Abstract
Physical exercise induces physiologic adaptations and is effective at reducing the risk of premature death from all causes. Pharmacological exercise mimetics may be effective in the treatment of a range of diseases including obesity and metabolic syndrome. Previously, we described the development of SLU-PP-332, an agonist for the estrogen-related receptor (ERR)α, β, and γ nuclear receptors that activates an acute aerobic exercise program. Here we examine the effects of this exercise mimetic in mouse models of obesity and metabolic syndrome. Diet-induced obese or ob/ob mice were administered SLU-PP-332, and the effects on a range of metabolic parameters were assessed. SLU-PP-332 administration mimics exercise-induced benefits on whole-body metabolism in mice including increased energy expenditure and fatty acid oxidation. These effects were accompanied by decreased fat mass accumulation. Additionally, the ERR agonist effectively reduced obesity and improved insulin sensitivity in models of metabolic syndrome. Pharmacological activation of ERR may be an effective method to treat metabolic syndrome and obesity.
SIGNIFICANCE STATEMENT
An estrogen receptor-related orphan receptor agonist, SLU-PP-332, with exercise mimetic activity, holds promise as a therapeutic to treat metabolic diseases by decreasing fat mass in mouse models of obesity.
Introduction
Obesity is a major health issue and is a predisposing risk factor for early mortality due to multiple diseases including cancers, cardiovascular disease, and type 2 diabetes (Booth et al., 2012). Obesity is associated with the accumulation of fat in the skeletal muscle that leads to reduced metabolic efficiency and insulin resistance (Taylor, 1999). Skeletal muscle is one of the most flexible organs for fuel selection, and, depending on the environmental conditions (diet, exercise, etc.), it can use glucose or fatty acids as the main source of energy. In general, glucose is the fuel preferentially used by skeletal muscle and is selected for short- and high-load exercise (resistance exercise), and as the duration of energy demand lengthens (long and low-load exercise), skeletal muscle switches its preference to fatty acids (aerobic exercise) (Meex et al., 2010). Increased fatty acid utilization decreases fat storage in the tissues and improves overall metabolism functionality and is typically associated with improved glucose tolerance and insulin sensitivity. A key adaptation of skeletal muscle in response to aerobic exercise is increased oxidative metabolic capacity mediated by enhanced mitochondrial respiratory capacity, which allows for more efficient energy production and enhanced exercise endurance (Meex et al., 2010).
The estrogen receptor-related receptors (ERRα, ERRβ, and ERRγ) are members of the nuclear receptors superfamily. ERRs are constitutively active orphan receptors most closely related to estrogen receptors (ERs) in terms of sequence homology but do not display the estrogen-binding properties of the ERs (Giguère et al., 1988). High energy demand tissues, such as muscle, heart, or liver, display high levels of expression ERRs (Giguère et al., 1988; Sladek et al., 1997; Chen et al., 1999). Classic ERR target genes encode proteins involved in fatty acid oxidation, mitochondrial biogenesis, and the Krebs cycle (Audet-Walsh and Giguére, 2015; Fan and Evans, 2015).
Several genetic gain- and loss-of-function mouse models of ERR have been developed in the past decade. ERRβ knockout mice display embryonic lethality while ERRγ and ERRα KO mice display varying degrees of susceptibility to the development of heart failure (Luo et al., 1997; Alaynick et al., 2007; Sakamoto et al., 2020). Additionally, several studies have shown a role of ERR in skeletal muscle function and endurance via modulation of mitochondrial biogenesis and lipid oxidation (Narkar et al., 2011; LaBarge et al., 2014). Importantly, mice overexpressing ERRγ in the skeletal muscle display an increase in oxidative muscle fibers and exhibit increased exercise endurance (Narkar et al., 2011; Rangwala et al., 2010).
Increased skeletal muscle oxidative capacity is associated with improved glucose tolerance and insulin sensitivity and reduced obesity (Donnelly et al., 2013), thus we hypothesized that synthetic ERR agonists that increase their transcriptional activity above their very high constitutive level may hold significant utility in the treatment of metabolic syndrome.
In continuation of our efforts to develop ERR pan agonists (Shahien et al., 2020), we recently reported the development of an ERR agonist, SLU-PP-332, that targets all three ERR isoforms, allowing for evaluation of this unique class of agent in vivo. SLU-PP-332 activates all three ERRs in cell-based assays with slightly more potency at ERRα. Moreover, we previously demonstrated that SLU-PP-332 displays both plasma and muscle exposure (0.2 μM and 0.6 μM respectively) in vivo 6 hours after intraperitoneal injection (30 mg/kg) (Billon et al., 2023).
We have shown that frequent pharmacological activation of ERR provides similar improvements to repeated bouts of aerobic exercise associated with increased skeletal muscle oxidative capacity (Billon et al., 2023). Moreover, a recent study has shown that ERRα activation via a synthetic agonist improved fatty liver disease in vivo (Mao et al., 2022). In this study, we examined the ability of SLU-PP-332 to modulate energy expenditure in vivo. We observed an increased resting energy expenditure in mouse models of obesity/metabolic syndrome that was associated with increased fatty acid oxidation leading to improvements in glucose tolerance and decreasing adiposity.
Materials and Methods
Mice
Male mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All procedures were approved and conducted per the St. Louis University and Washington University Animal Care Use Committees.
General Mouse Studies
For all experiments, 8 to 10 male C57BL6/J or ob/ob mice per group (12 weeks of age for chow) were administered SLU-PP-332 at 50 mg/kg (i.p., twice daily) or vehicle for 28 days or 12 days (for the ob/ob). We chose dose and frequency of dosing based on our previously published data (Billon et al., 2023). The ∼1-month dosing regimen was selected based on our previous experience with monitoring the effects of exercise mimetics in wild-type (WT) and diet-induced obesity (DIO) mice (Solt et al., 2012). Studies with the ob/ob mice were shortened to 12 days due to reduced tolerance of twice per day intraperitoneal administration. At the termination of the experiment, tissues were collected and gene expression analysis was completed by real-time quantitative polymerase chain reaction (qPCR) using methods. Food intake and body weight were monitored daily. Body composition was measured before initiation and at the termination of the experiments using NMR (Bruker BioSpinLF50). Plasma was collected for triglyceride (TG) and cholesterol measurements as well. All twice-daily dosing was performed with dosing occurring at Zeitgeber time 0 and Zeitgeber time 12.
