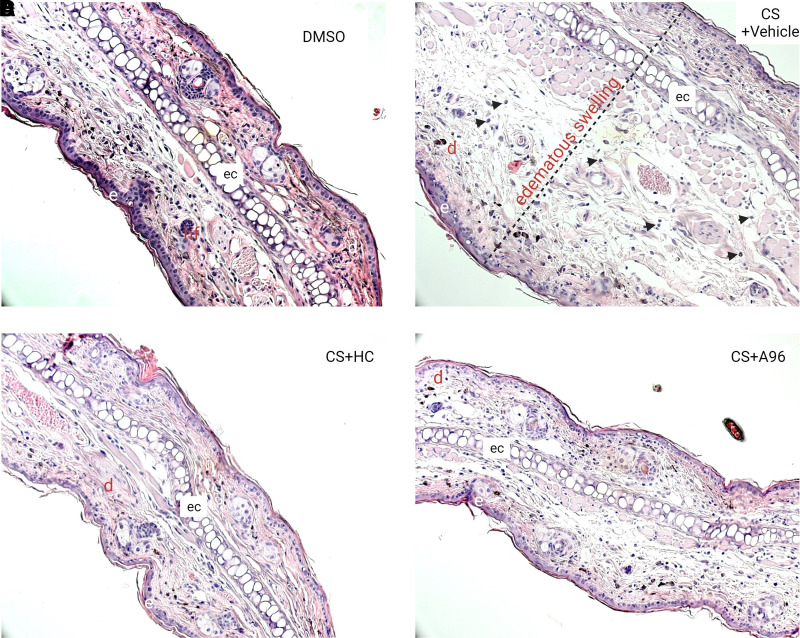
Effects of TRPA1 antagonists on CS tear gas agent–induced histopathology features. Right ears of C57BL/6 male mice were exposed to CS (200 mM, 20 μl) and left ears to DMSO (solvent for CS, 20 μl). At 0.5 and 4 hours after CS exposure, mice were treated with vehicle (0.5% methylcellulose), HC-030031 (HC), or A967079 (A96) intraperitoneally (i.p.). At 6.5 hours after CS exposure, mice were euthanized and ear punch biopsies were collected for histopathological analysis. Representative H&E-stained histopathologic sections from (A) DMSO (solvent for CS), (B) CS + vehicle, (C) CS + HC030031, and (D) CS + A967079 groups are presented at 20× magnification. Black arrow heads = infiltration of leukocytes. d, dermis; e, epidermis; ec, elastic cartilage; hf, hair follicle.
CS Tear Gas–Induced Acute Inflammation Was Reduced after Treatment with TRPA1 Antagonists.
HC-030031, a first-generation TRPA1 inhibitor, and A967079, a second-generation TRPA1 inhibitor, were tested for mitigating CS tear gas–induced skin injuries. After treatment with these TRPA1 antagonists, edema measured by ear thickness (HC, 119% reduction and A96, 53% reduction compared with the vehicle-treated group) and ear punch biopsy weights (HC, 72% reduction and A96, 41% reduction compared with the vehicle-treated group) was significantly reduced (P < 0.05) (Fig. 1, B and C). Treatment with HC or A96 mitigated a profound inflammatory response measured by proinflammatory cytokine marker levels (MMP9, KC/CXCL1, and IL-1β). MMP9 is implicated in skin blisters and vesicant injuries and has been shown as a promoter of separation of the epidermis and dermis after skin injuries. HC and A96 treatment reduced MMP9 levels by 146% and 64%, respectively, compared with vehicle treatment. A96 treatment significantly reduced CXCL1/KC protein levels (47% reduction, P < 0.05), whereas HC treatment did not reduce. IL-1β levels were significantly reduced with HC treatment (213% reduction, P < 0.05) whereas A96 treatment showed a trend in reduction (Fig. 1, D–F). Similar to other studied parameters, the tissue extravasation (intensity of IRDye 800 CW dye fluorescence) was significantly reduced with both HC and A96 treatment (P < 0.05) (Fig. 2). The prominent edematous swelling that was seen in gross punch biopsy samples was evident in the histopathology profiles. Treatment with TRPA1 antagonists reduced edema. Edema reduction with TRPA1 antagonist treatment is also further supported by the reduction in ear thickness and ear punch biopsy weights (Fig. 3).
Chronic Inflammation after CS Tear Gas Agent Exposure Was Reduced after Treatment with TRPA1 Antagonists.
