Extract
Inhaled corticosteroids (ICS) prevent COPD exacerbations [1]. Randomised controlled trials (RCTs) have shown that higher blood eosinophil counts (BECs) are associated with better clinical responses to ICS treatment in COPD patients with a history of exacerbations [1]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) report recommends using BEC to identify individuals most likely to benefit from ICS treatment [1]. Higher BECs are associated with increased type 2 pulmonary inflammation [2–4], potentially explaining the differential response to ICS.
Tweetable abstract
Current smoking reduces small airway intraepithelial eosinophil counts in COPD patients and controls. This provides evidence of an attenuation of type-2 related inflammation in the small airways imposed by current smoking, which may affect ICS response. https://bit.ly/49YSKwG
To the Editor:
Inhaled corticosteroids (ICS) prevent COPD exacerbations [1]. Randomised controlled trials (RCTs) have shown that higher blood eosinophil counts (BECs) are associated with better clinical responses to ICS treatment in COPD patients with a history of exacerbations [1]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) report recommends using BEC to identify individuals most likely to benefit from ICS treatment [1]. Higher BECs are associated with increased type 2 pulmonary inflammation [2–4], potentially explaining the differential response to ICS.
RCT analyses have demonstrated that ICS have a greater effect on exacerbation prevention in ex- versus current smokers [5, 6]. A post hoc analysis of the SUMMIT study reported a blunted response to ICS-containing therapies with regards to improvement in forced expiratory volume in 1 s (FEV1) and exacerbation reduction in current versus ex-smokers [5], with similar observations in pooled and meta-analysis of COPD RCTs [6, 7].
The small airways (<2 mm diameter) show enhanced immune cell infiltration in COPD versus controls [8], associated with airway remodelling causing airflow obstruction and gas trapping [9]. Few COPD studies investigating small airway inflammation have quantified eosinophil counts. Hogg et al. [8] reported that the proportion of small airways with positive eosinophil staining were similar in COPD versus controls, without quantifying the number of cells per airway. This is relevant for small airway eosinophils in COPD, which exist in discrete microenvironments characterised by type 2 inflammation [10]. Quantitative analysis reported increased small airway eosinophil counts in very severe COPD [10]. Current smoking may be an uncontrolled confounder in these studies [8, 10, 11]. We hypothesised that current smoking in COPD alters the pattern of type 2 inflammation in the small airways, thereby influencing responses to ICS. To address this hypothesis, we investigated small airway eosinophil counts in COPD patients and controls, focusing on the effects of current smoking.
Tissue blocks were obtained from 27 controls and 48 COPD patients undergoing surgical resection for suspected lung cancer, recruited from Manchester University NHS Foundation Trust Hospital, UK. Patients provided written informed consent using protocols approved by Manchester (03/SM/396) and Northwest (20/NW/0302) ethics committees. COPD patients (mean age 68.0 years; mean smoking history 53.6 pack-years) met GOLD diagnostic criteria: 54.2% were current smokers with ≥10-pack-year history, and had an FEV1/forced vital capacity ratio <0.7, with mean FEV1 64.8% predicted, comprising 10, 23 and 15 GOLD stage 1, 2 and 3 patients, respectively. Controls (mean age 66.8 years; mean smoking history 40.6 pack-years) were current (n=14, 51.9%) or ex-smokers (n=13, 48.1%) with ≥10 pack-years and no airflow obstruction. Smoking status was self-reported and ex-smoking constituted ≥1 year(s) of cessation. No subjects had a history of asthma. Age, pack-year history and sex were not different between groups.
Eosinophils in the small airway (< 2 mm diameter) intra- and subepithelial regions were identified by Luna staining. Intra- and subepithelial cell counts were normalised to the area of epithelium or lamina propria, respectively, and quality control checked with an interuser disagreement <10%. Differences between groups were assessed using unpaired t-tests or Mann–Whitney U-tests with significance set at p<0.05. Multiple linear regression analyses assessed associations between intraepithelial eosinophil counts (dependent variable) and smoking status (independent variable), whilst controlling for potentially confounding variables: FEV1 % predicted and ICS use in COPD.