Diet-Induced Obesity Model
Twenty-week-old male C57BL6/J were maintained on a high-fat diet (HFD; 20% carbohydrate, 60% fat) for 8 weeks (average weight = 38g). The mice were maintained on the HFD for the duration of the experiment, which included SLU-PP-332 administration for 28 days (50 mg/kg, i.p., twice daily).
For acute insulin response, mice were dosed for 7 days (50 mg/kg i.p, twice daily.), fasted for 7 hours, and then acutely dosed with insulin (0.75 U/kg of fat-free mass) and sacrificed 30 minutes after. Upon termination of the study, tissue samples were collected for gene expression as assessed by real-time qPCR. Blood samples were collected for further metabolite screening.
Comprehensive Laboratory Animal Monitoring System
The Columbus Instruments (Columbus, OH) comprehensive laboratory animal monitoring system (CLAMS) was used to assess the metabolic parameters of mice. Male C57BL6/J (10 or 20 weeks of age for chow or HFD, respectively) were housed individually in metabolic cages on a 12-hour day–night cycle, fed with either a normal chow or HFD. Mice were acclimated for 5 days in the CLAMS unit prior to administration of SLU-PP-332 or vehicle (10% Tween, 10% DMSO, 80% PBS). The administration of SLU-PP-332 was continued for 10 days. Eight animals were included per group. The hourly or average values during light and dark periods were calculated. Two-way ANOVA followed by the Bonferroni post-test was used to calculate the P value.
Glucose Tolerance Test
After a 6-hour fast, vehicle and SLU-PP-332-treated mice (n = 8) were injected intraperitoneally with glucose (2 g/kg of fat-free mass) (Sigma-Aldrich, St. Louis, MO, USA) to examine glucose tolerance. Blood was collected by tail snip, and glucose was measured before the injection (t = 0 minute) and 15 minutes, 30 minutes, 60 minutes, and 120 minutes post-injection using a OneTouch Ultra2 glucometer.
Lipid Assays
Plasma TGs, total cholesterol, and liver enzymes were assessed using an Analox instrument (GM7 MicroStat, St. Louis University School of Medicine, St. Louis, MO, USA).
Real-Time PCR
Total RNA extraction from mouse tissues was performed using Trizol reagent methods (Invitrogen). The RNA samples were reverse transcribed using the qScript cDNA kit (Quanta). All samples were run in duplicates, and the analysis was completed by determining ΔΔCt values. The reference gene used was 36B4, a ribosomal protein gene.
Statistical Analysis
Data are expressed as mean +/− S.E.M. Student’s test or two-way ANOVA was used to calculate statistical significance. P < 0.05 was considered significant.
Results
SLU-PP-332 Induces Fatty Acid Metabolism In Vivo
Increased whole-body fatty acid oxidation is a characteristic metabolic adaptation to aerobic exercise and aerobic training (Egan and Zierath, 2013). Previously, we demonstrated that SLU-PP-332 induces an acute aerobic exercise genetic program and increased exercise endurance (Billon et al., 2023). Here we began by assessing if SLU-PP-332 administration would result in metabolic adaptations typically observed with aerobic training. To monitor both acute and chronic effects of drug administration, we examined the effect of SLU-PP-332 (50 mg/kg twice daily) on metabolic parameters in C57Bl/6 mice using CLAMS. Mice were treated with either SLU-PP-332 or vehicle for 28 days. To avoid any interference of ERR with thermogenesis, mice were housed at thermoneutrality (Gantner et al., 2016). No difference in total body weight was observed after 28 days of treatment with SLU-PP-332 (50 mg/kg twice daily) (Fig. 1A; Supplemental Fig. 1A), but a decrease in fat mass gain was observed using NMR (Fig. 1B). As expected for mice maintained at thermoneutrality, the interscapular brown adipose tissue (BAT) depot was small, and SLU-PP-332 treatment did not alter BAT mass (Supplemental Fig. 1B). No difference in daily food intake or lean mass was observed over the 28-day dosing period (Supplemental Fig. 1C and 1D). Only relatively minor changes in plasma cholesterol and liver enzyme levels were noted in SLU-PP-332-treated mice compared with vehicle-treated (Fig. 1, C and D). There was no effect on locomotor activity in the mice as well (Fig. 1E). Within 2 hours following the first dose of SLU-PP-332 in the mice, we observed an acute decrease in the respiratory exchange ratio (RER) indicative of a shift in fuel utilization toward lipids (Fig. 1F). The lower RER was maintained during the length of the dosing during both light and dark periods and as shown in Fig. 1G. The amount of fatty acid oxidized in drug versus vehicle-treated mice was calculated using Frayn’s equation (Frayn, 1983), and SLU-PP-332 treatment increased fatty acid oxidation by 25% compared with vehicle-treated animals (Fig. 1H). Reciprocally, a decrease in carbohydrate utilization was observed (Fig. 1I). An increase in energy expenditure was observed (Fig. 1J), but there was no difference in food intake (Fig. 1K). No change in white adipocyte size was observed between vehicle and SLU-PP-332-treated mice (Fig. 1L)
Fig. 1.