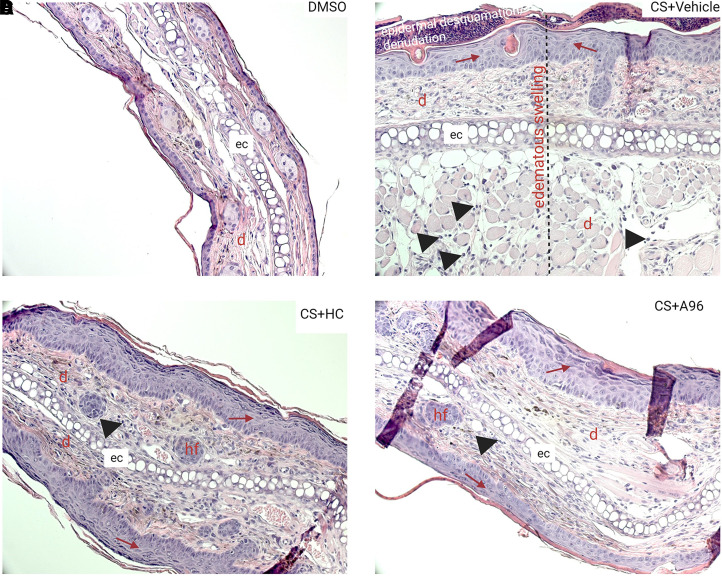
In a 5-day chronic observation study after CS tear gas agent exposure, a profound and chronic inflammation was noted. Ear thickness and ear punch biopsy weights that were used as surrogate markers of edema have significantly increased by 182% and 136%, respectively, with CS exposure. Treatment with either HC or A96 reduced ear thickness (HC, 38% reduction and A96, 42% reduction) and ear punch biopsy weights (HC, 30% reduction and A96, 31% reduction) (P < 0.05). Although treatment with HC and A96 decreased CXCL2/MIP2 (P < 0.05), they showed trends in reduction of KC/CXCL1 and IL-1β (data not shown) (Fig. 4). Representative H&E-stained histopathological profiles were presented (Fig. 5). At the same magnification (20×) of all histopathological profiles, the epidermises on both surfaces of the ears were not visible in the CS + vehicle group, whereas treatment with TRPA1 antagonists decreased edematous swelling. CS exposure also caused epidermal thickening, keratinolysis, and epidermal desquamation/denudation. Treatment with TRPA1 antagonists partially decreased or did not decrease epidermal thickening. TRPA1 antagonist treatment decreased infiltration of cells and epidermal desquamation.
Fig. 5.
Histologic images of skin injuries after CS tear gas agent exposure and treatment with TRPA1 antagonists. Right ears of C57BL/6 male mice were exposed to CS (200 mM, 20 μl) and left ears to DMSO (solvent for CS, 20 μl). At 0.5, 4, 24, and 48 hours after CS exposure, mice were treated with vehicle (0.5% methylcellulose), HC-030031 (HC), or A967079 (A96) intraperitoneally (i.p.). Representative H&E-stained histopathologic sections from (A) DMSO, (B) CS + vehicle, (C) CS + HC-030031, and (D) CS + A967079 groups are presented at 20× magnification. Black arrow heads = infiltration of leukocytes; red arrow = epidermal thickening. d, dermis; e, epidermis; ec, elastic cartilage; hf = hair follicle.
Water Washing Is Not an Effective Decontamination Strategy.
Decontamination of CS-exposed mouse ear skin with water washing did not improve the studied parameters such as ear thickness, ear punch biopsy weights, and IL-1β proinflammatory cytokine levels measured in ear punch biopsy homogenate (P > 0.05) (Supplemental Fig. 3).
Activation of TRPA1 Ion Channels after Exposure to CS Tear Gas Agent Was Inhibited in Cells Treated with Advanced TRPA1 Antagonists.
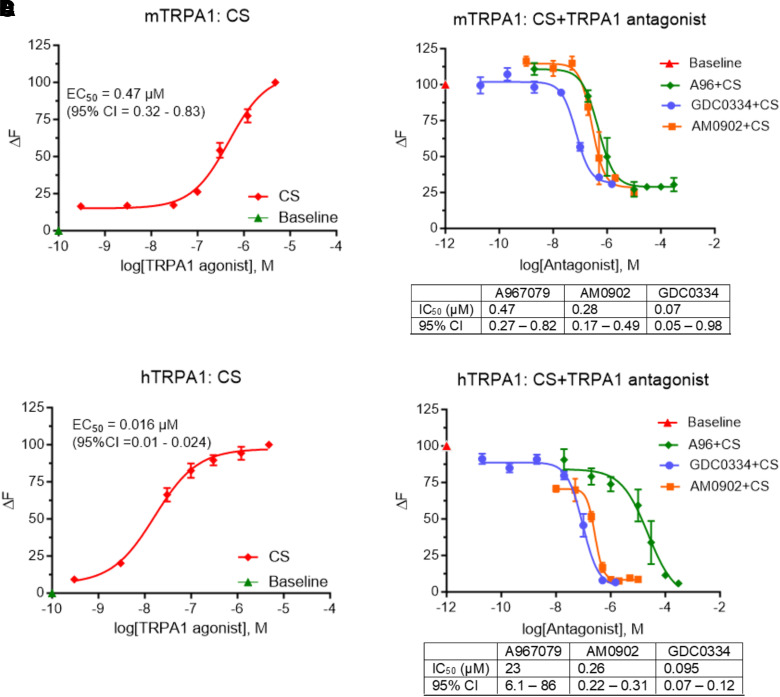
In vitro calcium functional assays showed that the human TRPA1 ion channel is highly sensitive to CS tear gas agent (EC50 = 0.016 μM; 95% confidence intervals (CI) = 0.01 – 0.024 μM]) compared with the mouse TRPA1 ion channel (EC50 = 0.47 μM, 95% CI = 0.32 – 0.83 μM). Advanced TRPA1 antagonists such as A967079 (mouse IC50 = 0.47 μM; human IC50 = 23 μM), AMG0902 (mouse IC50 = 0.28 μM; human IC50 = 0.26 μM), and GDC0334 (mouse IC50 = 0.07 μM; human IC50 = 0.095 μM) inhibited CS tear gas agent–induced intracellular calcium influx in a dose-dependent manner (Fig. 6).
