Abstract
The number of drug overdoses and deaths has increased significantly over the past decade and co-use of opioids and stimulants is associated with greater likelihood of overdose and decreased likelihood of accessing treatment, compared with use of opioids alone. Potential adverse effects of opioid/stimulant mixtures, particularly methamphetamine, are not well characterized. Two structurally different drugs with agonist properties at µ-opioid receptors (MOR), fentanyl and heroin, and d-methamphetamine, alone and in mixtures, were assessed for their effects on ventilation in rats breathing normal air. Whole-body phethysmography chambers were equipped with a tower and swivel allowing infusions to indwelling intravenous catheters. After a 45-minute habituation period, saline, fentanyl, heroin, or d-methamphetamine, alone and in mixtures, was administered. Five minutes later, the opioid receptor antagonist naloxone or vehicle was injected. Fentanyl (0.0032–0.1 mg/kg) and heroin (0.32–3.2 mg/kg) decreased ventilation [frequency (f) and tidal volume (VT)] in a dose-related manner whereas d-methamphetamine (0.1–3.2 mg/kg) increased f to >400% of control and decreased VT to <60% of control, overall increasing minute volume (product of f and VT) to >240% of control. When combined, d-methamphetamine (0.1–3.2 mg/kg) attenuated the ventilatory depressant effects of fentanyl (0.1 mg/kg) and heroin (3.2 mg/kg). d-Methamphetamine did not alter the potency of naloxone to reverse the ventilatory depressant effects of fentanyl or heroin. These studies demonstrate that d-methamphetamine can attenuate the ventilatory depressant effects of moderate doses of opioid receptor agonists while not altering the potency of naloxone to reverse opioid hypoventilation.
SIGNIFICANCE STATEMENT
Co-use of opioids and stimulants is associated with greater likelihood of overdose and decreased likelihood of accessing treatment, compared with use of opioids alone. Potential adverse effects of opioid/stimulant mixtures are not well characterized. This study reports that 1) d-methamphetamine attenuates the ventilatory depressant effects of moderate doses of two structurally different opioid receptor agonists, fentanyl and heroin, and 2) d-methamphetamine does not alter potency or effectiveness of naloxone to reverse the ventilatory depressant effects of these opioid receptor agonists.
Introduction
In part as a response to the opioid epidemic, prescriptions for opioid receptor agonists decreased by 44.4% between 2011 and 2020 (AMA, 2021). However, opioid misuse remains a significant public health challenge, with 107,622 drug overdose deaths reported in the US in 2021, 80,816 attributed to opioids (https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm). The predominant life-threatening effect of opioids is hypoventilation, often resulting in death (Stoeckel et al., 1982; Pattinson, 2008). Opioid-induced hypoventilation results from activation of µ-opioid receptors (MORs) located throughout the pontomedullary area (Mansour et al., 1994; Lonergan et al., 2003) and is characterized by decreased frequency (f) and volume (tidal volume [VT]), resulting in an overall decrease in ventilation (minute volume [VE], the product of f and VT) that is often accompanied by an irregular breathing pattern (Stoeckel et al., 1982; Bouillon et al., 2003; Pattinson, 2008) and upper airway dysfunction (Savilampi et al., 2013, 2014).
Although the opioid epidemic evolved from the misuse of prescription opioids to the misuse of heroin, today synthetic MOR agonists (e.g., non-morphinan phenylpiperidine fentanyl and its analogs) are most commonly detected in overdose victims (Jones et al., 2018; Hedegaard et al., 2021). The opioid receptor antagonist naloxone (Narcan) is one of two (nalmefene was recently approved) Food and Drug Administration-approved medications for treating opioid overdose. There are suggestions that naloxone is less effective in reversing the ventilatory depressant effect of fentanyl and its analogs compared with its ability to reverse ventilatory depressant effects of morphinan opioids, including heroin and morphine. One epidemiologic study (Coffin et al., 2022) reported that naloxone was less effective at reducing the annual overdose death rate of fentanyl (12.0%) relative to that of heroin (26.4%). Under hypercapnic conditions (i.e., 5% carbon dioxide [CO2]) in mice, naloxone was less effective at reversing the ventilatory depressant effects of fentanyl compared with morphine (Hill et al., 2020).
Many overdose deaths are caused by fentanyl being an adulterant not only with another opioid but increasingly with other classes of drugs (Jones et al., 2018). A growing number of overdoses and deaths occur in individuals using more than one drug, intentionally or unintentionally, often an opioid with a stimulant (Hoots et al., 2020; Lockwood et al., 2021). Stimulants are the second leading class of drugs causing overdose deaths, contributing to 32,856 overdose deaths in 2021 (https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm). In contrast to opioid overdose, stimulant overdose is characterized by hyperthermia (Buchanan and Brown, 1988) and cardiac dysfunction, often leading to death (e.g., Dalal et al., 2020). Overdoses involving stimulants increasingly involve an opioid although the contribution of stimulants to the adverse effects of opioids is not fully characterized. Conversely, it is not entirely clear whether or how opioids contribute to stimulant overdose.
In contrast to the ventilatory depressant effects of opioid receptor agonists, stimulant drugs alone can increase ventilation. For example, d-methamphetamine and d-amphetamine increase f in human subjects breathing normal air (Martin et al., 1971) and d-amphetamine also increases f in rats breathing normal air (Schierok et al., 2000). d-Amphetamine also increases VE in humans under hypercapnic conditions (3–7% CO2) that increase ventilation (Bourke et al., 1983). Clinical studies have shown that d-amphetamine can attenuate the ventilatory depressant effect of morphine under both normal air and hypercapnic conditions (Bourke et al., 1983; Jasinski and Preston, 1986). In rats breathing normal air, d-amphetamine attenuates fentanyl-induced changes in ex vivo respiratory parameters (i.e., increase in partial pressure CO2 and decrease in percent oxygen saturation; Moody et al., 2020). However, a recent study using hypercapnic (5% CO2) conditions in mice reported that both (±)-methamphetamine and d-amphetamine produced bi-phasic changes in VE in a dose- and time-dependent manner (Elder et al., 2023). Moreover, doses of stimulant drugs that decreased VE exacerbated the ventilatory depressant effects of fentanyl (Elder et al., 2023). Another study in mice reported that methamphetamine potentiates the antinociceptive effects of morphine and methadone (Sprague and Takermori, 1978). Thus, under some conditions stimulant drugs can enhance the ventilatory effects of agonists acting at MOR.
The present study assessed the ability of d-methamphetamine to modify the ventilatory depressant effects of fentanyl and heroin under normal air conditions that model the physiologic conditions under which drug overdoses typically occur in humans. Additionally, d-methamphetamine was evaluated for whether it alters the potency or effectiveness of naloxone to reverse the ventilatory depressant effects of fentanyl and heroin. Rectal temperature was also compared before and after ventilation experiments to provide another assessment of possible interactions between agonists acting at MOR and d-methamphetamine, both of which can produce hyperthermia (Solis et al., 2017, 2018).
Materials and Methods
Subjects
Eight adult male Sprague-Dawley rats (weighing 338–356 g at the beginning of the study and 430–508 g six months later at the end of the study) were purchased from Envigo RMS, LLC (Indianapolis, IN). The sample size was determined by a power analysis (α = 0.05, 80% power) and was sufficient to detect a shift of at least twofold in the dose-effect function for the effects of opioid agonists on ventilation (G*Power version 3.1.9.6). Rats were housed individually in a vivarium maintained at 23.0 ± 1.5°C (mean ± standard deviation) and 40 ± 20% humidity under a 14/10 hour light/dark cycle (lights on at 0600 hours). Experiments were conducted during the early light cycle (0700–1130 hours). Rats had free access to food (7912 irradiated LM-485 Mouse/Rat Sterilizable Diet, Envigo RMS, LLC) and filtered reverse osmosis tap water (Edstrom BFS-675 Bottle Filling Station, Edstrom Industries, Inc., Waterford, WI) in the home cage. Subjects were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee at The University of Texas Health Science Center at San Antonio and the guidelines of the Committee on Care and Use of Laboratory Animal Resources and the Guide for Use of Laboratory Animals (National Research Council, Department of Health, Education and Welfare, 85-23, revised 2011).
Surgery
Following a minimum of 5 days in the vivarium, rats received a chronic indwelling intravenous catheter. Anesthesia was induced and maintained via inhalation of isoflurane (Fluriso, VetOne, Boise, ID [2.5–5%v/v and 0.5–3.5%v/v, up to 0.4–1 L/min, respectively]) using SomnoSuite Low-Flow Digital Vaporizer (Kent Scientific Co., Torrington, CT). Once the absence of a righting reflex was observed, rats were placed on a heated area of SurgiSuite (Kent Scientific Co.) set at 37°C to provide heat support and transferred to an anesthesia nose cone. Veterinary ophthalmic ointment (Puralube, Dechra Pharmaceuticals, Leawood, KS) was applied to the eyes and a thermosensor probe was inserted into the rectum to monitor body temperature. Using aseptic techniques, a catheter (CNC-3H-30-6/6.5, Access Technologies, Skokie, IL) was implanted in the right external jugular vein as described previously (Hiranita et al., 2010; Jimenez et al., 2021). The catheter was attached to a 22-G access port (VABR1B/22, Instech Laboratories, Inc., Plymouth Meeting, PA) anchored in a subcutaneous pocket and the port exited through the midscapular region. Immediately after surgery, rats received subcutaneous injections of 5.0 mg/kg Baytril (Bayer HealthCare LLC, Shawnee, KS) and 1.0 mg/kg meloxicam (Covetrus, Portland, ME). After surgery, rats were allowed at least 5 days to recover before experiments commenced. Additionally, 5.0 mg/kg Baytril and 1.0 mg/kg meloxicam were administered 24 and 12 hours, respectively, after surgery. Catheters were flushed daily with 0.5 mL heparinized saline (100 U/mL; Mylan, Canonsburg, PA) immediately before experiments and also on nonexperimental days. If a catheter malfunctioned (e.g., blockage or leakage), a new catheter was implanted in the right or left femoral or left external jugular vein. Rats were handled and habituated to the experimental chamber and conditions for 5–7 days prior to beginning the study.