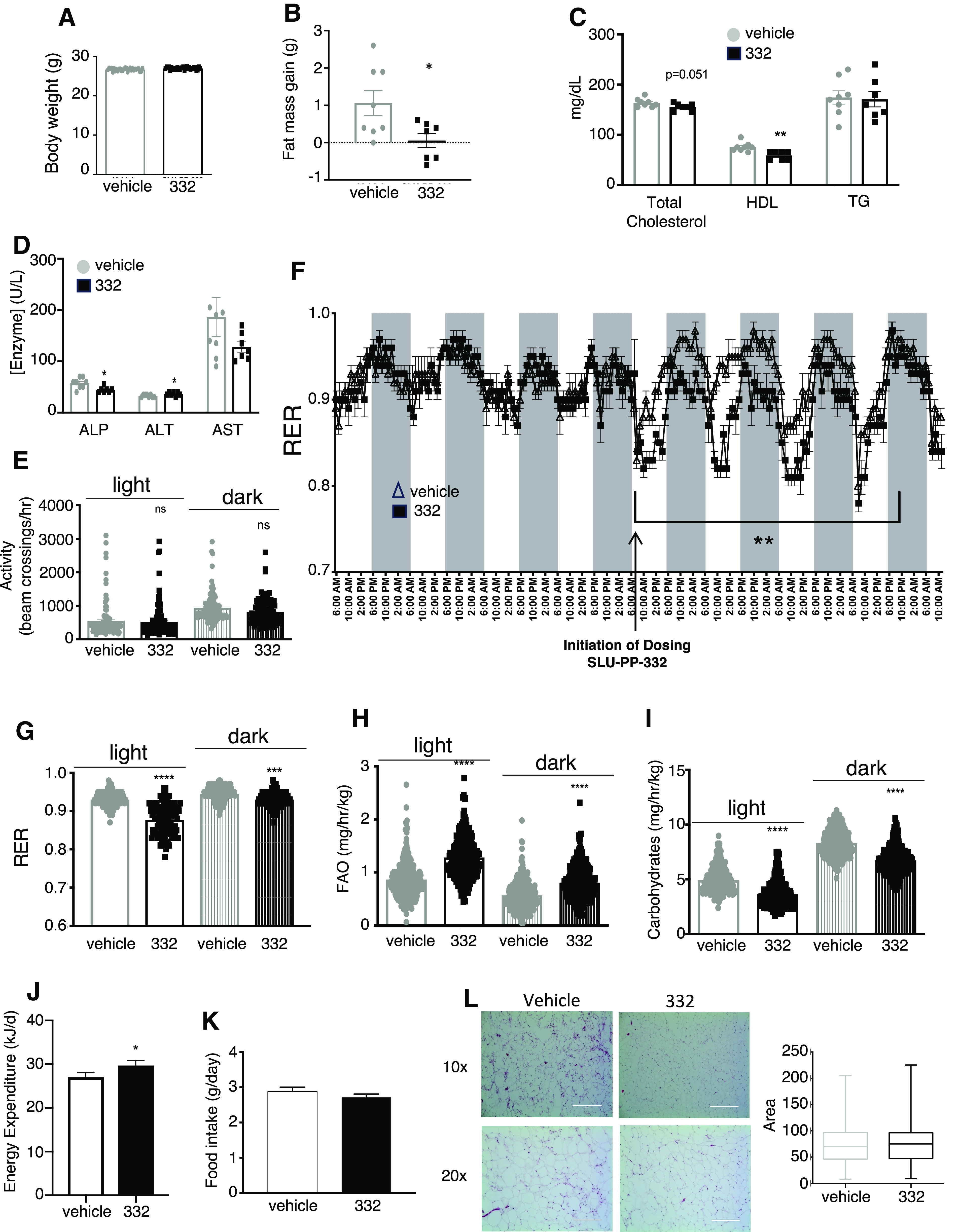
SLU-PP-332 improves muscle function and increases fatty acid metabolism in vivo. Results from a 28-day SLU-PP-332 dosing regimen in C57Bl6 mice on a normal chow diet. Mice were kept at thermoneutrality and also assessed in CLAMS. Body weight (A), fat mass gain (B), blood lipid profile (C) (total cholesterol, high-density lipoprotein, and TG), and liver enzymes (D) of 3-month-old males treated with vehicle (white bar, n = 8) or SLU-PP-332 (black bar, n = 8) for 28 days. Results from the CLAMS of the same animals treated with vehicle (white bar, n = 8) and SLU-PP-332 (black bar, n = 8) under chow diet during the day (solid bar) and night (shaded bar) over the 5 days at thermoneutrality are illustrated in (E) through (J). Locomotor activity (E); RER [(F) and (G)]; fatty acid oxidation (H); carbohydrate oxidation (I) in these mice is illustrated. Energy expenditure (J) and average food consumption (K) over the 28 days from the same mice. In (F) arrow points to the first dosing. (L), Hematoxylin and Eosin staining of WAT from vehicle (left) or SLU-PP-332 (right) treated animals (n = 4) and quantitation of adipocytes size. * P < 0.05, ** P < 0.01, ***P < 0.001, ****P < 0.0001.
SLU-PP-332 Does Not Alter Glucose Metabolism In Vivo
SLU-PP-332 did not affect fed or fasted blood glucose levels (Fig. 2A) or insulin levels (Fig. 2B). We also assessed the potential effect of SLU-PP-332 glucose metabolism by performing insulin sensitivity and glucose/pyruvate tolerance tests in the same mice described earlier. Three weeks of SLU-PP-332 treatment did not affect glucose tolerance (Fig. 2C). ERRs have been suggested to be involved in gluconeogenesis (Kim et al., 2012), but we observed no significant alteration in pyruvate tolerance in response to SLU-PP-332, suggesting hepatic glucose output was not affected (Fig. 2D). We also examined pancreatic islet structure and observed no distinctions between drug and vehicle treatment groups (Fig. 2E). Consistent with this observation, the expression of the rate-limiting enzyme of gluconeogenesis, phosphoenolpyruvate carboxykinase (Pck1), was not significantly altered with SLU-PP-332 treatment (Supplemental Fig. 1E). Although there were no indications of hepatic steatosis in the WT mice, SLU-PP-332 treatment did cause a reduction of hepatic TGs (Supplemental Fig. 1F). We next assessed the effect of SLU-PP-332 in muscle metabolism in vivo. Mice treated with SLU-PP-332 displayed increased muscle pyruvate (Fig. 2F) and decreased glycogen content (Fig. 2G) compared with the vehicle-treated group. We tested in vivo glucose uptake using fluorescently labeled 6-deoxyglucose. Mice treated with SLU-PP-332 displayed increased glucose uptake by the quadricep muscle after 2 weeks of treatment (Fig. 2H) compared with the vehicle-treated group. These data indicate that SLU-PP-332 induced an alteration in preference for fuel utilization consistent with physiologic adaptation to exercise that results in increased whole-body fatty acid oxidation (review in Astorino and Schubert, 2018) and also suggest that ERR agonists may hold utility in the treatment of metabolic diseases.
Fig. 2.