Fig. 6.
Effects of advanced TRPA1 antagonists on CS tear gas agent–induced calcium influx. HEK293T cells were transfected with mouse (A and B) and human (C and D) TRPA1 plasmid. (A and C) Show dose-response curve of CS tear gas [mouse: EC50 = 0.47 μM, 95% confidence intervals (CI) = 0.32–0.83 μM; human: EC50 = 0.016 μM, 95% CI = 0.01–0.024 μM]. (B and D) TRPA1 antagonists [A967079 (A96), AMG0902, or GDC0334] inhibited the calcium influx response elicited by CS tear gas in mouse and human TRPA1-transfected cells. IC50 and 95% CI values are presented in tables under Fig. 6, B and D. Data are presented as mean ± S.E.M. Each experiment was performed with a minimum of three replicates.
Discussion
Mouse-ear inflammation models are routinely used for skin toxicity studies (Achanta et al., 2018). The ears of C57BL/6 mice have sparse hair and are therefore suitable for modeling exposures to human skin. In real riot-control situations, the actual concentration of the exposure to CS tear gas agent is unknown and is highly variable. Based on our in vitro studies, we found that CS tear gas activates human TRPA1 ion channel at lower concentrations compared with mouse [mouse: EC50 = 0.47 μM, 95% confidence intervals (CI) = 0.32–0.83 μM; human: EC50 = 0.016 μM, 95% CI = 0.01–0.024 μM). The EC50 value for activating TRPA1 in mice was 0.47 μM of CS tear gas agent, whereas in our in vivo mouse model, we exposed to 200 mM of CS tear gas. Our exposure resulted in profound edema, inflammation, and tissue extravasation. Our CS exposure concentrations may have caused saturated skin injuries. Thus, our model represents a severe skin injury phenotype in humans.
In this study, we tested the therapeutic potential of 2 TRPA1 antagonists to mitigate CS-induced skin inflammation. Consistent with in vitro studies published by our laboratory and other investigators, TRPA1 ion channels were shown here to be a key mediator of CS tear gas agent–induced inflammation in skin based on in vitro calcium functional assays and in vivo mouse studies (Brône et al., 2008; Bessac et al., 2009). TRPA1 antagonists HC-030031 and A-967079 decreased tissue edema (ear thickness and punch biopsy weights), inflammatory cytokine marker levels, and intensity of IRDye 800CW PEG dye fluorescence in the treated group compared with the vehicle group. Histopathology was improved, particularly edematous swelling, in the treated group compared with the vehicle group. Edema was the prominent feature evidenced by the increase in ear thickness and ear punch biopsy weight measurements and edematous swelling in histopathology. Treatment with TRPA1 inhibitors significantly reduced edema. Both HC and A96 have been widely studied in in vitro and in vivo studies for target engagement studies and various disease indications (Achanta et al., 2018; Achanta and Jordt, 2020; Chen and Terrett, 2020; Talavera et al., 2020; Maglie et al., 2021).
We developed in vivo imaging with infrared fluorescent dye to visualize the extravasation of inflammatory exudate and to monitor real-time healing. Although such imaging is common in tumor biology studies, this is the first study to use this in vivo imaging technique in inflammatory studies (Vasey et al., 1999). Vascular leakage is a common feature of acute inflammation. The nonspecific fluorescent dye used in this study, IRDye 800 CW PEG contrast agent (25–60 kDa), reaches the sites of acute inflammation due to vascular leakage and has a minimal return to the vasculature due to changes in colloid osmotic pressure. The method was optimized to track the progression of inflammation in real time using these less invasive procedures. In this study, we injected a 1:2 dilution of IRDye 800CW PEG agent (0.5 nmol per animal), which is half the manufacturer’s suggested amount (1 nmol per animal), to avoid signal saturation. Further, since IRDye 800 CW is excreted in the urine, individual caging of mice after injecting the dye is suggested to prevent background noise from urine excreted by cage mates. CS exposure caused profound vascular leakage and treatment with HC or A96attenuated vascular leakage, suggesting possible improvement of tissue integrity and barrier protection.