Rectal Temperature
Rectal temperature was measured immediately before and immediately after each ventilation experiment using a digital thermometer with an uninsulated microprobe (BAT7001H, Physitemp, Clifton, NJ). McKesson lubricating jelly (16-8919, McKesson, Irving, TX) was applied to the tip of the microprobe prior to each use.
Ventilation
Prior to testing drugs, rats were habituated to whole-body plethysmography chambers (Buxco Small Animal WBP 4-Site System, Data Sciences International [DSI], St. Paul, MN) located in sound-attenuating cubicles that were not illuminated during experiments. Chambers were connected with Tygon tubing to a 2-SITE FPWBP Controller (Part # 601–1401-001, DSI). A continuous flow (2.2 L/minute) of normal air was circulated through the chambers by the 2-SITE FPWBP Controller. The top of each chamber was equipped with a single-channel fluid swivel (KVABR1T/25-MBS, Instech Laboratories, Inc.) that was connected with Tygon tubing to one of three valves from a Namic 3-way stopcock (Navilyst Medical, Marlborough, MA). One of the two valves from the stopcock was connected to a 30 mL syringe (BD, Franklin Lakes, NJ) mounted on an infusion pump (PHM-100, Med Associates, Inc., Fairfax, VT), located outside the chamber, which delivered a saline flush through the stopcock. The third stopcock valve was used to deliver test compounds. The tip of each swivel was connected to a 22-G access port exiting the rat midscapular region with Tygon tubing protected by a surrounding metal spring tether. Infusion pumps were controlled by a 28V T/T interface cabinet (SG-6510D, Med Associates, Inc.), Med PC IV software (Med Associates, Inc.), and a computer (Dell OptiPlex GX620, Dell, Round Rock, TX) and delivered programmed infusions of 0.5 mL over five seconds. Ventilation was monitored and recorded by changes in pressure in the chamber as detected and amplified by the 2-SITE FPWBP controller and digitized by FinePointe Software New (DSI) in a computer (Dell Optiplex 7010). Chambers were calibrated using the FinePointe Software New immediately before each experiment. Two rats were randomly assigned to one of four chambers used in this study and 60% isopropyl alcohol (Sigma-Aldrich, St. Louis, MO) in tap water was used to thoroughly clean chambers between consecutive sessions. Stopcocks and Tygon tubing were flushed with at least 1.0 mL of saline after each experiment.
Immediately after measuring rectal temperature, rats were placed in the whole-body plethysmography chambers and studied under normal air conditions (Supplemental Fig. 1). Rats were habituated to the chamber for 45 minutes (habituation period: −45 to 0 minutes) followed by administration of vehicle or drugs(s) and a 60-minute test period (test period: 0 to 60 minutes). Test compounds were administered intravenously at the beginning of the test period (time 0) and 5 minutes later. The first infusion was saline vehicle, fentanyl alone (0.0032–0.1 mg/kg), heroin alone (0.1–3.2 mg/kg), d-methamphetamine alone (0.1–3.2 mg/kg), or a mixture of d-methamphetamine (0.1–3.2 mg/kg) and fentanyl (0.032 or 0.1 mg/kg) or heroin (0.56 or 3.2 mg/kg). The second infusion was 10% β-cyclodextrin vehicle alone or with naloxone (0.0001–0.1 mg/kg). Vehicle and drug infusions were followed by an infusion of saline (0.5 mL over 5 seconds). Immediately after the test period, rats were removed from chambers and rectal temperature was again measured. Test sessions were separated by at least 4 days with the order of drugs, doses, and tests with drugs alone and in mixtures varying nonsystematically across subjects. A control session was conducted on the day prior to each drug test session; in control sessions rats received an infusion of saline (time 0) followed by an infusion of 10% β-cyclodextrin vehicle (time 5 minutes).
Data Analyses
The primary dependent variables were ventilatory frequency (f, breaths per minute), tidal volume (VT, mL/breath/kg), and resulting minute volume (VE = f × VT, mL/minute/kg) for ventilation and change from baseline (°C) for rectal temperature. Ventilatory parameters were averaged in 5-minute bins and normalized to individual averages during the ventilation test period under the saline/vehicle condition. Data normalization reflects individual changes more precisely compared with presentation of group averages of absolute values. The baseline temperature for individual subjects was the average of all baseline values immediately before each ventilation experiment. Changes from baseline for rectal temperature were the temperatures measured after ventilation experiments (°C) subtracted by the baseline temperature (°C). Figures were created with GraphPad Prism version 9 for Windows (GraphPad Software, La Jolla, CA). Data are expressed as the mean ± 1 S.E.M. for eight rats. Time- and dose-effect functions were statistically analyzed with SigmaPlot version 12.0 (Systat Software Inc., San Jose, CA) by one- (dose) or two- (dose and time, or presence of d-methamphetamine) way repeated-measures ANOVA followed by post hoc Bonferroni t tests for pairwise comparisons. Using the GraphPad Prism software, the dose-effect functions were further analyzed with linear regression (Snedecor and Cochran, 1967) to calculate effective doses as follows: 1) ED50, potency of heroin and fentanyl to decrease ventilation; 2) ED80, potency for naloxone to reverse opioid agonist-induced hypoventilation; and 3) ED150, potency for d-methamphetamine to increase ventilation and modify the ventilatory depressant effects of heroin and fentanyl. Also calculated were the slopes and their 95% confidence intervals (CI). For the dose-effect analyses of test compounds alone, the first 10-minute average of the area under the curve (AUC) for VE in the test period (5- and 10-minute time points, AUC5-10) was used for individual subjects. For the dose-effect analyses of naloxone reversal, the first 10-minute average of AUC for VE after administration of naloxone or vehicle (10- and 15-minute time points, AUC10-15) was used for individual subjects. For all analyses, the criterion for statistical significance was P < 0.05.
Drugs
Fentanyl hydrochloride (HCl), heroin HCl, d-methamphetamine HCl, and naloxone HCl were generously provided by the Drug Supply Program of the National Institute on Drug Abuse (Rockville, MD). Fentanyl, heroin, and d-methamphetamine were dissolved in physiologic saline (0.9% sodium chloride, 2B1323, Baxter Healthcare Corporation, Deerfield, IL), and naloxone was dissolved in 10% w/v β-cyclodextrin (Janssen Research & Development, LLC, Raritan, NJ) in sterile water (B. Braun Medical, Inc., Irvine, CA). All drugs and their vehicles were administered intravenously in a volume of 0.5 m/kg body weight. Solutions were passed through a syringe filter (CH2225-PES, polyethersulfone syringe filter, 0.22 µm, 25 mm, ThermoFisher Scientific, Waltham, MA) prior to intravenous infusions. Doses are expressed in mg/kg body weight as the salt forms.
Results
Baseline Ventilation
The left panels in Supplemental Fig. 2 show ventilation time-effect functions under baseline conditions when an infusion of saline was followed by an infusion of 10% β-cyclodextrin (saline/vehicle). During the habituation period (−40 to 0 minutes), f decreased by approximately 100 breaths/minute over the first 30 minutes before stabilizing at approximately 105 breaths/minute for the remainder of the session. In contrast, VT was relatively stable at 4.2 to 5.2 mL/breath/kg for the entire session including the habituation period. As a result, VE decreased by approximately 400 mL/minute/kg for the first 30 minutes before stabilizing at approximately 440 mL/minute/kg for the reminder of the control session. Table 1 shows the mean ± 1 S.E.M. of baseline (no drug) ventilation parameters of the test period as well as baseline rectal temperature. Rectal temperature was similar before and after ventilation control sessions (difference of -0.04°C ± 0.07°C).
TABLE 1.
Baseline values for frequency (f), tidal volume (VT), minute volume (VE), and rectal temperature (see Supplemental Fig. 2 for source data) The value for pre-session rectal temperature includes all measurements made prior to all daily sessions including test sessions and values for ventilatory parameters (f, VT, and VE) and post-session rectal temperature are from saline/vehicle control test sessions only (i.e., average of ventilatory values from 1 to 60 minutes and of changes from pre-session temperature). Shown are the mean ± 1 S.E.M. for eight rats.
| Parameter | Baseline value |
|---|---|
| f (breaths/minute) | 105.4 ± 1.9 |
| VT (mL/breath/kg) | 4.96 ± 0.03 |
| VE (mL/minute/kg) | 442.6 ± 4.2 |
| Rectal temperature (°C) prior to sessions | 37.07 ± 0.03 |
| Post-session temperature, change from pre-session (°C) | −0.04 ± 0.07 |
Effects of Fentanyl Alone, Heroin Alone, d-Methamphetamine Alone, and Naloxone Alone
The effects of fentanyl, heroin, and d-methamphetamine on ventilation are shown in Fig. 1 (filled symbols represent significant differences from saline/vehicle). Values in each panel are normalized to the saline/vehicle conditions shown in the left panels of Supplemental Fig. 2. Supplemental Table 1 summarizes results of statistical analyses of the time-effect functions.
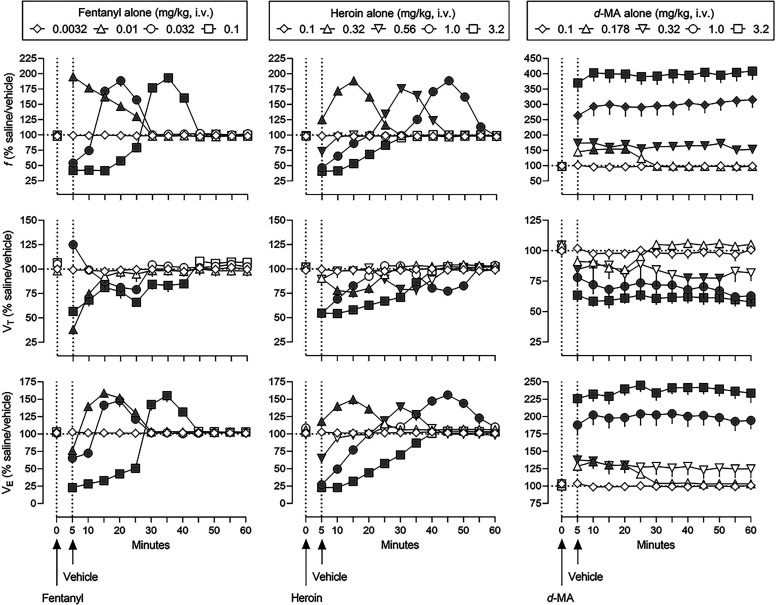
Fig. 1.