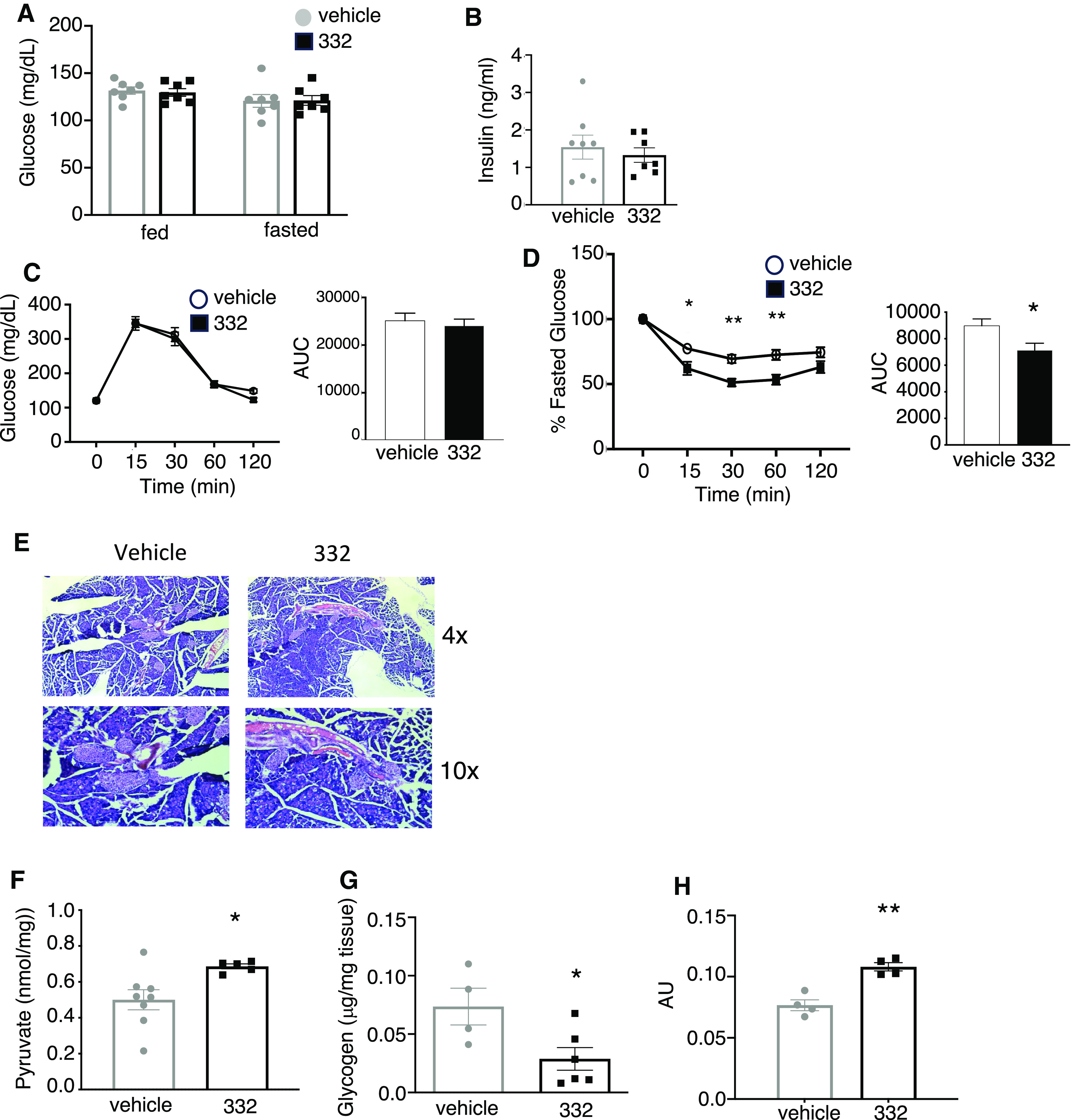
SLU-PP-332 does not improve glucose metabolism in vivo in chow-fed mice. Fed and fasted blood glucose levels (A) of the animals treated with vehicle (white bar, n = 8) and SLU-PP-332 (black bar, n = 8) maintained on a chow diet (fed) or after 8 hours of fasting (fast). Blood insulin level (B) of the animals treated with vehicle (white bar, n = 8) and SLU-PP-332 (black bar, n = 8) under chow diet (fed) after 28 days of treatment. Intraperitoneal glucose (C) and pyruvate (D) tolerance tests from vehicle (open circle/white bar) or SLU-PP-332 (black square/bar) treated animal (n = 8/group) under chow diet. The area under the curve is represented on each graph. (E) Hematoxylin and Eosin staining of the pancreas from vehicle (left) or SLU-PP-332 (right) treated animals (n = 4). Muscle pyruvate (F) and glycogen (G) content from animals treated with vehicle (white bar, n = 8) and SLU-PP-332 (black bar, n = 8) under chow diet (fed) after 28 days of treatment. (H) In vivo muscle glucose uptake from vehicle (white bar, n = 4) or SLU-PP-332 (black bar, n = 4) treated mice for 15 days.
SLU-PP-332 Reduces Fat Mass and Improves Glucose Metabolism in Several Mouse Models of Obesity
Based on the exercise mimetic activity and alterations in energy metabolism we observed in mice treated with SLU-PP-332, we hypothesized that such compounds may have a beneficial effect in models of obesity/metabolic syndrome. We used both the DIO and ob/ob mouse models to examine the effects of SLU-PP-332 on various metabolic parameters. For the DIO model, we initiated a study with 20-week-old C57BL6 mice that had been maintained on a HFD for 8 weeks (20% carbohydrate, 60% fat) before initiation of SLU-PP-332 treatment and housed at thermoneutrality. The mice were maintained on the HFD throughout the SLU-PP-332 treatment (50 mg/kg, i.p., twice daily). We noted a progressive weight loss in both DIO and ob/ob mice treated with SLU-PP-332 (Supplemental Fig. 2A), and after 28 days of treatment the drug treated mice weighed ∼12% less (Fig. 3A). After 28 days of treatment, vehicle-treated mice had gained ∼5 g of fat mass while drug-treated mice had gained less than 0.5 g of fat mass (Fig. 3B). As expected for mice maintained at thermoneutrality, the interscapular BAT depot was small and SLU-PP-332 treatment did not alter BAT mass (Supplemental Fig. 2B). No significant differences in food intake were observed during the duration of the treatment period (Supplemental Fig. 2C). There were no significant changes in lean mass (Supplemental Fig. 2D). In addition to the decrease in adiposity, we also observed a decrease in plasma total cholesterol, high-density lipoprotein and TGs but no change in low-density lipoprotein (Fig. 3C). There were also no increases in liver enzyme levels (Fig. 3D). Fasting plasma glucose and insulin levels were lower in SLU-PP-332-treated animals, but no differences were observed in fed blood glucose levels (Fig. 3, E and F).
Fig. 3.