CS tear gas agent–induced irritation and inflammation effects have been perceived to be transient and acute with no or minimal toxic effects. However, we showed here that the CS-exposed animals did not recover either rapidly or spontaneously and that skin exposure to CS caused persistent inflammation and lesions. In the chronic (extended observation) studies, edematous swelling, epidermal thickening, keratinolysis, epidermal desquamation, and infiltration of cells were evident in the control group, whereas improvement was noted in the TRPA1 antagonist-treated group. In both acute and chronic studies, edematous swelling was very prominent. In chronic studies, at 20× magnification, epidermises on both sides were not visible in the same field of view in the CS + vehicle group due to prominent edematous swelling (Fig. 5). In the DMSO group, a single or two-layer epithelial cell layer was present in the epidermis. However, CS exposure resulted in obvious epidermal thickness with multiple layers of epithelial cells. Significant keratinolysis was evident in CS-exposed mice, but treatment with TRPA1 antagonists relatively decreased keratinolysis. These findings suggest that therapeutic intervention, or an effective decontamination strategy that is not currently available, is mandatory in cases of exposure to a CS tear gas agent. Here in our study, we exposed mouse ears to one-time application of CS. Recent reports suggest that repeated exposure to CS may cause contact dermatitis (Arbak et al., 2014; Lam et al., 2020).
The mice in the treatment group that received HC-030031 after CS tear gas exposure showed decreased activity levels compared with the control group, although this was not objectively quantified. Lower doses of HC and A96 may be effective in reducing CS tear gas –induced inflammation; however, our dosing regimen was adopted based on previously published studies (Caceres et al., 2009; Chen et al., 2011; Liu et al., 2016; Achanta et al., 2018; Gyamfi et al., 2020). Nevertheless, both compounds showed decent therapeutic effects in our severe CS skin injury models.
Not only is there a lack of potential medical countermeasures for CS tear gas skin injuries, but there are also no approved decontamination strategies for CS tear gas skin exposures. Water washing of the CS tear gas–exposed mouse ears did not resolve inflammation and may therefore not be an effective decontamination strategy. Water washing coupled with scrubbing with soap might offer additional benefits, but we did not investigate in the current study. A couple of decontamination strategies have been assessed but not prospectively studied in preclinical studies or humans. Reactive Skin Decontamination Lotion (RSDL; Emergent BioSolutions, Gaithersburg, MD) is a US Food and Drug Administration (FDA)-approved emerging product that has been indicated for decontaminating nerve gas exposure. Published in vitro studies show that when RSDL is incubated with CS, the potency of the CS tear gas agent is substantially reduced (Cao et al., 2018; Gebremedhin et al., 2020). However, this has not been confirmed in any preclinical or clinical studies. Diphoterine (PREVOR Laboratory, Valmondois, France) is a commercially available hypertonic, amphoteric, and chelating agent that has shown some benefits in limited human studies but has not yet been studied in preclinical or clinical studies prospectively (Viala et al., 2005; Luka et al., 2007). In our mouse skin exposure studies, decontamination with water did not resolve inflammation. Thus, more definitive studies on decontamination strategies are urgently needed to protect against the effects of CS tear gas exposure in addition to therapeutic interventions.
TRPA1 ion channels are implicated in several inflammatory models, and investigational TRPA1 antagonists are being studied widely for several indications (Bautista et al., 2006, 2013; Dai et al., 2007; Macpherson et al., 2007; Bessac and Jordt, 2008; Bessac et al., 2008; Brône et al., 2008; Escalera et al., 2008; Caceres et al., 2009; Liu et al., 2013; Radresa et al., 2013; Holzer and Izzo, 2014; Achanta et al., 2018). Expression of TRPA1 is believed to be primarily in the sensory nerve endings of the skin. However, recent studies showed that non-neuronal skin cells such as keratinocytes, melanocytes, mast cells, dendritic cells, and endothelial cells also express TRPA1 (Atoyan et al., 2009; Maglie et al., 2021). These ion channels play an important role in cutaneous physiologic functions (Bautista et al., 2013; Maglie et al., 2021). Therefore, pharmacological targeting of TRPA1 ion channels offers great promise to treat CS tear gas–induced skin injuries. Pharmacological inhibition of TRPA1 showed promising results in other chemical injuries such as sulfur mustard analog–induced skin injuries and many other chemical injuries (Bautista et al., 2006; Stenger et al., 2015, 2017; Tai et al., 2017; Achanta et al., 2018; Yadav et al., 2023).