Effects of fentanyl alone, heroin alone, and d-methamphetamine alone on ventilation in rats breathing normal air (normal air conditions). Abscissae: time in minutes. Ordinates: top panels, frequency of ventilation (f); middle panels, tidal volume (VT); and lower panels, minute volume (VE). Data are expressed as a percentage of values in control conditions (horizontal dashed lines) when rats received an infusion of saline followed 5 minutes later by an infusion of 10% β-cyclodextrin (saline/vehicle) as shown in Supplemental Fig. 2. Symbols represent the mean ± 1 S.E.M. for eight rats. Fentanyl (left), heroin (center), and d-methamphetamine (right) were administered intravenously at time 0 and 10% β-cyclodextrin was administered intravenously 5 minutes later (indicated by arrows on abscissae and vertical dashed lines). Gray symbols indicate a significant difference from saline/vehicle values (see Supplemental Table 1 for details of statistical analyses).
Fentanyl altered ventilation in a dose- and time- related manner (left panels, Fig. 1). The smallest dose (0.0032 mg/kg) of fentanyl (diamonds) had no effect on ventilation, whereas 0.01 mg/kg (triangles) significantly increased f to 195% of control for 5 to 25 minutes (upper left panel, Fig. 1). A dose of 0.032 mg/kg fentanyl (circles) initially decreased f to 54% of control for 5 to 10 minutes then increased f to 189% of control for 15 to 25 minutes. A dose of 0.1 mg/kg fentanyl (squares) had a more sustained decrease and subsequently increase in ventilation although the magnitude of changes was similar across the two larger doses of fentanyl. The time-effect functions of fentanyl on VT varied somewhat from its effects on f. A dose of 0.01 mg/kg fentanyl (triangles) significantly decreased VT to 62% of control for 5 to 10 minutes (middle left panel, Fig. 1); 0.032 mg/kg (circles) initially increased VT to 125% of control at 5 minutes then decreased VT to 79% of control from 15 to 25 minutes. At a dose of 0.1 mg/kg, fentanyl (squares) had a more sustained effect in decreasing VT to 57% of control from 5 to 40 minutes. The time-effect function for fentanyl on VE (lower left panel, Fig. 1) was most similar to the effect of fentanyl on f, with VE initially decreased then increased in a dose- and time-related manner. The duration and magnitude of the effects of fentanyl on VE were time- and dose-related.
Similar to fentanyl, heroin altered ventilation in a dose- and time-related manner although the duration of action of heroin was longer compared with fentanyl. The smallest dose (0.1 mg/kg) of heroin (diamonds) had no effect on ventilation (upper, middle, and lower center panels, Fig. 1), whereas 0.32 mg/kg (triangles) significantly increased f to 189% of control from 5 to 25 minutes. A dose of 0.56 mg/kg heroin (inverted triangles) initially decreased f to 73% of control at the 5-minute time point before increasing f to 175% of control for 25 to 40 minutes. A dose of 1.0 mg/kg heroin (circles) had a more sustained decrease in f, compared with smaller doses; the subsequent increase in f was similar to smaller doses although occurring later after drug administration. The upper center panel of Fig. 1 clearly shows the dose- and time-related decrease in f by increasing doses of heroin. The largest dose of heroin, 3.2 mg/kg (squares), decreased f for 5 to 25 minutes and did not increase f during the 60-minute session. In contrast to dose-related decreases followed by increases in f, heroin only decreased VT (middle center panel, Fig. 1). A dose of 0.32 mg/kg heroin (triangles) significantly decreased VT to 76% of control for 10 to 20 minutes whereas 0.56 mg/kg (inverted triangles) decreased VT to 79% of control for 25 to 35 minutes. A slightly different patten of effect was obtained with 1.0 mg/kg heroin (circles) on VT, with an initial decrease to 54% of control from 5 to 15 minutes followed by a slight recovery then a second significant decrease (the second coinciding with a significant increase in f; compare circles in upper and middle center panels, Fig. 1). A dose of 3.2 mg/kg heroin (squares) significantly decreased VT to a maximum of 55% of control for 5 to 35 minutes. The time-effect functions for heroin on VE were more similar to the effects of heroin on f, compared with effects on VT (lower center panel, Fig. 1). A dose of 0.32 mg/kg heroin (triangles) significantly increased VE to a maximum of 150% of control for 5 to 25 minutes. VE was initially decreased then increased in a dose- and time-related manner across the three larger doses of heroin. The magnitude and duration of increases in VE were relatively similar across the three larger doses of heroin.
d-Methamphetamine significantly altered ventilation (right panels, Fig. 1), although the time- and dose-effect functions for d-methamphetamine were qualitatively different from those of fentanyl and heroin. For example, the effects of d-methamphetamine were monophasic and longer-lasting, compared with the biphasic and relatively shorter duration of effects observed with fentanyl and heroin (Fig. 1). The smallest dose (0.1 mg/kg) of d-methamphetamine (diamonds) had no significant effect on ventilation while 0.178 mg/kg (triangles) significantly increased f to 154% of control for 10 to 20 minutes; 0.32 mg/kg (inverted triangles) increased f up to 175% of control for the entire 60-minute test period (upper right panel, Fig. 1). Doses of 1.0 and 3.2 mg/kg d-methamphetamine (circles and squares, respectively) further increased f to 315 and 409% of control, respectively. In marked contrast to increases in f, d-methamphetamine decreased VT in a dose-related manner (middle right panel, Fig. 1). The two smallest doses of d-methamphetamine (0.1 and 0.178 mg/kg, diamonds and triangles, respectively) had no effect on VT, whereas 0.32 mg/kg (inverted triangles) significantly decreased VT to a maximum of 77% of control at 5, 40, 45, and 50 minutes. Further decreases in VT were obtained with 1.0 and 3.2 mg/kg d-methamphetamine (circles and squares, respectively), to a maximum of 57% and 62% of control, respectively. Significant increases in f and significant, although more modest, decreases in VT, resulted in overall marked increases in VE (lower right panel, Fig. 1). The smallest dose (0.1 mg/kg) of d-methamphetamine (diamonds) had no significant effect, whereas 0.178 mg/kg d-methamphetamine (triangles) increased VE to 135% of control for 10 to 20 minutes. Similar to 0.178 mg/kg, 0.32 mg/kg d-methamphetamine (inverted triangles) increased VE to 138% of control for 5 to 20 minutes. Further increases in VE were obtained with 1.0 and 3.2 mg/kg d-methamphetamine (circles and squares, respectively), to a maximum of 204% and 245% of control, respectively.
The right panels in Supplemental Fig. 2 show the effects of naloxone (0.0001–0.1 mg/kg) on ventilation. Across a 1000-fold dose range, naloxone did not significantly alter ventilation (Supplemental Table 2).
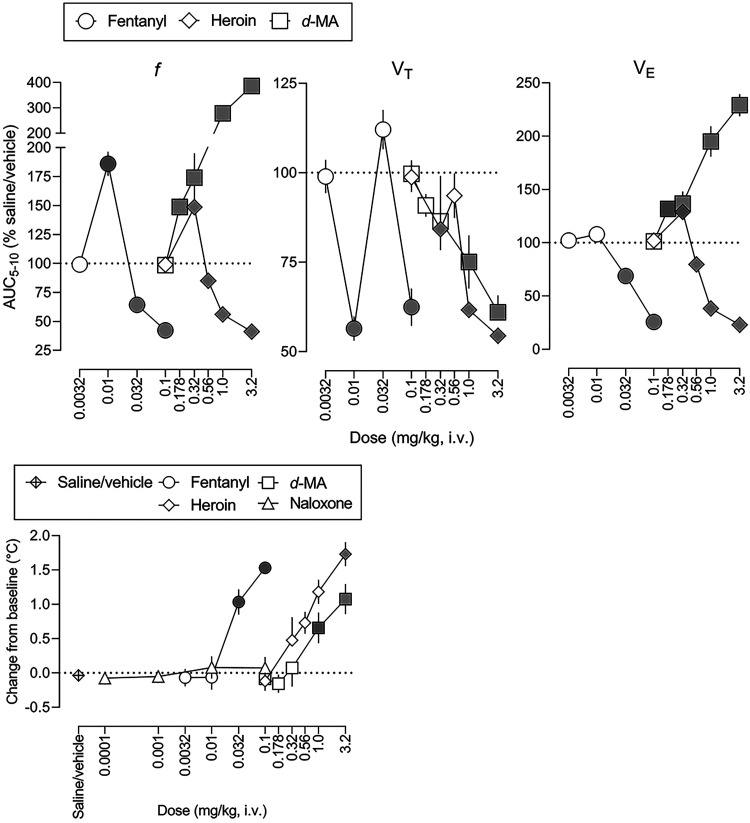
The three upper panels in Fig. 2 show dose-effect functions for fentanyl alone, heroin alone, and d-methamphetamine alone on ventilation, plotted as the AUC for minutes 5 to 10 and expressed as a percentage of the saline/vehicle control condition (calculated for individual subjects then averaged among 8 subjects). Table 2 and Supplemental Table 3 summarize results of statistical analyses of the dose-effect functions in Fig. 2. Fentanyl significantly (denoted by filled symbols) increased f to 186% of control at a dose of 0.01 mg/kg and significantly decreased f to 42% of control at a dose of 0.1 mg/kg (circles, leftmost panel, Fig. 2; ED50 value = 0.0840 mg/kg, Table 2). Similarly, heroin significantly increased f to 149% of control at a dose of 0.32 mg/kg and significantly decreased f to 41% of control at a dose of 3.2 mg/kg (diamonds, upper center panel, Fig. 2; ED50 value = 2.51 mg/kg). In contrast, d-methamphetamine only increased f, to a maximum of 387% of control at a dose of 3.2 mg/kg (squares, leftmost panel, Fig. 2). Fentanyl significantly decreased VT to 56% of and 62% of control at 0.01 and 0.1 mg/kg, respectively (circles, center panel, Fig. 2), heroin significantly decreased VT at doses of 0.32, 1.0, and 3.2 mg/kg, to a maximum of 54% of control, and d-methamphetamine significantly decreased VT to a maximum of 61% of control at a dose of 3.2 mg/kg (center panel, Fig. 2). Fentanyl significantly decreased VE up to a maximum of 26% of control at 0.1 mg/kg (right panel, Fig. 2; ED50 value = 0.0679 mg/kg, Table 2) and heroin significantly decreased VE to a maximum of 23% of control at 3.2 mg/kg (ED50 value = 1.90 mg/kg), whereas d-methamphetamine increased VE up to a maximum of 229% of control at 3.2 mg/kg. There was no significant difference in slope among the fentanyl, heroin, and d-methamphetamine dose-effect functions, with fentanyl being 30-fold more potent than heroin to decrease f and VE.