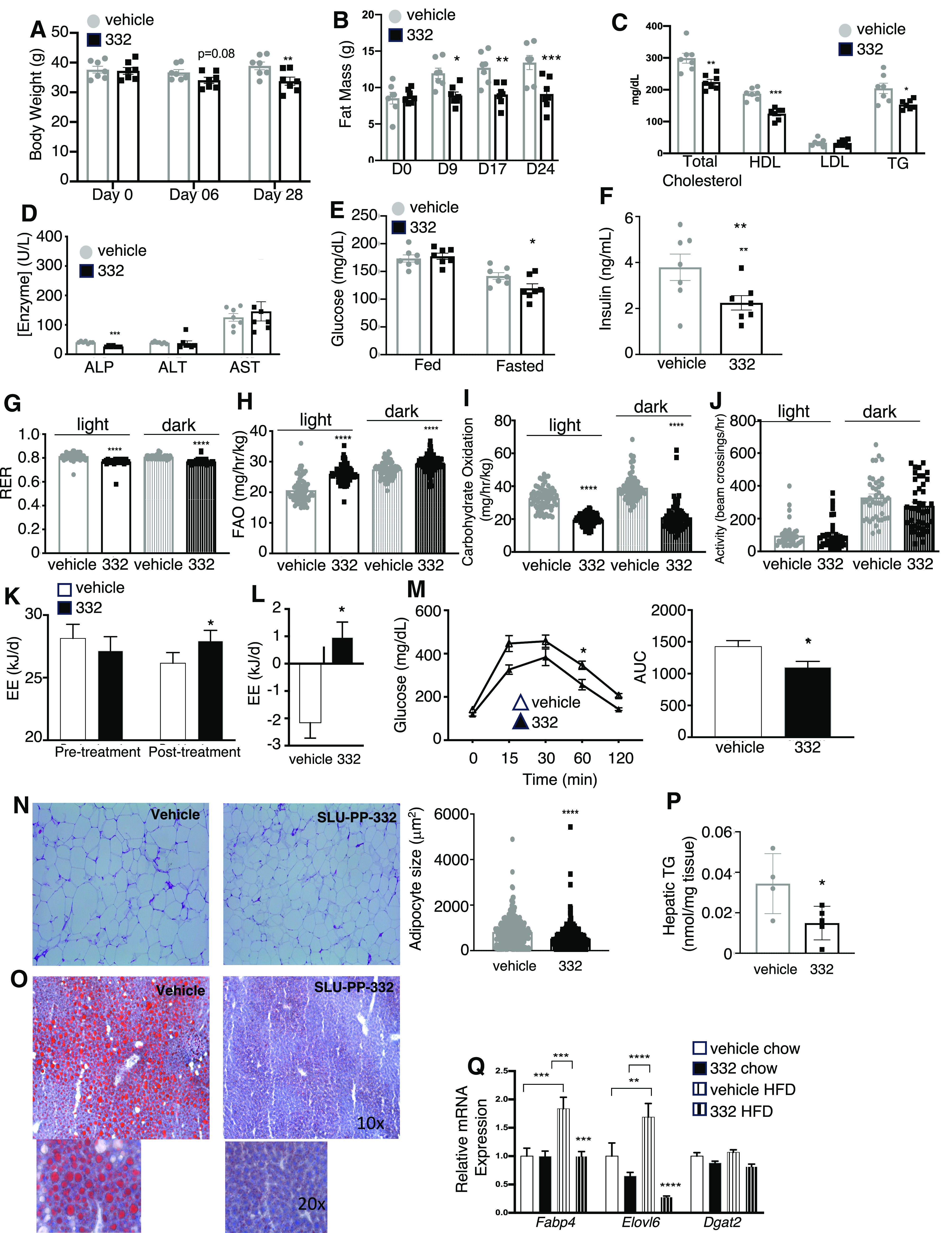
SLU-PP-332 increases fatty acid metabolism in a DIO mouse model. Body weight (A), fat mass gain (B), blood lipid profile (C) (total cholesterol, high-density lipoprotein, low-density lipoprotein, and TG), and liver enzymes (D) of DIO males treated with vehicle (gray bar, n = 7) or SLU-PP-332 (black bar, n = 7) for 28 days. Mice were kept at thermoneutrality and fed with an HFD. Blood glucose (E) and insulin (F) levels of animals treated with vehicle (gray bar, n = 7) and SLU-PP-332 (black bar, n = 7) under HFD (fed) or after 8 hours of fasting (fast). Only fasting insulin levels are shown. (G–L) Indirect calorimetry measurement from the same animals treated with vehicle (gray bar, n = 7) and SLU-PP-332 (black bar, n = 7) during the day (solid bar) and night (shaded bar) over 5 days. RER (G); fatty acid oxidation (H); carbohydrate oxidation (I), activity and (J), and energy expenditure (K). (L) Energy expenditure difference between before and after dosing normalized by food intake and body weight from the same animals. Glucose tolerance test (M) from the same animals treated with vehicle (gray bar, n = 7) and SLU-PP-332 (black bar, n = 7) maintained on an HFD. The area under the curve for the glucose tolerance test is also shown. WAT (N) from mice dosed with vehicle (gray bars, n = 7) or SLU-PP-332 (black bar, n = 7) maintained on an HFD. The bar graph represents the average size of adipocytes (vehicle: white and SLU-PP-332: black bar). Liver from these mice stained with Oil Red O (O) and liver triglyceride (P) content from mice administered vehicle (gray bars, n = 7) or SLU-PP-332 (black bar, n = 7) under HFD. (Q) Liver gene expression from mice under chow diet (solid bar, n = 8) or fed with HFD (striped bar, n = 7), treated with vehicle (white bar) or SLU-PP-332 (black bar) for 28 days.* P < 0.05, ** P < 0.01, ***P < 0.001, ****P < 0.0001.
We next assessed whether SLU-PP-332 treatment affected whole-body fuel selection and energy expenditure using CLAMS. As observed for chow-fed mice, SLU-PP-332 treatment reduced the RER (Fig. 3G), indicating an increase in fatty acid oxidation (Fig. 3H) and reduced glucose oxidation (Fig. 3I) compared with vehicle-treated mice. No changes were observed in locomotor activity (Fig. 3J). RER was similarly reduced by SLU-PP-332 treatment to that observed with mice on a regular chow diet (Supplemental Fig. 2E). Resting energy expenditure was significantly higher in the SLU-PP-332 treatment group (Fig. 3, K and L). Additionally, SLU-PP-332-treated mice displayed improved glucose tolerance (Fig. 3M), although there was no change in an insulin tolerance test (Supplemental Fig. 2F, left). There was also no effect on pyruvate tolerance or hepatic pck1 expression (Supplemental Fig. 2G and 2H) and there were no significant changes in pancreatic histology (Supplemental Fig. 2I). Since the SLU-PP-332 treated mice did not gain fat mass, we examined the effect of the ERR agonist on white adipose tissue (WAT). Paraffin-sections of visceral WAT show a significant decrease of adipocytes size in SLU-PP-332-treated mice when they were maintained on the HFD (Fig. 3N). We also noted a decrease in hepatic steatosis (Fig. 3O) and a decrease in hepatic TGs (Fig. 3P) consistent with increased lipid utilization. Given that conflicting data regarding insulin sensitivity (decreased fasting plasma glucose levels combined with lower fasting plasma insulin and a decrease in adipocyte size but no alteration in the insulin tolerance test), we assessed the effect of SLU-PP-332 treatment on AKT phosphorylation in skeletal muscle (quadricep) in response to insulin. We observed only a trend toward an increase in AKT phosphorylation in skeletal muscle from DIO mice (8 weeks on HFD prior to 7 days SLU-PP-332 administration) (Supplemental Fig. 2J).