Although HC-030031, A967079, and other TRPA1 antagonists have been widely tested in several preclinical models with positive outcomes, they also have some off-target effects such as thermoregulation, altered taste and smell, reduced pain perception, chemosensory deficits, etc. The newly discovered TRPA1 antagonists are highly selective and potent with minimal or no side effects. Therefore, there is a need to investigate the newly disclosed TRPA1 antagonists in our CS skin injury models (Supplemental Table 1). Using in vitro calcium functional assays, we determined the pharmacological activity of advanced TRPA1 antagonists such as AMG0902 and GDC0334 (Lehto et al., 2016; Schenkel et al., 2016; Balestrini et al., 2021). Both of these compounds effectively inhibited the influx of intracellular calcium ions resulting from CS tear gas–induced activation of TRPA1 ion channels. Consistent with published IC50 values for GDC0334, this antagonist is effective at nanomolar concentrations for inhibiting CS-evoked intracellular calcium influx (Balestrini et al., 2021). In a healthy volunteer Phase 1 study, treatment with GDC0334 reduced TRPA1 agonist-induced dermal blood flow (DBF), pain, and itch, demonstrating GDC0334 target engagement in humans. This is the first TRPA1 inhibitor that has exhibited an ideal safety profile and pharmacokinetic parameters (Balestrini et al., 2021). GDC0334 showed strong therapeutic effects in four different preclinical studies (Balestrini et al., 2021). GDC6599 (Compound 20) is a tetrahydrofuran-based TRPA1 antagonist that entered into a clinical trial study to evaluate the efficacy, safety, pharmacokinetics, and pharmacodynamic effects in patients with chronic cough (clinical trial number: NCT05660850). GDC6599 showed therapeutic effects against ovalbumin-induced asthma and mustard oil-induced pain model. GDC6599 also exhibited decent safety and pharmacokinetic profile in preclinical models (Terrett et al., 2021). Additional TRPA1 antagonists such as CB-189, CB-625, AP18, GRC-17536 (also known as ISC 17536), BI01305834, and BAY-390 are undergoing active preclinical studies by several pharmaceutical companies (Liu et al., 2013; Skerratt, 2017; Achanta and Jordt, 2020; Chen and Terrett, 2020; van den Berg et al., 2020; Jain et al., 2022; Mesch et al., 2023) (Supplemental Table 1). Therefore, future studies are warranted to test the therapeutic effects of these latest TRPA1 antagonists in in vivo mouse and nonrodent animal models of CS tear gas exposure.
With the increased and uncontrolled deployment of CS tear gas agents for riot control, casualties are also increasing. In this study, we offer to advance the development of treatments for injuries from exposure to CS tear gas agent based on mechanistic studies.
Conclusions
We demonstrated that the TRPA1 ion channel as a key mediator of tear gas–induced inflammation and TRPA1 antagonists such as HC-030031 and A-967079 decreased tissue edema, inflammatory cytokine markers, and vascular extravasation in CS tear gas–induced skin injuries while improving tissue histopathology. These findings will pave the way for studying advanced TRPA1 inhibitors in rodent and nonrodent species for treating human CS tear gas–induced skin injuries under FDA’s animal rule as potential therapeutic agents.
Acknowledgments
The authors would like to thank Sairam V Jabba, Research Scientist at Duke University School of Medicine, for his thoughtful discussions on in vitro calcium functional assay using FlexStation, as well as Hydra Biosciences LLC (Cambridge, MA) and Genentech (South San Francisco, CA) for the generous gifts of HC-030031 and GDC-0334, respectively.
Data Availability
The authors declare that all of the data that support the findings of this study are available within the paper and its Supplemental Material.
Abbreviations
- A96
A967079
- CI
confidence interval
- CS
2-chlorobenzalmalononitrile
- CXCL2/MIP-2
chemokine (C-X-C motif) ligand 2/macrophage inflammatory protein 2
- FDA
US Food and Drug Administration
- HC
HC-030031
- HEK293T
human embryonic kidney 293T
- IL-1β
interleukin-1 beta
- KC/CXCL1
keratinocyte chemoattractant/chemokine (C-X-C motif) ligand 1
- MMP9
matrix metalloproteinase 9
- TRPA1
transient receptor potential ion channel ankyrin 1
Authorship Contributions
Participated in research design: Achanta, Chintagari, Jordt.
Conducted experiments: Achanta, Chintagari, Balakrishna, Liu.
Contributed new reagents or analytic tools: Achanta, Chintagari, Balakrishna, Liu, Jordt.
Performed data analysis: Achanta, Chintagari, Balakrishna.
Wrote or contributed to the writing of the manuscript: Achanta, Balakrishna.
Footnotes
This work was supported by National Institutes of Health (NIH) Countermeasures Against Chemical Threats (CounterACT) Program and National Institute of Environmental Health Sciences (NIEHS) [Grants U01ES030672, 1R21-ES033020, and R01ES034387] (to S.A., S.-E.J.). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the US Food and Drug Administration (FDA).
The authors have declared a conflict of interest. S.-E.J. served on the scientific advisory board of Hydra Biosciences LLC (Cambridge, MA), a biopharmaceutical firm developing TRP ion channel inhibitors for the treatment of pain. Other authors do not have any conflicts of interest.