Fig. 2.
Dose-effect functions (from time-effect functions shown in Fig. 1) for the effects of fentanyl, heroin, and d-methamphetamine on ventilation (f, left; VT, center; and VE, right) and on rectal body temperature (lower panel). Abscissae: dose in mg/kg body weight (i.v.). Ordinates: three left panels, area under the curve (AUC) from 5 to 10 minutes after drug administration expressed as a percent of control (saline/vehicle; horizontal dashed lines) AUC; lower panel, change from baseline for rectal temperature in degrees C. Symbols represent the mean ± 1 S.E.M. for eight rats. Gray symbols represent a significant difference from saline/vehicle values (see Table 2; Supplemental Tables 3 and 4 for details of statistical analyses).
TABLE 2.
ED50 and slope values (95% CI) for the effects of fentanyl alone and heroin alone on ventilation (calculated from data shown in Fig. 2)
| Compound | f | VT | VE | |
|---|---|---|---|---|
| fentanyl alone | ED50 | 0.0840 (0.0676, 0.113) | Not determined (no less than 56.9% of control) | 0.0679 (0.0616, 0.0752) |
| Slope | −144 (-175, −113) | −82.4 (−90.5, −74.3) | ||
| heroin alone | ED50 | 2.51 (2.02, 3.38) | Not determined (no less than 54.5% of control) | 1.90 (1.51, 2.44) |
| Slope | −99.3 (−120, −78.9) | −103 (−122, −83.0) | ||
TABLE 3.
ED150 and slope values (95% CI) for the effects on ventilation of d-methamphetamine administered alone and in mixtures with fentanyl or heroin (calculated from data shown in Fig. 4)
Potency and slope ratios represent the values determined with mixture divided by values determined with d-methamphetamine alone.
| Compound | f | VE | |
|---|---|---|---|
| d-methamphetamine alone | ED150 | 0.189 (0.148, 0.455) | 0.793 (0.467, 1.10) |
| Slope | 180 (150, 211) | 81.0 (65.5, 96.5) | |
| d-methamphetamine + 0.1 mg/kg fentanyl | ED150 | 0.698 (0.387, 0.964) | 2.10 (1.79, 2.50) |
| ED150 ratio | 3.69 (0.851, 6.51) | 2.65 (1.63, 5.35) | |
| Slope | 181 (158, 204) | 105 (92.6, 118) | |
| Slope ratio | 1.01 (0.749, 1.36) | 1.30 (0.960, 1.80) | |
| d-methamphetamine + 3.2 mg/kg heroin | ED150 | 0.770 (0.477, 1.03) | 2.21 (1.91, 2.60) |
| ED150 ratio | 4.07 (1.05, 6.96) | 2.79 (1.74, 5.53) | |
| Slope | 178 (159, 197) | 103 (92.8, 114) | |
| Slope ratio | 0.989 (0.754, 1.31) | 1.27 (0.962, 1.74) | |
The lower panel in Fig. 2 shows the effects of fentanyl alone, heroin alone, and d-methamphetamine alone on rectal temperature (Supplemental Table 4 summarizes results of statistical analyses of the dose-effect functions). There was no significant change in rectal temperature in saline/vehicle control sessions (diamonds with cross-hatch) or sessions preceded by an infusion of naloxone (triangles). In contrast, fentanyl, heroin, and d-methamphetamine significantly increased rectal temperature up to 1.5, 1.7, and 1.1°C, respectively.
Effects of d-Methamphetamine on Fentanyl and Heroin
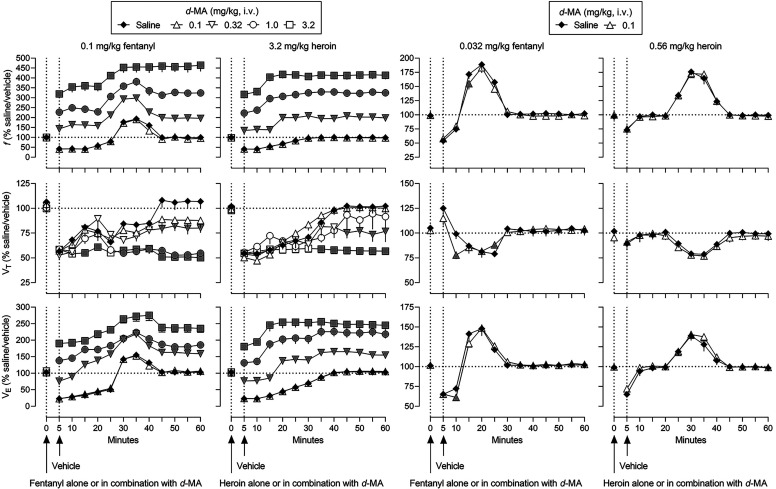
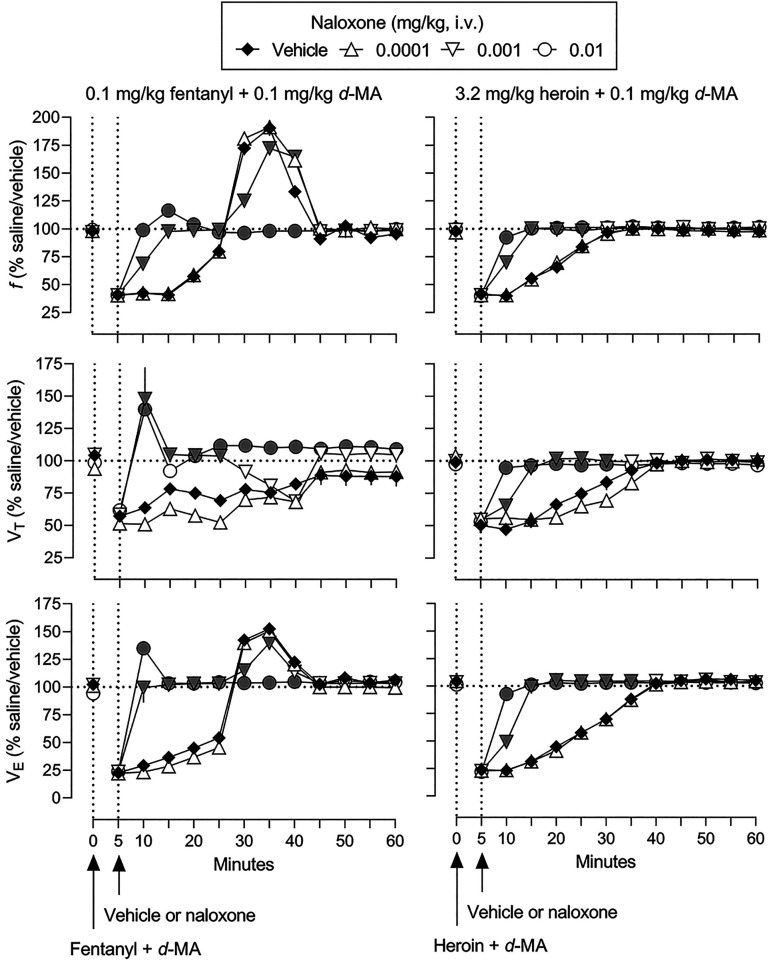
The effects of mixtures of d-methamphetamine with fentanyl or heroin on ventilation are shown in Fig. 3 (gray symbols represent significant differences from saline/vehicle; Supplemental Table 5 summarizes results of statistical analyses of the time-effect functions). First, the most effective doses of fentanyl (0.1 mg/kg) and heroin (3.2 mg/kg) were combined with different doses of d-methamphetamine (two leftmost panels, Fig. 3). The smallest dose of d-methamphetamine (0.1 mg/kg) did not affect the time-effect functions of fentanyl or heroin on any ventilatory parameter. d-Methamphetamine dose-dependently and significantly shifted upward the time-effect function of fentanyl on f, to a maximum of 465% of control (top leftmost panel, Fig. 3). In contrast, d-methamphetamine with fentanyl dose-dependently and significantly further decreased VT (middle leftmost panel, Fig. 3). Mixtures of d-methamphetamine and fentanyl increased VE, compared with fentanyl alone, to a maximum of 275% of control (bottom leftmost panel, Fig. 3). Combining d-methamphetamine with heroin resulted in a similar pattern of effects with f and VE significantly increased and VT significantly decreased, compared with heroin alone (second leftmost panels, Fig. 3). Mixtures of d-methamphetamine and heroin resulted in maximum increases in f to 418% of control (top second leftmost panel), in VE to 255% of control (middle second leftmost panel), and a maximum decrease in VT to 55% of control (bottom leftmost panel). The temporal pattern of effect on ventilation by 0.1 mg/kg fentanyl and 3.2 mg/kg heroin (i.e., increases in f and VE) was not markedly changed by combing each opioid receptor agonist with d-methamphetamine (top and bottom leftmost and second leftmost panels, respectively, Fig. 3).