The second metabolic disease model we used to assess the effects of SLU-PP-332 was the ob/ob model. Three-month-old male ob/ob mice maintained on a regular chow diet and maintained at thermoneutrality were administered SLU-PP-332 (50mg/kg, i.p., twice daily) or vehicle for 12 days. Although total body weight only displays a trend toward a decrease after 12 days of administration of the drug (Fig. 4A), adiposity was significantly decreased as well as liver weight (Fig. 4, B and C) even though food intake was unaltered (Fig. 4D). In a manner similar to what was observed in WT and DIO mice, we noted a decrease in RER within 2 hours of administration of SLU-PP-332 (Fig. 4, E and F). Fatty acid oxidation was increased with drug treatment while carbohydrate oxidation was lower (Fig. 4, G and H). Resting energy expenditure was elevated (Fig. 4I). The mice displayed substantial hepatic steatosis that was reduced by SLU-PP-332 treatment (Fig. 4J). These data demonstrating the efficacy of pharmacological activation of ERR in two mouse models of metabolic disease/obesity suggest that such compounds may be useful in treating metabolic diseases.
Fig. 4.
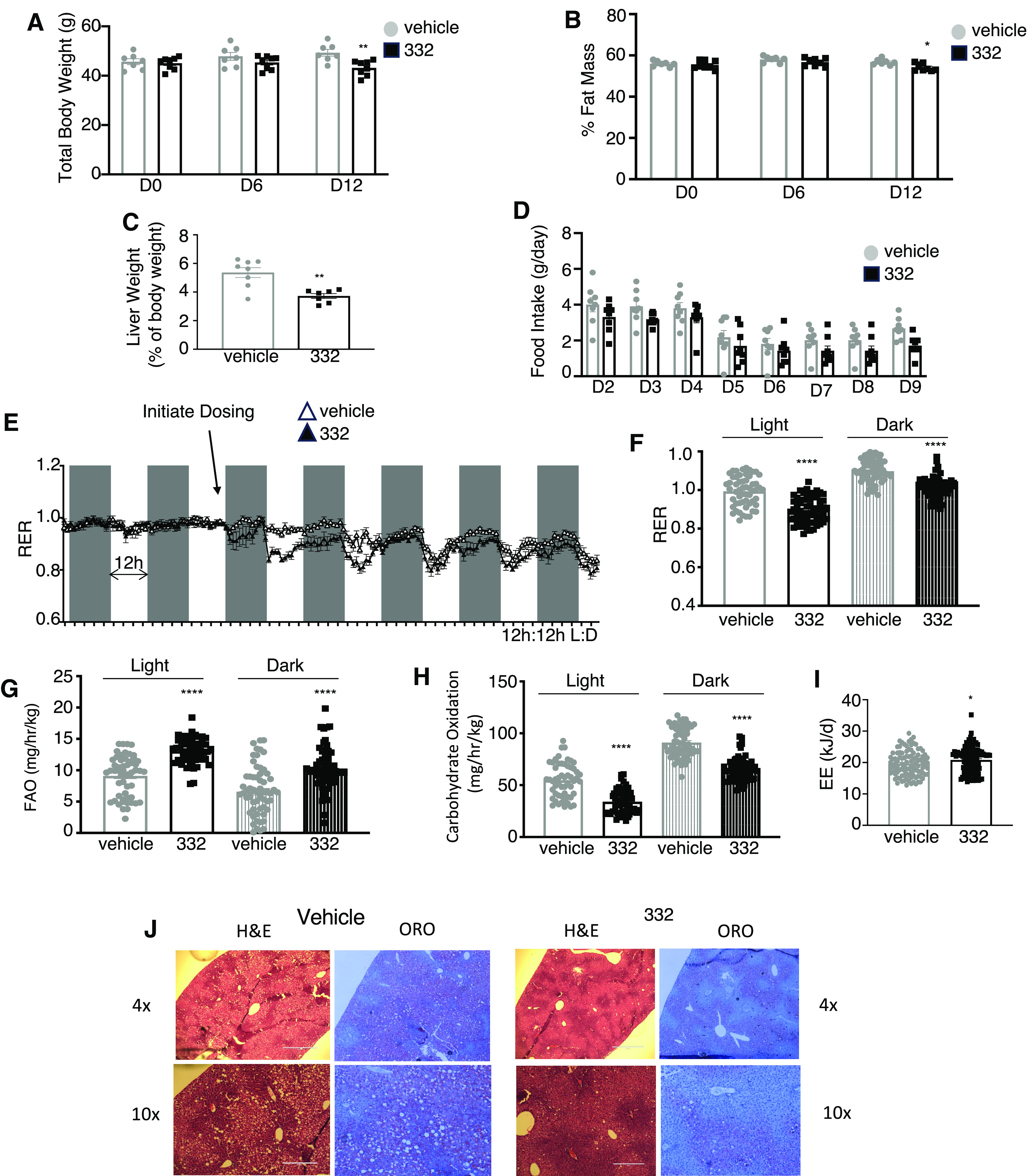
SLU-PP-332 increases fatty acid metabolism and energy expenditure in ob/ob mice. Body weight (A), fat mass (B), liver weight (C), and daily food intake (D) of 3-month-old ob/ob male mice treated with vehicle (white bar, n = 8) or SLU-PP-332 (black bar, n = 8) for 15 days. Mice were maintained at thermoneutrality and fed a chow diet. Results from indirect calorimetry (E), RER (F), fatty acid oxidation (G), carbohydrates oxidation (H), resting energy expenditure (I) of the same animals treated with vehicle (white bar, n = 8), and SLU-PP-332 (black bar, n = 8) maintained on an HFD during day and night over 15 days at thermoneutrality. Liver histology (J) Hematoxylin and Eosin and Oil Red O staining of frozen liver sections from mice treated with vehicle or SLU-PP-332 for 15 days. * P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Obesity and fat accumulation are associated with a higher risk of diabetes and cardiovascular diseases (Hill and Wyatt, 2013). Lipid accumulation in the muscle induces insulin resistance and impairs glucose uptake (Callahan et al., 2017), while exercise and decreased caloric intake (negative energy balance) are known to decrease fat accumulation and improve glucose and insulin signaling (Heath et al., 1983).