Current affiliation: US Food and Drug Administration (FDA), Bethesda, Maryland (N.R.C. contributed to this work when he was at Yale University School of Medicine, New Haven, Connecticut).
Current affiliation: Office of Animal Research Support, Yale University, New Haven, Connecticut.
Current affiliation: Department of Neurobiology and Acupuncture Research, The Third Clinical Medical College, Zhejiang Chinese Medical University, Key Laboratory of Acupuncture and Neurology of Zhejiang Province, Hangzhou, China.
Some elements of this work have been submitted as an abstract: Achanta S, Balakrishna S, Chintagari N, Bessac BF, Sui A, and Jordt SA (2014) TRPA1 Inhibitors Counteract Inflammation and Edema in a Mouse Model of CS Tear Gas Agent-Induced Cutaneous Injury (Abstract). 53rd Society of Toxicology Annual Meeting; 2014 Mar 23–27; Phoenix, AZ. Won the “Stratacor Postdoctoral Award,” presented by the Dermal Toxicology specialty section, Society of Toxicology.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Achanta S, Chintagari NR, Brackmann M, Balakrishna S, Jordt SE (2018) TRPA1 and CGRP antagonists counteract vesicant-induced skin injury and inflammation. Toxicol Lett 293:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achanta S, Jordt SE (2017) TRPA1: Acrolein meets its target. Toxicol Appl Pharmacol 324:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achanta S, Jordt SE (2020) Transient receptor potential channels in pulmonary chemical injuries and as countermeasure targets. Ann N Y Acad Sci 1480:73–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal Y, Thornton D, Phipps A (2009) CS gas–completely safe? A burn case report and literature review. Burns 35:895–897. [DOI] [PubMed] [Google Scholar]
- Aktan AO (2013) Tear gas is a chemical weapon, and Turkey should not use it to torture civilians. BMJ 346:f3801. [DOI] [PubMed] [Google Scholar]
- Arbak P, Başer I, Kumbasar OO, Ülger F, Kılıçaslan Z, Evyapan F (2014) Long term effects of tear gases on respiratory system: analysis of 93 cases. ScientificWorldJournal 2014:963638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoyan R, Shander D, Botchkareva NV (2009) Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol 129:2312–2315. [DOI] [PubMed] [Google Scholar]
- Balestrini AJoseph VDourado MReese RMShields SDRougé LBravo DDChernov-Rogan TAustin CDChen H, et al. (2021) A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J Exp Med 218:e20201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Pellegrino M, Tsunozaki M (2013) TRPA1: a gatekeeper for inflammation. Annu Rev Physiol 75:181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE (2008) Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE (2010) Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc 7:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE (2009) Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J 23:1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE (2008) TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava K, Banerjee P, White IR (2012) Investigating contact allergy to CS spray. Contact Dermat 66:109–110. [DOI] [PubMed] [Google Scholar]
- Blain PG (2003) Tear gases and irritant incapacitants. 1-chloroacetophenone, 2-chlorobenzylidene malononitrile and dibenz[b,f]-1,4-oxazepine. Toxicol Rev 22:103–110. [DOI] [PubMed] [Google Scholar]
- Brône B, Peeters PJ, Marrannes R, Mercken M, Nuydens R, Meert T, Gijsen HJ (2008) Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol Appl Pharmacol 231:150–156. [DOI] [PubMed] [Google Scholar]
- Caceres AIBrackmann MElia MDBessac BFdel Camino DD’Amours MWitek JSFanger CMChong JAHayward NJ, et al. (2009) A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA 106:9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres AI, Liu B, Jabba SV, Achanta S, Morris JB, Jordt SE (2017) Transient receptor potential cation channel subfamily M member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br J Pharmacol 174:867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Hui X, Zhu H, Elmahdy A, Maibach H (2018) In vitro human skin permeation and decontamination of 2-chloroethyl ethyl sulfide (CEES) using Dermal Decontamination Gel (DDGel) and Reactive Skin Decontamination Lotion (RSDL). Toxicol Lett 291:86–91. [DOI] [PubMed] [Google Scholar]
- Carron PN, Yersin B (2009) Management of the effects of exposure to tear gas. BMJ 338:b2283. [DOI] [PubMed] [Google Scholar]
- Chen H, Terrett JA (2020) Transient receptor potential ankyrin 1 (TRPA1) antagonists: a patent review (2015-2019). Expert Opin Ther Pat 30:643–657. [DOI] [PubMed] [Google Scholar]