Fig. 3.
Ventilatory effects of 0.1 mg/kg fentanyl (leftmost), 3.2 mg/kg heroin (second leftmost), 0.032 mg/kg fentanyl (third leftmost), and 0.56 mg/kg heroin (rightmost) alone and in mixtures with various doses of d-methamphetamine. Effects of fentanyl and heroin after vehicle (solid diamonds) are replotted from Fig. 1. See Fig. 1 for other details (see Supplemental Table 5 for details of statistical analyses).
d-Methamphetamine was also combined with smaller, less effective doses of fentanyl (0.032 mg/kg) and heroin (0.56 mg/kg) to ascertain whether the nature of interaction was related to the dose of opioid receptor agonist (two right panels, Fig. 3). With the exceptions of the 10-minute time point when f was modestly but significantly decreased from 171 to 155% of control, the 10-minute time point when VT was modestly decreased from 99 to 78% of control, the 25-minute time point when VT was modestly increased from 79 to 88% of control, and the 10-minute time point when VE decreased from 72 to 61% of control, the effects of mixtures of 0.1 mg/kg d-methamphetamine and 0.032 mg/kg fentanyl were not different from the effects of that dose of fentanyl alone (third leftmost panels, Fig. 3). The effects of mixtures of 0.1 mg/kg d-methamphetamine and 0.56 mg/kg heroin also were not different from the effects of that dose of heroin alone (third rightmost panels, Fig. 3).
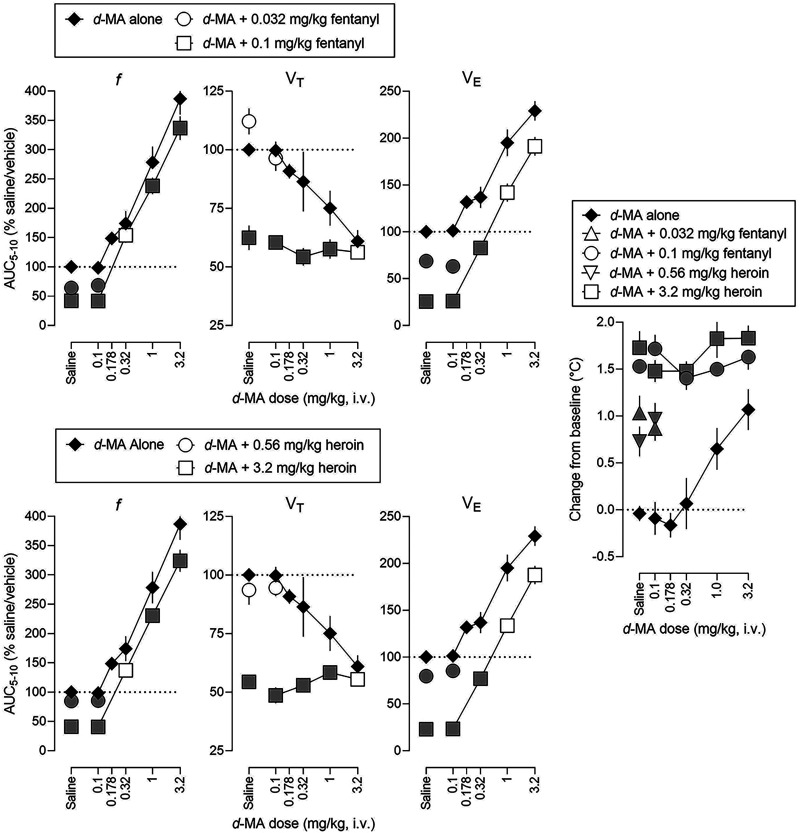
The leftmost six panels in Fig. 4 represent dose-effect functions (AUC for 5–10 minutes) for the effects on ventilation of mixtures of d-methamphetamine and fentanyl or heroin (Table 3 and Supplemental Table 6 summarize results of statistical analyses of the time-effect functions). Based on comparisons of ED150 and slope values (Table 3), dose-effect functions for f and VE were not different between d-methamphetamine alone and d-methamphetamine in combination with 0.1 mg/kg fentanyl (upper panels) or 3.2 mg/kg heroin (lower panels).
Fig. 4.
Dose-effect functions for ventilatory effects of d-methamphetamine alone and in mixtures with fentanyl (top, three left panels) and heroin (bottom, three left panels) and for hyperthermic effects of d-methamphetamine alone and in mixtures with fentanyl and heroin (rightmost panel). Effects of fentanyl alone (symbols above Saline), heroin alone (symbols above Saline), and d-methamphetamine (solid diamonds) after vehicle are replotted from Fig. 2. See Figs. 2 and 3 for other details (see Table 3; Supplemental Table 6 for details of statistical analyses).
The rightmost panel in Fig. 4 shows the effects on rectal temperature of d-methamphetamine alone and in mixtures with fentanyl or heroin (Supplemental Table 6 summarizes results of statistical analyses of the time effect functions). Increases in body temperature by fentanyl or heroin alone (data points above “Saline”, Fig. 4) were not significantly altered by d-methamphetamine.
Naloxone Reversal of Drug Effects
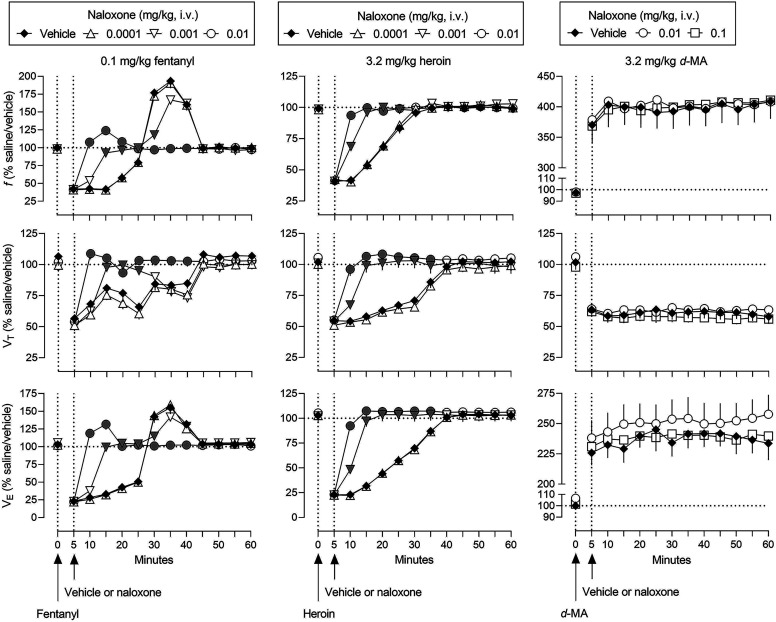
The effects of naloxone on the ventilatory effects of 0.1 mg/kg fentanyl, 3.2 mg/kg heroin, and 3.2 mg/kg d-methamphetamine are shown in Fig. 5 (gray symbols represent significant differences from solid diamonds; Supplemental Table 7 summarizes results of statistical analyses of the time effect functions). Naloxone reversed the ventilatory depressant effects of fentanyl and heroin in a dose- and time-related manner. The smallest dose of naloxone studied (0.0001 mg/kg, triangles) had no effect, whereas a 10-fold larger dose (0.001 mg/kg, inverted triangles) fully reversed the effects of fentanyl and heroin on f, VT, and VE within 15 minutes (left and middle panels, respectively, Fig. 5). A 10-fold larger dose of naloxone (0.01 mg/kg, circles) fully reversed the ventilatory depressant effects of fentanyl and heroin within 10 minutes. Up to a dose (0.1 mg/kg) 10-fold larger than the dose that fully reversed the ventilatory effects of opioid receptor agonists within 10 minutes, naloxone did not significantly alter the effects of d-methamphetamine on ventilation (squares, right panels, Fig. 5).
Fig. 5.
Ventilatory effects of naloxone administered 5 minutes after administration of 0.1 mg/kg fentanyl (left panels), 3.2 mg/kg heroin (center panels), or 3.2 mg/kg d-methamphetamine (right panels). Effects of fentanyl, heroin, and d-methamphetamine after vehicle (solid diamonds) are replotted from Fig. 1. See Figs. 1 and 3 for other details (see Supplemental Table 7 for details of statistical analyses).
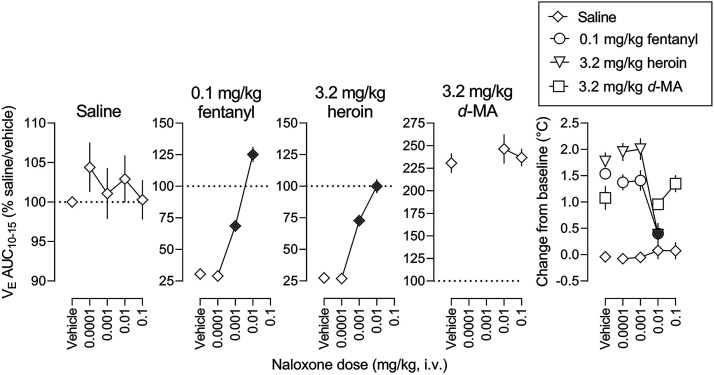
The four leftmost panels in Fig. 6 show dose-effect functions for naloxone alone and in combination with 0.1 mg/kg fentanyl, 3.2 mg/kg heroin, and 3.2 mg/kg d-methamphetamine. The AUC values in each panel are expressed as a percentage of values obtained under saline/vehicle conditions for 10 to 15 minutes (gray symbols represent significant differences from vehicle [no naloxone]; Table 4 and Supplemental Table 8 summarize results of statistical analyses of the dose-effect functions). Naloxone alone had no significant effect on VE (leftmost panel, Fig. 6). However, naloxone dose-dependently reversed decreases in VE by 0.1 mg/kg fentanyl and 3.2 mg/kg heroin (second and third leftmost panels, respectively, Fig. 6). ED80 values were not significantly different for naloxone to reverse the effects of fentanyl or the effects of heroin. In contrast, up to a dose of 0.1 mg/kg, naloxone did not significantly alter the effects of d-methamphetamine on ventilation (second rightmost panel, Fig. 6).