The ERRs play essential roles in the regulation of energy metabolism and fuel selection. Loss of ERRα or ERRγ function results in reduced muscle oxidative function and reduced functional endurance (Perry et al., 2014; Yoshihara et al., 2016). Therefore, pharmacological activation of ERRs may provide beneficial metabolic effects associated with increased skeletal muscle activity for the treatment of metabolic diseases.
Our data clearly suggest that such compounds may hold utility in the treatment of diseases such as type 2 diabetes and obesity where exercise is typically prescribed. Obesity and fat accumulation are associated with a higher risk of development of diabetes and cardiovascular diseases (Hill and Wyatt, 2013). Lipid accumulation in the muscle induces insulin resistance and impairs glucose uptake (Callahan et al., 2017), while exercise and decreased caloric intake are known to decrease fat accumulation and improve glucose and insulin signaling (Heath et al., 1983). WT mice maintained either on the normal diet or HFD and ob/ob mice were resistant to gaining fat mass when administered SLU-PP-332. Importantly, SLU-PP-332 treatment increases resting energy expenditure that mimics the recovery phenotype after exercise but without increasing physical activity (Gillette et al., 1994).
The beneficial effects of SLU-PP-332 on the metabolic profile could not necessarily have been predicted based on conflicting data with regard to the role of ERRα. For example, it has been reported that ERRα-null mice have reduced fat mass and are resistant to DIO (Luo et al., 2003). Additionally, selective ERRα inverse agonists have been characterized that display antidiabetes activity (Patch et al., 2011, 2017). In both of these cases, it appears that several ERRα target genes are actually elevated rather than reduced, suggesting that there may be some engagement of compensatory mechanisms. Interestingly, these ERRα-null mice also display reduced muscle oxidative capacity, an effect that is also induced by C29, a synthetic ERRα inverse agonist (Patch et al., 2011) These contradictory effects, at least in terms of a potential treatment of metabolic diseases, are also aggravated by the potential for an ERRα inverse agonist to induce heart failure (Huss et al., 2007). Thus, based on our data with SLU-PP-332 along with the preponderance of the genetic gain- and loss-of-function data, ERR agonists are more likely to be beneficial in the treatment of metabolic disease.
The relative balance of ERRα versus ERRγ agonist activity of a therapeutic compound may be important to avoid potential side effects. Although there has been considerable redundancy in the function described, distinct regulatory roles of the ERR isoforms have been demonstrated as well, such as their effects on gluconeogenesis. ERRα suppresses whereas ERRγ activates Pck1 gene expression, providing a mechanism where the isoforms have opposing effects on gluconeogenesis (Herzog et al., 2006; Kim et al., 2012). These observations are consistent with increased Pck1 expression observed in the ERRα-null mice (Perry et al., 2014) as well as the ability of the ERRγ inverse agonist GSK5182 to suppress gluconeogenesis (Kim et al., 2012). These effects of GSK5182 appear to conflict with the report that an ERRβ/γ agonist increases muscle oxidative function (Rangwala et al., 2010). We note that with SLU-PP-332 there is no change in Pck1 expression or pyruvate tolerance, suggesting no effect on hepatic glucose output (Supplemental Figs. 1E, 2F and 2G, Fig. 2F) This might be due to competing effects of ERRα and ERRγ that effectively cancel the effect on these genes, or it may be due to alterations in the gluconeogenic response to whole-body alterations in metabolic activity. The fact that SLU-PP-332 exhibits a degree of preference for ERRα over ERRγ may help to eliminate the potential side effect of induction of gluconeogenesis.
In summary, the ERR agonist SLU-PP-332 functions as an exercise mimetic inducing an acute aerobic exercise program that leads to myriad physiologic adaptations that are associated with exercise including increased skeletal muscle oxidative fibers, increased fatty acid oxidation, and enhanced exercise endurance. Although much of the exercise mimetic activity may be associated with targeting ERR in the skeletal muscle, additional target tissues such as the liver may also play a role. In obese mice, SLU-PP-332 reduced fat mass and improved glucose tolerance, suggesting a potential use of ERR agonists in the treatment of a range of metabolic diseases including obesity and type 2 diabetes.
Acknowledgments
The authors thank Sherry Burris for her help with sectioning.
Data Availability
The authors declare that all the data supporting the findings of this study are available within the paper and its Supplemental Material.
Abbreviations
- BAT
brown adipose tissue
- CLAMS
Comprehensive Laboratory Animal Monitoring System
- DIO
diet-induced obesity
- ER
estrogen receptor
- ERR
estrogen-related receptor
- HFD
high-fat diet
- Pck1
phosphoenolpyruvate carboxykinase
- qPCR
quantitative polymerase chain reaction
- RER
respiratory exchange ratio
- TG
triglyceride
- WAT
white adipose tissue
- WT
wild-type
Authorship Contributions
Participated in research design: Billon, Burris.
Conducted experiments: Billon, Schoepke, Avdagic.
Contributed to new reagents or analytic tools: Chatterjee, Butler, Elgendy, Walker.
Performed data analysis: Billon, Butler, Burris.
Wrote or contributed to the writing of the manuscript: Billon, Burris.
Footnotes
This work was supported by National Institutes of Health National Institute of Aging and National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grants AG077160, AR069280, and AG060769] (to T.P.B.).