- Chen JJoshi SKDiDomenico SPerner RJMikusa JPGauvin DMSegreti JAHan PZhang XFNiforatos W, et al. (2011) Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 152:1165–1172. [DOI] [PubMed] [Google Scholar]
- CS gas: infringing the gas laws. (1970) Nature 226:890. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K (2007) Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 117:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalera J, von Hehn CA, Bessac BF, Sivula M, Jordt SE (2008) TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J Biol Chem 283:24136–24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin M, Fentabil M, Cochrane L, Lau V, Toth D, Barry J (2020) In vitro decontamination efficacy of the RSDL® (Reactive Skin Decontamination Lotion Kit) lotion component against riot control agents: capsaicin, Mace™ (CN) and CS. Toxicol Lett 332:36–41. [DOI] [PubMed] [Google Scholar]
- Gyamfi OA, Bortey-Sam N, Donkor AB, White CW, Logue BA (2020) Analysis of TRPA1 antagonist, A-967079, in plasma using high-performance liquid chromatography tandem mass-spectrometry. J Pharm Anal 10:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Izzo AA (2014) The pharmacology of TRP channels. Br J Pharmacol 171:2469–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hout JJ, Kluchinsky T, LaPuma PT, White DW (2011) Evaluation of CS (o-chlorobenzylidene malononitrile) concentrations during U.S. Army mask confidence training. J Environ Health 74:18–21. [PubMed] [Google Scholar]
- Jain SM, Balamurugan R, Tandon M, Mozaffarian N, Gudi G, Salhi Y, Holland R, Freeman R, Baron R (2022) Randomized, double-blind, placebo-controlled trial of ISC 17536, an oral inhibitor of transient receptor potential ankyrin 1, in patients with painful diabetic peripheral neuropathy: impact of preserved small nerve fiber function. Pain 163:e738–e747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GR (1996) CS sprays: antidote and decontaminant. Lancet 347:968–969. [DOI] [PubMed] [Google Scholar]
- Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384. [DOI] [PubMed] [Google Scholar]
- Kain N, Mishra A, James MI (2010) Guidance needed on secondary effects of CS gas on staff. BMJ 340:c1189. [DOI] [PubMed] [Google Scholar]
- Karagama YG, Newton JR, Newbegin CJ (2003) Short-term and long-term physical effects of exposure to CS spray. J R Soc Med 96:172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman E, Erturan S, Duman C, Yaman M, Duman GU (2009) Acute laryngeal and bronchial obstruction after CS (o-chlorobenzylidenemalononitrile) gas inhalation. Eur Arch Otorhinolaryngol 266:301–304. [DOI] [PubMed] [Google Scholar]
- Lam RPK, Wong KW, Wan CK (2020) Allergic contact dermatitis and tracheobronchitis associated with repeated exposure to tear gas. Lancet 396:e12. [DOI] [PubMed] [Google Scholar]
- Lehto SGWeyer ADYoungblood BDZhang MYin RWang WTeffera YCooke MStucky CLSchenkel L, et al. (2016) Selective antagonism of TRPA1 produces limited efficacy in models of inflammatory- and neuropathic-induced mechanical hypersensitivity in rats. Mol Pain 12:1744806916677761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BEscalera JBalakrishna SFan LCaceres AIRobinson ESui AMcKay MCMcAlexander MAHerrick CA, et al. (2013) TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J 27:3549–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Tai Y, Caceres AI, Achanta S, Balakrishna S, Shao X, Fang J, Jordt SE (2016) Oxidized phospholipid oxPAPC activates TRPA1 and contributes to chronic inflammatory pain in mice. pLoS One 11:e0165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka A, Stolbach A, Hoffman RS (2007) Response to “Prevention of CS ‘tear gas’ eye and skin effects and active decontamination with Diphoterine: preliminary studies in 5 French gendarmes”. J Emerg Med 32:309–310, author reply 310–311. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445:541–545. [DOI] [PubMed] [Google Scholar]
- Maglie R, Souza Monteiro de Araujo D, Antiga E, Geppetti P, Nassini R, De Logu F (2021) The role of TRPA1 in skin physiology and pathology. Int J Mol Sci 22:3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesch SWalter DLaux-Biehlmann ABasting DFlanagan SMiyatake Ondozabal HBäurle SPearson CJenkins JElves P, et al. (2023) Discovery of BAY-390, a selective CNS penetrant chemical probe as transient receptor potential ankyrin 1 (TRPA1) antagonist. J Med Chem 66:1583–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajos EJ, Salem H (2001) Riot control agents: pharmacology, toxicology, biochemistry and chemistry. J Appl Toxicol 21:355–391. [DOI] [PubMed] [Google Scholar]