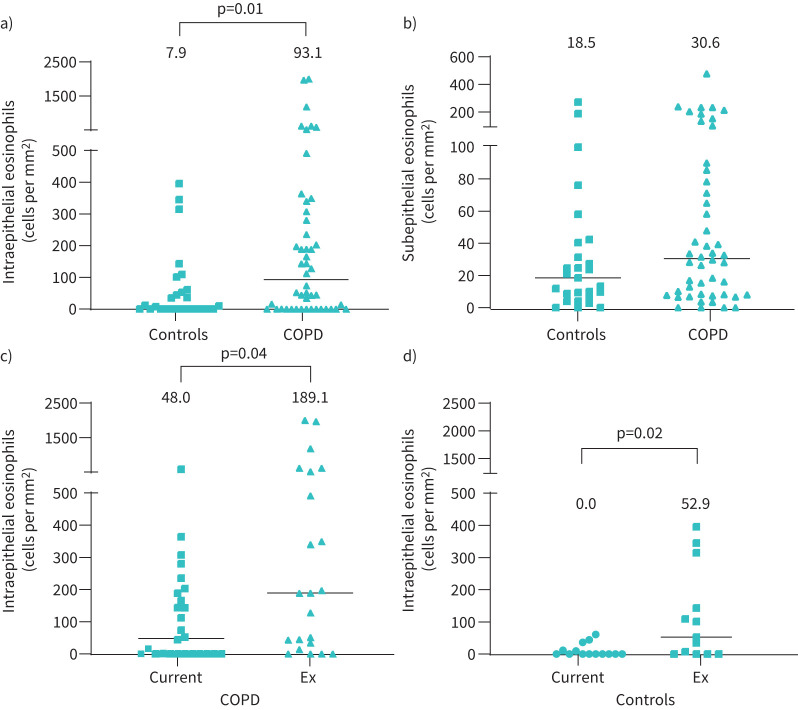
COPD patients had higher intraepithelial eosinophil counts versus controls (median 93.1 versus 7.9 cells per mm2 respectively, p=0.01) (figure 1a). Subepithelial eosinophil counts were not significantly different between groups (30.6 versus 18.5 cells per mm2 respectively, p=0.15) (figure 1b), although numerically higher in COPD patients. There were significant associations between intra- and subepithelial eosinophil counts in COPD patients (ρ=0.52, p<0.001) and controls (ρ=0.62, p<0.001).
FIGURE 1.
Comparison of small airway eosinophil counts between different groups defined by presence of COPD and smoking status. a) Intraepithelial eosinophil counts for COPD versus control, b) subepithelial eosinophil counts for COPD versus control (27 control subjects and 48 COPD patients), intraepithelial eosinophil counts for c) COPD current versus ex-smokers (n=26 and n=22, respectively), and for d) current versus ex-smoking controls (n=14 and n=13, respectively). Data represent individual patients or controls, black horizontal lines represent median eosinophil counts and individual datasets are labelled with median eosinophil count per mm2.
Intraepithelial eosinophil counts were lower in current versus ex-smokers, both in COPD patients (median 48.0 versus 189.1 cells per mm2 respectively, p=0.04) (figure 1c) and controls (median 0.0 versus 52.9 cells per mm2 respectively, p=0.02) (figure 1d). Current smoking did not influence subepithelial eosinophil counts in COPD patients (median 32.8 versus 28.9 cells per mm2 for current and ex-smokers respectively, p=0.96) or controls (median 12.6 versus 24.7 cells per mm2 for current and ex-smokers respectively, p=0.40). Clinical characteristics were no different between ex- versus current-smoking COPD patients or controls.
Univariate analysis revealed no association between eosinophil counts and FEV1 (absolute or % predicted, or GOLD stage) or ICS use in COPD. Furthermore, the relationship between intraepithelial eosinophil counts and smoking status remained significant when adjusted for potential confounding factors (COPD, p=0.01; controls, p=0.02). The median within-subject difference was not statistically different between different tissue blocks, regardless of proximity to the excised tumour.
We report higher small airway intraepithelial eosinophil counts in COPD patients versus controls. The key novel finding was that intraepithelial eosinophil counts were lower in current smokers, observed in both COPD patients and controls.
The strengths of this study include the stratification by current smoking status, combined with the careful quantification of eosinophil numbers in both the intra- and subepithelium. Eosinophilic airway inflammation exists in a subgroup of COPD patients [1, 2]; our results suggest this is more prominent in the intraepithelial region of the small airways. We have previously demonstrated a type 2 gene expression signature, using bronchial brushings, that is associated with higher blood and bronchial tissue eosinophil counts [2]. The airway epithelium therefore appears to be an important location for the presence of type 2 inflammation in COPD.