Fig. 6.
Dose-effect functions for the effects of naloxone on ventilation (from time-effect functions shown in Fig. 5) and rectal temperature when administered 5 minutes after administration of saline, 0.1 mg/kg fentanyl, 3.2 mg/kg heroin, or 3.2 mg/kg d-methamphetamine. See Fig. 2 for other details (see Table 4 and Supplemental Table 8 for details of statistical analyses).
TABLE 4.
ED80 and slope values (95% CI) for reversal by naloxone of the ventilatory effects of 0.1 mg/kg fentanyl alone, 0.1 mg/kg fentanyl with 0.1 mg/kg d-methamphetamine, 3.2 mg/kg heroin alone, and 3.2 mg/kg heroin with 0.1 mg/kg d-methamphetamine (calculated from data shown in Fig. 8)
Potency and slope ratios represent the values determined with mixtures of d-methamphetamine with fentanyl or heroin divided by values determined with fentanyl or heroin alone, respectively.
| Compound | VE | |
|---|---|---|
| naloxone versus 0.1 mg/kg fentanyl alone | ED80 | 4.39 (3.49, 5.34) |
| Slope | 8.30 (6.67, 9.94) | |
| naloxone versus 0.1 mg/kg fentanyl + 0.1 mg/kg d-methamphetamine | ED80 | 3.39 (0.859, 5.75) |
| ED80 ratio | 0.772 (0.161, 1.65) | |
| Slope | 6.43 (3.40, 9.46) | |
| Slope ratio | 0.775 (0.342, 1.42) | |
| naloxone versus 3.2 mg/kg heroin alone | ED80 | 6.10 (4.51, 8.34) |
| Slope | 5.61 (3.69, 7.54) | |
| naloxone versus 3.2 mg/kg heroin + 0.1 mg/kg d-methamphetamine | ED80 | 6.33 (4.68, 8.76) |
| ED80 ratio | 1.04 (0.561, 1.94) | |
| Slope | 5.22 (3.37, 7.07) | |
| Slope ratio | 0.930 (0.447, 1.92) | |
The rightmost panel in Fig. 6 shows effects on rectal body temperature of naloxone alone and with 0.1 mg/kg fentanyl, 3.2 mg/kg heroin, and 3.2 mg/kg d-methamphetamine (gray symbols represent significant differences from vehicle [no naloxone]; Supplemental Table 8 summarizes results of statistical analyses of the dose-effect functions). Whereas naloxone alone did not significantly affect rectal temperature, fentanyl, heroin, and d-methamphetamine significantly increased rectal temperature (data points above “Vehicle”). Naloxone reversed fentanyl- (circles) and heroin- (inverted triangles) induced hyperthermia but not d-methamphetamine-induced hyperthermia (squares).
Naloxone was also studied for its ability to reverse the ventilatory and hyperthermic effects of mixtures of heron, morphine, and d-methamphetamine. Fig. 7 shows time-effect functions for vehicle and naloxone administered 5 minutes after infusion of a mixture of 0.1 mg/kg d-methamphetamine and either 0.1 mg/kg fentanyl or 3.2 mg/kg heroin (gray symbols represent significant differences from solid diamonds; Supplemental Table 9 summarizes results of statistical analyses of the time-effect functions). Naloxone reversed in a dose- and time-related manner the ventilatory depressant effects of mixtures of d-methamphetamine and either fentanyl or heroin. The smallest dose of naloxone (0.0001 mg/kg, upward triangles) had no effect on the effect of drug mixtures whereas 10-fold and 100-fold larger doses of naloxone (0.001 and 0.01 mg/kg, inverted triangles and circles, respectively) fully reversed the ventilatory depressant effects of drug mixtures. Dose-effect functions for naloxone reversal of the effects of drug mixtures on ventilation and rectal temperature are shown in Fig. 8 (AUC values in each panel are expressed as a percentage of the saline/vehicle for minutes 10 to 15; gray symbols represent significant differences from 0.1 mg/kg fentanyl alone; Table 4 and Supplemental Table 10 summarize results of statistical analyses of the dose-effect functions). With the exception of VE for the mixture of d-methamphetamine and fentanyl, reversal by naloxone was not different between drug mixtures and each opioid receptor agonist alone (Supplemental Table 10). Analyses of ED80 and slope values revealed no significant difference in the ED80 or slope ratio for the dose-effect function for naloxone to reverse the effects of fentanyl or heroin (Table 4).
Fig. 7.
Ventilatory effects of naloxone administered 5 minutes after administration of either 0.1 mg/kg fentanyl combined with 0.1 mg/kg d-methamphetamine (left panels) or 3.2 mg/kg heroin combined with 0.1 mg/kg d-methamphetamine (right panels). See Figs. 1 and 3 for other details (see Supplemental Table 9 for details of statistical analyses).
Fig. 8.
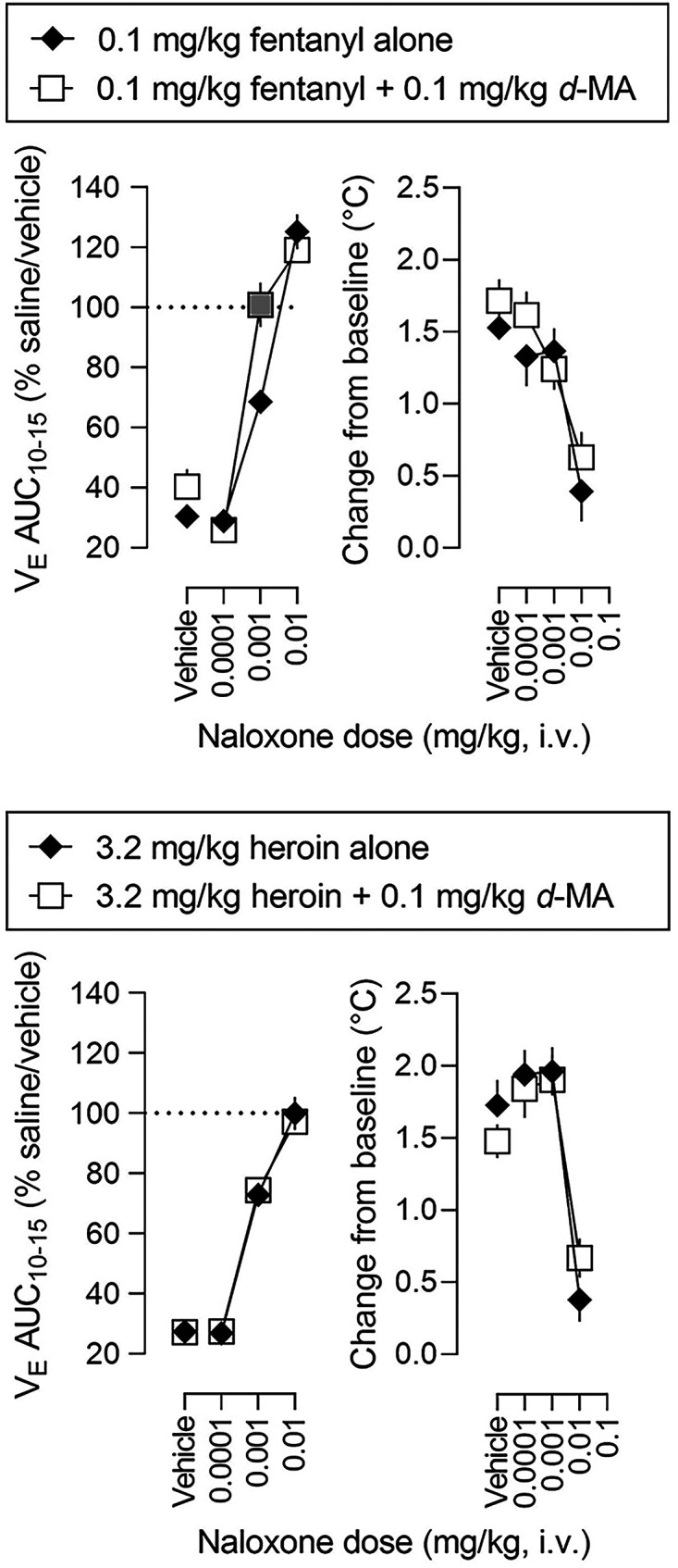
Dose-effect functions for the effects of naloxone on ventilation (left panels, from time-effect functions shown in Fig. 7) and on rectal temperature (right panels) when administered alone and when administered 5 minutes after administration of 0.1 mg/kg fentanyl alone (upper panels, replotted from Fig. 6), 0.1 mg/kg fentanyl combined with 0.1 mg/kg d-methamphetamine (upper panels), 3.2 mg/kg heroin alone (lower panels, replotted from Fig. 6), or 3.2 mg/kg heroin combined with 0.1 mg/kg d-methamphetamine (lower panels). See Figs. 2 and 4 for other details (see Table 4 and Supplemental Table 10 for details of statistical analyses).
Discussion
The major findings of this study are the following. First, the pattern of effects on ventilation of d-methamphetamine alone was qualitatively different from the pattern of effects of heroin and fentanyl alone. Whereas d-methamphetamine increased f and decreased VT, resulting in an overall increase in VE (the product of f and VT), fentanyl and heroin decreased f, VT, and VE. Second, d-methamphetamine attenuated decreases in f by fentanyl or heroin, resulting in an overall attenuation of the effects of each opioid receptor agonist on VE. Third, d-methamphetamine did not affect the potency of naloxone to reverse the ventilatory depressant effects of fentanyl or heroin. Fourth, d-methamphetamine did not alter hyperthermic effects of fentanyl or heroin. Fifth, d-methamphetamine did not affect the potency of naloxone to reverse opioid agonist-induced hyperthermia. These results suggest that d-methamphetamine, under normal air conditions, can attenuate the ventilatory depressant effects of opioid receptor agonists acting at MOR without altering the potency of naloxone to reverse ventilatory depression.