- Radresa O, Dahllöf H, Nyman E, Nolting A, Albert JS, Raboisson P (2013) Roles of TRPA1 in pain pathophysiology and implications for the development of a new class of analgesic drugs. Open Pain J 6:137–153 DOI: 10.2174/1876386301306010137. [DOI] [Google Scholar]
- Rappert B (2003) Health and safety in policing: lessons from the regulation of CS sprays in the UK. Soc Sci Med 56:1269–1278. [DOI] [PubMed] [Google Scholar]
- Rothenberg C, Achanta S, Svendsen ER, Jordt SE (2016) Tear gas: an epidemiological and mechanistic reassessment. Ann N Y Acad Sci 1378:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel LBOlivieri PRBoezio AADeak HLEmkey RGraceffa RFGunaydin HGuzman-Perez ALee JHTeffera Y, et al. (2016) Optimization of a novel quinazolinone-based series of transient receptor potential A1 (TRPA1) antagonists demonstrating potent in vivo activity. J Med Chem 59:2794–2809. [DOI] [PubMed] [Google Scholar]
- Schep LJ, Slaughter RJ, McBride DI (2015) Riot control agents: the tear gases CN, CS and OC–a medical review. J R Army Med Corps 161:94–99. [DOI] [PubMed] [Google Scholar]
- Shambhu S, Kurtis R (2011) Allergic contact dermatitis due to CS spray. Emerg Med J 28:345. [DOI] [PubMed] [Google Scholar]
- Silny W, Czarnecka-Operacz M, Silny P (2005) The new scoring system for evaluation of skin inflammation extent and severity in patients with atopic dermatitis. Acta Dermatovenerol Croat 13:219–224. [PubMed] [Google Scholar]
- Skerratt S (2017) Recent progress in the discovery and development of TRPA1 modulators. Prog Med Chem 56:81–115. [DOI] [PubMed] [Google Scholar]
- Smith J, Greaves I (2002) The use of chemical incapacitant sprays: a review. J Trauma 52:595–600. [DOI] [PubMed] [Google Scholar]
- Southward RD (2001) Cutaneous burns from CS incapacitant spray. Med Sci Law 41:74–77. [DOI] [PubMed] [Google Scholar]
- Stenger B, Popp T, John H, Siegert M, Tsoutsoulopoulos A, Schmidt A, Mückter H, Gudermann T, Thiermann H, Steinritz D (2017) N-acetyl-L-cysteine inhibits sulfur mustard-induced and TRPA1-dependent calcium influx. Arch Toxicol 91:2179–2189. [DOI] [PubMed] [Google Scholar]
- Stenger B, Zehfuss F, Mückter H, Schmidt A, Balszuweit F, Schäfer E, Büch T, Gudermann T, Thiermann H, Steinritz D (2015) Activation of the chemosensing transient receptor potential channel A1 (TRPA1) by alkylating agents. Arch Toxicol 89:1631–1643. [DOI] [PubMed] [Google Scholar]
- Tai Y, Wang C, Wang Z, Liang Y, Du J, He D, Fan X, Jordt SE, Liu B (2017) Involvement of transient receptor potential cation channel member A1 activation in the irritation and pain response elicited by skin-lightening reagent hydroquinone. Sci Rep 7:7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, Naert R, Nilius B (2020) Mammalian transient receptor potential TRPA1 channels: from structure to disease. Physiol Rev 100:725–803. [DOI] [PubMed] [Google Scholar]
- Terrett JAChen HShore DGVillemure ELarouche-Gauthier RDéry MBeaumier FConstantineau-Forget LGrand-Maître CLépissier L, et al. (2021) Tetrahydrofuran-based transient receptor potential ankyrin 1 (TRPA1) antagonists: ligand-based discovery, activity in a rodent asthma model, and mechanism-of-action via cryogenic electron microscopy. J Med Chem 64:3843–3869. [DOI] [PubMed] [Google Scholar]
- Torgrimson-Ojerio BN, Mularski KS, Peyton MR, Keast EM, Hassan A, Ivlev I (2021) Health issues and healthcare utilization among adults who reported exposure to tear gas during 2020 Portland (OR) protests: a cross-sectional survey. BMC Public Health 21:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M, Nijboer-Brinksma S, Bos S, van den Berge M, Lamb D, van Faassen M, Kema I, Gosens R, Kistemaker L (2020) Withdrawn: the novel TRPA1 antagonist BI01305834 inhibits ovalbumin-induced bronchoconstriction in guinea pigs. Br J Pharmacol 177:4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S, Holt PJ (2001) Severe cutaneous reaction to CS gas. Clin Exp Dermatol 26:248–250. [DOI] [PubMed] [Google Scholar]
- Vasey PAKaye SBMorrison RTwelves CWilson PDuncan RThomson AHMurray LSHilditch TEMurray T, et al. (1999) Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin Cancer Res 5:83–94. [PubMed] [Google Scholar]
- Viala B, Blomet J, Mathieu L, Hall AH (2005) Prevention of CS “tear gas” eye and skin effects and active decontamination with Diphoterine: preliminary studies in 5 French gendarmes. J Emerg Med 29:5–8. [DOI] [PubMed] [Google Scholar]
- Watson K, Rycroft R (2005) Unintended cutaneous reactions to CS spray. Contact Dermat 53:9–13. [DOI] [PubMed] [Google Scholar]
- Yadav M, Chaudhary PP, D’Souza BN, Ratley G, Spathies J, Ganesan S, Zeldin J, Myles IA (2023) Diisocyanates influence models of atopic dermatitis through direct activation of TRPA1. pLoS One 18:e0282569. [DOI] [PMC free article] [PubMed] [Google Scholar]