Current smoking may reduce eosinophil numbers per se or alter eosinophil retention in different airway compartments. Bronchoalveolar lavage eosinophils are increased in COPD current versus ex-smokers, suggesting increased transit into the airway lumen, possibly due to increased activation [12, 13]. Once these signals are removed (smoking cessation), eosinophils may resume tissue homeostatic functions with longer residency in the epithelium. Limitations of this analysis include: smoking status was self-reported and we were unable to analyse confounding clinical factors due to limited information available. Matched BECs were not available for these samples. However, the relationship between BECs and small airway eosinophil counts has been recently reported, showing a positive correlation [14]. Reassuringly, eosinophil counts were repeatable when assessed from multiple tissue blocks, regardless of proximity to the excised tumour.
The present analysis shows that current smoking attenuates eosinophil counts in the small airway epithelium, which may contribute to the blunted clinical response to ICS in current smokers [5, 6].
Footnotes
Provenance: Submitted article, peer reviewed.
Data availability: Our dataset has been deposited with Dryad (https://doi.org/10.5061/dryad.f7m0cfz3b).
Ethics statement: Patients provided written informed consent using protocols approved by Manchester (03/SM/396) and Northwest (20/NW/0302) ethics committees.
Conflict of interest: A. Higham has received personal fees from Chiesi.
Conflict of interest: D. Singh has received personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epidendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Synairgen, Teva, Theravance and Verona.
Conflict of interest: A. Beech and S. Booth have no conflicts of interest to declare.
Support statement: D. Singh is supported by the by the National Institute for Health Research Manchester Biomedical Research Centre.
References
- 1.Singh D, Agusti A, Martinez FJ, et al. Blood eosinophils and chronic obstructive pulmonary disease: a Global Initiative for Chronic Obstructive Lung Disease science committee 2022 review. Am J Respir Crit Care Med 2022; 206: 17–24. doi: 10.1164/rccm.202201-0209PP [DOI] [PubMed] [Google Scholar]
- 2.Higham A, Beech A, Wolosianka S, et al. Type 2 inflammation in eosinophilic chronic obstructive pulmonary disease. Allergy 2021; 76: 1861–1864. doi: 10.1111/all.14661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faiz A, Pavlidis S, Kuo CH, et al. Th2 high and mast cell gene signatures are associated with corticosteroid sensitivity in COPD. Thorax 2022; 78: 335–343. doi: 10.1136/thorax-2021-217736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higham A, Dungwa J, Pham TH, et al. Increased mast cell activation in eosinophilic chronic obstructive pulmonary disease. Clin Transl Immunol 2022; 11: e1417. doi: 10.1002/cti2.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt SP, Anderson JA, Brook RD, et al. Cigarette smoking and response to inhaled corticosteroids in COPD. Eur Respir J 2018; 51: 1701393. doi: 10.1183/13993003.01393-2017 [DOI] [PubMed] [Google Scholar]
- 6.Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018; 6: 117–126. doi: 10.1016/S2213-2600(18)30006-7 [DOI] [PubMed] [Google Scholar]
- 7.Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open 2020; 10: e037509. doi: 10.1136/bmjopen-2020-037509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive lung disease. N Engl J Med 2004; 350: 2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 9.Higham A, Quinn AM, Cancado JED, et al. The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res 2019; 20: 49. doi: 10.1186/s12931-019-1017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jogdand P, Siddhuraj P, Mori M, et al. Eosinophils, basophils and type 2 immune microenvironments in COPD-affected lung tissue. Eur Respir J 2020; 55: 1900110. doi: 10.1183/13993003.00110-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turato G, Semenzato U, Bazzan E, et al. Blood eosinophilia neither reflects tissue eosinophils nor worsens clinical outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1216–1219. doi: 10.1164/rccm.201708-1684LE [DOI] [PubMed] [Google Scholar]
- 12.Wen Y, Reid DW, Zhang D, et al. Assessment of airway inflammation using sputum, BAL, and endobronchial biopsies in current and ex-smokers with established COPD. Int J Chron Obstruct Pulmon Dis 2010; 5: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez CH, Li SX, Hirzel AJ, et al. Alveolar eosinophilia in current smokers with chronic obstructive pulmonary disease in the SPIROMICS cohort. J Allergy Clin Immunol 2018; 141: 429–432. doi: 10.1016/j.jaci.2017.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maetani T, Tanabe N, Sato A, et al. Association between blood eosinophil count and small airway eosinophils in smokers with and without COPD. ERJ Open Res 2023; 9: 00235-2023. doi: 10.1183/23120541.00235-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]