In the present study, d-methamphetamine increased f and decreased VT, resulting in an overall increase in VE. This pattern of hyperventilation is consistent with previous studies with stimulant drugs in humans and other species. For example, in human subjects d-methamphetamine and d-amphetamine (0.11–0.43 mg/kg, subcutaneously) increased f compared with placebo (Martin et al., 1971) and d-amphetamine (3 mg/kg, subcutaneously) also increased f in rats (Schierok et al., 2000). However, the generally consistent monotonic effects of stimulants on ventilation under normal air conditions is not always observed under hypercapnic (5% CO2) conditions in mice. For example, both (±)-methamphetamine and d-amphetamine (1.0–10 mg/kg, subcutaneously) had bi-phasic dose- and time- dependent effects on VE (Elder et al., 2023). The smallest dose of (±)-methamphetamine (1.0 mg/kg) modestly but significantly decreased VE by 10% to 15% of control for 10 to 30 minutes, whereas an intermediate dose of (±)-methamphetamine (3.0 mg/kg) did not significantly change VE. However, the largest dose of (±)-methamphetamine (10 mg/kg) significantly increased VE to approximately 150% of control for 15 to 60 minutes. Similarly, two smaller doses of d-amphetamine (1.0 and 3.0 mg/kg) modestly but significantly decreased VE by 10% to 20% of control for 10 to 25 minutes, whereas the largest dose of d-amphetamine (10 mg/kg) significantly increased VE to 150% of control for 15 to 60 minutes. The different patterns and time-effect functions of stimulant drugs on ventilation in the present study (monophasic increases for d-methamphetamine under the normal air conditions) and a previous study (bi-phasic changes for (±)-methamphetamine and d-amphetamine under hypercapnic conditions; Elder et al., 2023) might be related to the use of different CO2 levels in the two studies (normal air in the current study and hypercapnic in the previous study); however, d-amphetamine (0.046–0.215 mg/kg, i.v.) produced only monophasic increases in VE relative to placebo in humans even under hypercapnic conditions (3–7% CO2; Bourke et al., 1983). Another difference between the current study using rats and the previous study using mice, is that the experiments were conducted in the light cycle in the current study and in the dark cycle in the previous study (Elder et al., 2023). The combined impact of hypercapnic conditions and experiments being conducted during the dark cycle might have increased ventilation thereby increasing the likelihood of observing bi-phasic effects of (±)-methamphetamine and d-amphetamine on VE. Moreover, different housing conditions of subjects (group housed mice in the prior study and single housed rats in the current study) might have contributed to the different patterns of the effects of stimulant drugs on ventilation. For example, (±)-amphetamine is 12-fold more potent in group housed mice (five mice per cage; LD50 value: 21 mg/kg, orally) compared with single housed mice (LD50 value: 248 mg/kg; (Fanelli, 1973)). It is not entirely clear what factors account for differences in the effects of stimulant drugs between the present study and a previous study (Elder et al., 2023).
In the present study, d-methamphetamine attenuated the effects of fentanyl and heroin on f, resulting in an overall attenuation of the ventilatory depressant effects (VE) of these opioid receptor agonists. This effect of d-methamphetamine on opioid agonist-induced hypoventilation is consistent with previous studies in humans and other species. For example, d-amphetamine antagonized morphine-induced hypoventilation under both normal air and hypercapnic conditions in humans (Bourke et al., 1983; Jasinski and Preston, 1986). Under normal air conditions in rats, d-amphetamine antagonized fentanyl-induced changes in ex vivo respiratory parameters (increases in partial pressure of CO2 and decreases in percent oxygen saturation; Moody et al., 2020). In contrast, a previous study using hypercapnic conditions in mice (Elder et al., 2023) reported that both (±)-methamphetamine and d-amphetamine (1.0 mg/kg) modestly but significantly exacerbated the depressant effects of fentanyl (0.3 mg/kg, subcutaneously) on VE by 10% to 25% of control for 15 to 60 minutes. Moreover, the largest dose of each stimulant drug (10 mg/kg) fully antagonized fentanyl-induced hypoventilation. It remains to be determined precisely what factors contribute to whether stimulant drugs enhance or attenuate the ventilatory effects of opioid agonists acting at MOR.
In the present study, d-methamphetamine did not alter the potency of naloxone to reverse heroin- or fentanyl-induced hypoventilation. That d-methamphetamine did not alter the potency of naloxone to reverse ventilatory depression (by a mixture of d-methamphetamine and fentanyl or heroin) is consistent with the lack of effect of naloxone in altering the ventilatory (stimulant) effects of d-methamphetamine alone. Together these results support the view that any effect of stimulant drugs in modifying the ventilatory effects of opioid agonists acting at MOR is not mediated by MOR (i.e., functional antagonism). Other studies corroborate that view; for example, the opioid receptor antagonist naltrexone does not antagonize the reinforcing effects of d-methamphetamine (Hiranita et al., 2013) or cocaine (Tanda et al., 2016). Moreover, the lack of effect of d-methamphetamine on naloxone reverse of opioid hypoventilation is consistent with distinct patterns of overdose caused by stimulants and opioids. For example, opioid overdose (White and Irvine, 1999) but not stimulant overdose (Buchanan and Brown, 1988) is characterized by hypoventilation. In the present study, naloxone was equipotent in reversing the ventilatory effects of fentanyl and heroin; however, naloxone was less potent to reverse the depressant effects of fentanyl on VE compared with the depressant effects of the morphinan agonist morphine on VE in mice under hypercapnic conditions (5% CO2) in the dark cycle (unspecified housing conditions; Hill et al., 2020). Differences in the ability of naloxone to reverse the effects of fentanyl between the current study and a prior study (Hill et al., 2020) might also be due to CO2 levels, light/dark cycle differences, use of different species (mice versus rats), and/or housing conditions. However, the magnitude of rightward shifts with 0.1 mg/kg naltrexone did not significantly differ across the dose-effect functions of fentanyl or heroin on % fentanyl-appropriate responding in rats discriminating 0.01 mg/kg fentanyl from saline (Flynn and France, 2021), a study conducted under normal air condition during the light cycle and singly housed condition. Thus, hypercapnia and group-housed conditions might create a condition experimentally distinct from a normal air condition that alters sensitivity to drugs.
d-Methamphetamine did not alter opioid receptor agonist-induced hyperthermia or the potency of naloxone to antagonize that hyperthermia. Moreover, naloxone had no effect on d-methamphetamine-induced hyperthermia. The lack of effect (increasing or decreasing) of d-methamphetamine on opioid-induced hyperthermia and the lack of effect of naloxone in altering d-methamphetamine-induced hyperthermia, suggest that distinct mechanisms underly hyperthermia produced by opioid receptor agonists and d-methamphetamine, consistent with other studies under normal air conditions in humans (Jasinski and Preston, 1986).
In the present study, fentanyl and heroin markedly, but safely, decreased VE to approximately 23.0% of control without any adverse effect. However, the possibility remains that d-methamphetamine might interact differently with still larger (e.g., otherwise lethal or apnea-inducing) doses of fentanyl or heroin. The ventilatory depressant and particularly the lethal effects of opioids can vary dramatically depending on dose, dosing parameters, and other experimental conditions and rats can survive even after extended periods (e.g., 60 seconds) of opioid agonist-induced apnea (e.g., following an i.v. infusion of 0.3 mg/kg fentanyl; Haouzi et al., 2020). The largest dose of heroin used in the present study was smaller than a reported LD50 value of heroin (21.8 mg/kg, Barai et al., 2009) whereas the largest dose of fentanyl used in the present study was similar to the smaller LD50 values reported for fentanyl (0.005–3.1 mg/kg, Niemegeers et al., 1976; Yadav et al., 2018; Miner et al., 2021). Thus, generalizations from the results of this study might be limited to mixtures of non-lethal doses of drugs. Given the continued high number of drug overdose deaths, increasingly involving opioids and other (often stimulant) drugs, studies on larger doses might be warranted to fully understand the nature of interactions for drugs that continue having a significant impact on public health. Moreover, measures of arterial blood gas would provide potentially important information on the physiologic impact of these drugs on respiration. A recent study suggested that male rats are less sensitive than females to ventilatory depressant effects of heroin (Marchette et al., 2023), whereas the present study used only male rats. Thus, additional studies using females are also warranted. In summary, the present results suggest that under normal air conditions, d-methamphetamine attenuates the ventilatory depressant effects of “moderate” doses of opioid agonists acting at MOR and does not alter the potency of naloxone to reverse opioid agonist-induced hypoventilation.
Acknowledgments
The authors thank Julia R. Taylor and Cindal C. Dominguez for administrative assistance.
Data Availability
The authors declare that all of the data supporting the findings of this study are available within the paper and its Supplemental Material. Any further details are available upon request from the corresponding author.
Abbreviations
- AUC
area under a curve
- CI
confidence interval
- CO2
carbon dioxide
- f
frequency
- MOR
µ-opioid receptor
- VE
minute volume
- VT
tidal volume
Authorship Contributions
Participated in research design: Hiranita, France.
Conducted experiments: Hiranita, Ho.
Performed data analysis: Hiranita.
Wrote or contributed to the writing of the manuscript: Hiranita, Ho, France.
Footnotes
This study was supported by National Institutes of Health National Institute on Drug Abuse [Grants UG3-DA048387, UG3-DA048387-S1, R01-DA048417, and R01-DA058018] (to C.P.F.) and [Grant R01-DA005018] (to T.H.) and the Welch Foundation [Grant AQ-0039] (to C.P.F).
The authors have no conflicts of interest to declare.
Part of these data were presented as follows:
Ho NP, Hiranita T, and France CP (2022) Effects of fentanyl alone and in combination with methamphetamine on ventilation and rectal temperature in rat. Florida Chapter American College of Physicians 2022; 2022 July 27 (virtual presentation).