T.P.B., J.K.W., and B.E. are stockholders in Myonid Therapeutics, Inc. and Pelagos Pharmaceuticals, Inc.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguère V, et al. (2007) ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6:13–24. [DOI] [PubMed] [Google Scholar]
- Astorino TA, Schubert MM (2018) Changes in fat oxidation in response to various regimes of high intensity interval training (HIIT). Eur J Appl Physiol 118:51–63. [DOI] [PubMed] [Google Scholar]
- Audet-Walsh É, Giguére V (2015) The multiple universes of estrogen-related receptor α and γ in metabolic control and related diseases. Acta Pharmacol Sin 36:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon C, Sitaula S, Banerjee S, Welch R, Elgendy B, Hegazy L, Oh TG, Kazantzis M, Chatterjee A, Chrivia J, et al. (2023) Synthetic ERRα/β/γ agonist Induces an ERRα-dependent acute aerobic exercise response and enhances exercise capacity. ACS Chem Biol 18:756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, Laye MJ (2012) Lack of exercise is a major cause of chronic diseases. Compr Physiol 2:1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan ZJ, Oxendine MJ, Schaeffer PJ (2017) Intramuscular triglyceride content precedes impaired glucose metabolism without evidence for mitochondrial dysfunction during early development of a diabetic phenotype. Appl Physiol Nutr Metab 42:963–972. [DOI] [PubMed] [Google Scholar]
- Chen F, Zhang Q, McDonald T, Davidoff MJ, Bailey W, Bai C, Liu Q, Caskey CT (1999) Identification of two hERR2-related novel nuclear receptors utilizing bioinformatics and inverse PCR. Gene 228:101–109. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Honas JJ, Smith BK, Mayo MS, Gibson CA, Sullivan DK, Lee J, Herrmann SD, Lambourne K, Washburn RA (2013) Aerobic exercise alone results in clinically significant weight loss for men and women: Midwest Exercise Trial 2. Obesity (Silver Spring) 21:E219–E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17:162–184. [DOI] [PubMed] [Google Scholar]
- Fan W, Evans R (2015) PPARs and ERRs: molecular mediators of mitochondrial metabolism. Curr Opin Cell Biol 33:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634. [DOI] [PubMed] [Google Scholar]
- Gantner ML, Hazen BC, Eury E, Brown EL, Kralli A (2016) Complementary roles of estrogen-related receptors in brown adipocyte thermogenic function. Endocrinology 157:4770–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V, Yang N, Segui P, Evans RM (1988) Identification of a new class of steroid hormone receptors. Nature 331:91–94. [DOI] [PubMed] [Google Scholar]
- Gillette CA, Bullough RC, Melby CL (1994) Postexercise energy expenditure in response to acute aerobic or resistive exercise. Int J Sport Nutr 4:347–360. [DOI] [PubMed] [Google Scholar]
- Heath GW, Gavin JR 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO (1983) Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol 55:512–517. [DOI] [PubMed] [Google Scholar]
- Herzog B, Cardenas J, Hall RK, Villena JA, Budge PJ, Giguère V, Granner DK, Kralli A (2006) Estrogen-related receptor α is a repressor of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 281:99–106. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR (2013) The myth of healthy obesity. Ann Intern Med 159:789–790. [DOI] [PubMed] [Google Scholar]
- Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguère V, Murphy E, Kelly DP (2007) The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab 6:25–37. [DOI] [PubMed] [Google Scholar]
- Kim D-K, Ryu D, Koh M, Lee M-W, Lim D, Kim M-J, Kim Y-H, Cho W-J, Lee C-H, Park SB, et al. (2012) Orphan nuclear receptor estrogen-related receptor γ (ERRγ) is key regulator of hepatic gluconeogenesis. J Biol Chem 287:21628–21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarge S, McDonald M, Smith-Powell L, Auwerx J, Huss JM (2014) Estrogen-related receptor-α (ERRα) deficiency in skeletal muscle impairs regeneration in response to injury. FASEB J 28:1082–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Sladek R, Bader J-A, Matthyssen A, Rossant J, Giguère V (1997) Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature 388:778–782. [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader J-A, Richard D, Giguère V (2003) Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol 23:7947–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Peng L, Ren X, Chu Y, Nie T, Lin W, Zhao X, Libby A, Xu Y, Chang Y, et al. (2022) Discovery of JND003 as a new selective estrogen-related receptor α agonist alleviating nonalcoholic fatty liver disease and insulin resistance. ACS Bio Med Chem Au 2:282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P, Hesselink MK (2010) Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, Banayo E, Karunasiri MS, Lorca S, Evans RM (2011) Exercise and PGC-1α-independent synchronization of type I muscle metabolism and vasculature by ERRγ. Cell Metab 13:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patch RJ, Huang H, Patel S, Cheung W, Xu G, Zhao B-P, Beauchamp DA, Rentzeperis D, Geisler JG, Askari HB, et al. (2017) Indazole-based ligands for estrogen-related receptor α as potential anti-diabetic agents. Eur J Med Chem 138:830–853. [DOI] [PubMed] [Google Scholar]
- Patch RJ, Searle LL, Kim AJ, De D, Zhu X, Askari HB, O’Neill JC, Abad MC, Rentzeperis D, Liu J, et al. (2011) Identification of diaryl ether-based ligands for estrogen-related receptor α as potential antidiabetic agents. J Med Chem 54:788–808. [DOI] [PubMed] [Google Scholar]
- Perry M-C, Dufour CR, Tam IS, B’chir W, Giguère V (2014) Estrogen-related receptor-α coordinates transcriptional programs essential for exercise tolerance and muscle fitness. Mol Endocrinol 28:2060–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala SM, Wang X, Calvo JA, Lindsley L, Zhang Y, Deyneko G, Beaulieu V, Gao J, Turner G, Markovits J (2010) Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem 285:22619–22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Matsuura TR, Wan S, Ryba DM, Kim JU, Won KJ, Lai L, Petucci C, Petrenko N, Musunuru K, et al. (2020) A critical role for estrogen-related receptor signaling in cardiac maturation. Circ Res 126:1685–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahien M, Elagawany M, Sitaula S, Goher SS, Burris SL, Sanders R, Avdagic A, Billon C, Hegazy L, Burris TP, et al. (2020) Modulation of estrogen-related receptors subtype selectivity: conversion of an ERRβ/γ selective agonist to ERRα/β/γ pan agonists. Bioorg Chem 102:104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, Bader JA, Giguère V (1997) The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol 17:5400–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, et al. (2012) Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SI (1999) Deconstructing type 2 diabetes. Cell 97:9–12. [DOI] [PubMed] [Google Scholar]
- Yoshihara E, Wei Z, Lin CS, Fang S, Ahmadian M, Kida Y, Tseng T, Dai Y, Yu RT, Liddle C, et al. (2016) ERRγ is required for the metabolic maturation of therapeutically functional glucose-responsive β cells. Cell Metab 23:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]