Ho NP, Hiranita T, and France CP (2022) Effects of fentanyl alone and in combination with methamphetamine on ventilation and rectal temperature in rat. Florida Chapter American College of Physicians 2022; 2022 October 22; Ft. Lauderdale, FL.
Hiranita T, Ho NP, and France CP (2023) Comparison of the mu-opioid receptor antagonists methocinnamox (MCAM) and naloxone to reverse the ventilatory-depressant effects of fentanyl, heroin, and carfentanil in rats. 33rd Annual Texas Research Society on Alcoholism Scientific Meeting; 2023 February 17; College Station, TX.
Hiranita T, Ho NP, and France CP (2023) Comparison of methocinnamox (MCAM) and naloxone to reverse and prevent the ventilatory depressant effects of fentanyl, heroin, and carfentanil in rats. 15th Annual Behavior, Biology, and Chemistry Translational Research in Addiction meeting 2023; 2023 March 25–26; San Antonio, TX.
Hiranita T, Ho NP, and France CP (2023) Comparison of the mu-opioid receptor antagonists methocinnamox (MCAM) and naloxone to reverse the ventilatory depressant effects of fentanyl, heroin, carfentanil, and 3-methylfentanyl in male rats. 20th Annual Center for Biomedical Neuroscience Retreat 2023; 2023 May 12, 2023; San Antonio, TX.
Hiranita T, Ho NP, and France CP (2023) Comparison of the mu-opioid receptor antagonists methocinnamox (MCAM) and naloxone to reverse the ventilatory-depressant effects of fentanyl and heroin in male rats. Annual American Society for Pharmacology and Experimental Therapeutics (ASPET) 2023; 2023 May 18- 21; St. Louis, MO.
Hiranita T, Ho NP, and France CP (2023) Ventilatory effects of fentanyl, heroin, and d-methamphetamine, alone and in mixtures, in male rats. Annual College on Problems of Drug Dependence (CPDD) 2023; 2023 June 17–21; Denver, CO.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- AMA (2021) Opioid prescriptions decrease for 10th consecutive year.
- Barai SR, Suryawanshi SA, Pandey AK (2009) Responses of parathyroid gland, C cells, and plasma calcium and inorganic phosphate levels in rat to sub-lethal heroin administration. J Environ Biol 30(5, Suppl)917–922. [PubMed] [Google Scholar]
- Bouillon T, Bruhn J, Roepcke H, Hoeft A (2003) Opioid-induced respiratory depression is associated with increased tidal volume variability. Eur J Anaesthesiol 20:127–133. [DOI] [PubMed] [Google Scholar]
- Bourke DL, Allen PD, Rosenberg M, Mendes RW, Karabelas AN (1983) Dextroamphetamine with morphine: respiratory effects. J Clin Pharmacol 23:65–70. [DOI] [PubMed] [Google Scholar]
- Buchanan JF, Brown CR (1988) ‘Designer drugs’. A problem in clinical toxicology. Med Toxicol 3:1–17. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Maya S, Kahn JG (2022) Modeling of overdose and naloxone distribution in the setting of fentanyl compared to heroin. Drug Alcohol Depend 236:109478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S, Arustamyan M, Marmolejos G, Ramakrishna K (2020) Delayed cardiomyopathy and cardiogenic shock due to intravenous methamphetamine use requiring hemodynamic support with veno-arterial extracorporeal membrane oxygenation. J Am Coll Emerg Physicians Open 1:117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder HJ, Varshneya NB, Walentiny DM, Beardsley PM (2023) Amphetamines modulate fentanyl-depressed respiration in a bidirectional manner. Drug Alcohol Depend 243:109740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli O (1973) Pharmacological and toxicological study of a new psychotropic stimulant: the 2-phenyl-5-dimethyl-tetrahydro-1,4-oxazine, in comparison with d,l-amphetamine, phenmetrazine and pemoline-Mg. Arzneimittelforschung 23:810–816. [PubMed] [Google Scholar]
- Flynn SM, France CP (2021) Discriminative stimulus effects of carfentanil in rats discriminating fentanyl: Differential antagonism by naltrexone. Drug Alcohol Depend 221:108599. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Mellen N, McCann M, Sternick M, Guck D, Tubbs N (2020) Evidence for the emergence of an opioid-resistant respiratory rhythm following fentanyl overdose. Respir Physiol Neurobiol 277:103428. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, Spencer MR, Warner M (2021) Drug Overdose Deaths in the United States, 1999-2020. NCHS Data Brief (426):1–8. [PubMed] [Google Scholar]
- Hill R, Santhakumar R, Dewey W, Kelly E, Henderson G (2020) Fentanyl depression of respiration: Comparison with heroin and morphine. Br J Pharmacol 177:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL (2010) Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther 332:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL (2013) Stimulants as specific inducers of dopamine-independent σ agonist self-administration in rats. J Pharmacol Exp Ther 347:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoots B, Vivolo-Kantor A, Seth P (2020) The rise in non-fatal and fatal overdoses involving stimulants with and without opioids in the United States. Addiction 115:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR, Preston KL (1986) Evaluation of mixtures of morphine and d-amphetamine for subjective and physiological effects. Drug Alcohol Depend 17:1–13. [DOI] [PubMed] [Google Scholar]
- Jimenez VM Jr, Castaneda G, France CP (2021) Methocinnamox Reverses and Prevents Fentanyl-Induced Ventilatory Depression in Rats. J Pharmacol Exp Ther 377:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Einstein EB, Compton WM (2018) Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010–2016. JAMA 319:1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood TE, Huynh P, Richard A, Sightes E, Bailey K, Ray B, Lieberman M (2021) Community overdose surveillance: Comparing substances collected from the death scene investigation to toxicology results. Drug Alcohol Depend 224:108722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM (2003) Mu opioid receptors in rat ventral medulla: effects of endomorphin-1 on phrenic nerve activity. Respir Physiol Neurobiol 138:165–178. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 350:412–438. [DOI] [PubMed] [Google Scholar]
- Marchette RCN, Carlson ER, Frye EV, Hastings LE, Vendruscolo JCM, Mejias-Torres G, Lewis SJ, Hampson A, Volkow ND, Vendruscolo LF, et al. (2023) Heroin- and Fentanyl-Induced Respiratory Depression in a Rat Plethysmography Model: Potency, Tolerance, and Sex Differences. J Pharmacol Exp Ther 385:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR (1971) Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12:245–258. [DOI] [PubMed] [Google Scholar]
- Miner NB, Schutzer WE, Zarnegarnia Y, Janowsky A, Torralva R (2021) Fentanyl causes naloxone-resistant vocal cord closure: A platform for testing opioid overdose treatments. Drug Alcohol Depend 227:108974. [DOI] [PubMed] [Google Scholar]
- Moody OA, Zhang ER, Arora V, Kato R, Cotten JF, Solt K (2020) D-Amphetamine Accelerates Recovery of Consciousness and Respiratory Drive After High-Dose Fentanyl in Rats. Front Pharmacol 11:585356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemegeers CJ, Schellekens KH, Van Bever WF, Janssen PA (1976) Sufentanil, a very potent and extremely safe intravenous morphine-like compound in mice, rats and dogs. Arzneimittelforschung 26:1551–1556. [PubMed] [Google Scholar]
- Pattinson KT (2008) Opioids and the control of respiration. Br J Anaesth 100:747–758. [DOI] [PubMed] [Google Scholar]
- Savilampi J, Ahlstrand R, Magnuson A, Geijer H, Wattwil M (2014) Aspiration induced by remifentanil: a double-blind, randomized, crossover study in healthy volunteers. Anesthesiology 121:52–58. [DOI] [PubMed] [Google Scholar]
- Savilampi J, Ahlstrand R, Magnuson A, Wattwil M (2013) Effects of remifentanil on the esophagogastric junction and swallowing. Acta Anaesthesiol Scand 57:1002–1009. [DOI] [PubMed] [Google Scholar]
- Schierok H, Markert M, Pairet M, Guth B (2000) Continuous assessment of multiple vital physiological functions in conscious freely moving rats using telemetry and a plethysmography system. J Pharmacol Toxicol Methods 43:211–217. [DOI] [PubMed] [Google Scholar]
- Snedecor G, Cochran W (1967) Statistical methods. 1967, p 46, Iowa State College Press, Ames, Iowa. [Google Scholar]
- Solis E Jr, Cameron-Burr KT, Shaham Y, Kiyatkin EA (2017) Intravenous Heroin Induces Rapid Brain Hypoxia and Hyperglycemia that Precede Brain Metabolic Response. eNeuro 4:ENEURO.0151-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr, Cameron-Burr KT, Shaham Y, Kiyatkin EA (2018) Fentanyl-Induced Brain Hypoxia Triggers Brain Hyperglycemia and Biphasic Changes in Brain Temperature. Neuropsychopharmacology 43:810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague GL, Takermori AE (1978) Enhancement of morphine analgesia and brain levels by methamphetamine in mice. J Pharmacol Exp Ther 207:485–493. [PubMed] [Google Scholar]
- Stoeckel H, Schüttler J, Magnussen H, Hengstmann JH (1982) Plasma fentanyl concentrations and the occurrence of respiratory depression in volunteers. Br J Anaesth 54:1087–1095. [DOI] [PubMed] [Google Scholar]
- Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M, Katz JL (2016) Lack of Specific Involvement of (+)-Naloxone and (+)-Naltrexone on the Reinforcing and Neurochemical Effects of Cocaine and Opioids. Neuropsychopharmacology 41:2772–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Irvine RJ (1999) Mechanisms of fatal opioid overdose. Addiction 94:961–972. [PubMed] [Google Scholar]
- Yadav SK, Kumar D, Kumar P, Gupta PK, Bhattacharya R (2018) Biochemical, Oxidative, and Physiological Changes Caused by Acute Exposure of Fentanyl and Its 3 Analogs in Rodents. Int J Toxicol 37:28–37. [DOI] [PubMed] [Google Scholar]