Abstract
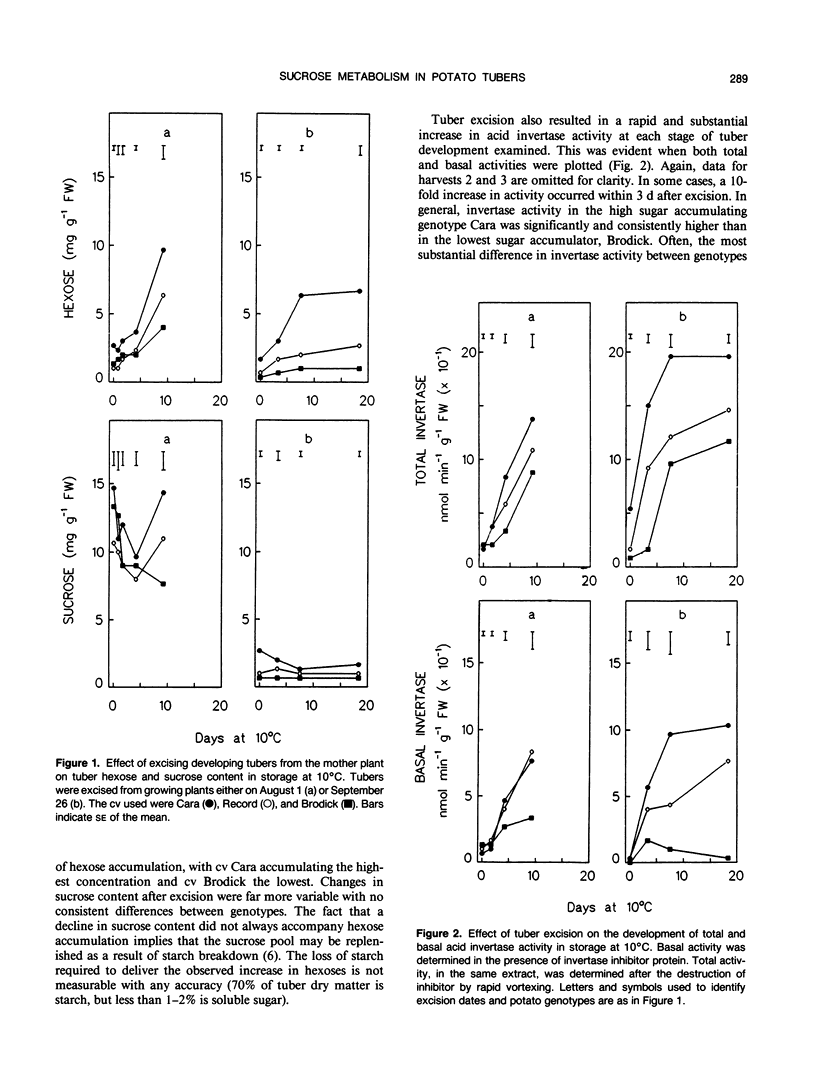
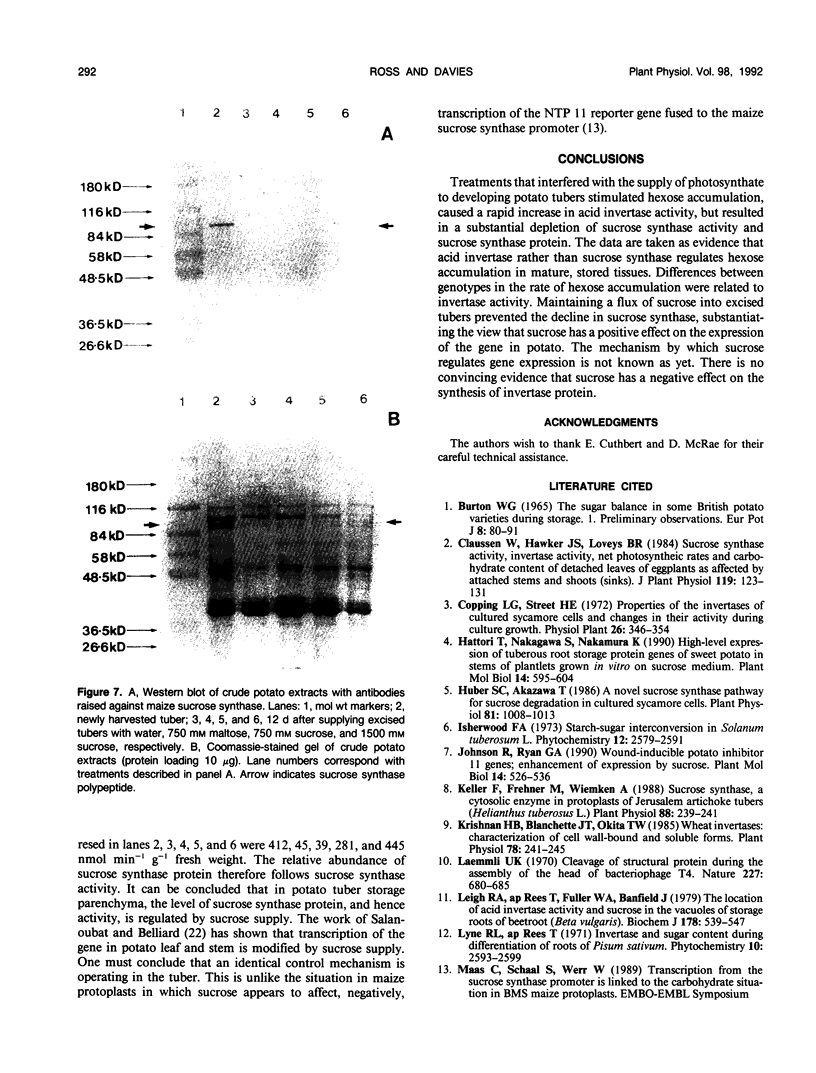
Excision of developing potato (Solanum tuberosum L.) tubers from the mother plant, followed by storage at 10°C, resulted in a rapid, substantial decrease in sucrose synthase activity and considerable increases in hexose content and acid invertase activity. A comparison of the response of three genotypes, known to accumulate different quantities of hexoses in storage, showed that both sucrose synthase activity and the extent to which activity declined following excision were similar in all cases. However, there was significant genotypic variation in the extent to which acid invertase activity developed, with tubers accumulating the highest hexose content also developing the highest extractable activity of invertase. Similar effects were found in nondetached tubers when growing plants were maintained in total darkness for a prolonged period. Furthermore, supplying sucrose to detached tubers through the cut stolon surface prevented the decline in sucrose synthase activity. Maltose proved to be ineffective. Western blots using antibodies raised against maize sucrose synthase showed that the decline in sucrose synthase activity was associated with the loss of protein rather than the effect of endogenous inhibitors. Although there were indications that maintaining a flux of sucrose into isolated tubers could prevent the increase in acid invertase activity, the results were not conclusive.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hattori T., Nakagawa S., Nakamura K. High-level expression of tuberous root storage protein genes of sweet potato in stems of plantlets grown in vitro on sucrose medium. Plant Mol Biol. 1990 Apr;14(4):595–604. doi: 10.1007/BF00027505. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986 Aug;81(4):1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Ryan C. A. Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol. 1990 Apr;14(4):527–536. doi: 10.1007/BF00027498. [DOI] [PubMed] [Google Scholar]
- Keller F., Frehner M., Wiemken A. Sucrose Synthase, a Cytosolic Enzyme in Protoplasts of Jerusalem Artichoke Tubers (Helianthus tuberosus L.). Plant Physiol. 1988 Oct;88(2):239–241. doi: 10.1104/pp.88.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Blanchette J. T., Okita T. W. Wheat invertases : characterization of cell wall-bound and soluble forms. Plant Physiol. 1985 Jun;78(2):241–245. doi: 10.1104/pp.78.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Rees T., Fuller W. A., Banfield J. The location of acid invertase activity and sucrose in the vacuoles of storage roots of beetroot (Beta vulgaris). Biochem J. 1979 Mar 15;178(3):539–547. doi: 10.1042/bj1780539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Röber B. T., Kossmann J., Hannah L. C., Willmitzer L., Sonnewald U. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet. 1990 Oct;224(1):136–146. doi: 10.1007/BF00259460. [DOI] [PubMed] [Google Scholar]
- Nomura T., Akazawa T. Enzymic mechanism of starch stynthesis in ripening rice grains. VII. Purification and enzymic properties of sucrose synthetase. Arch Biochem Biophys. 1973 Jun;156(2):644–652. doi: 10.1016/0003-9861(73)90316-0. [DOI] [PubMed] [Google Scholar]
- Pressey R. Potato sucrose synthetase: purification, properties, and changes in activity associated with maturation. Plant Physiol. 1969 May;44(5):759–764. doi: 10.1104/pp.44.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R., Shaw R. Effect of temperature on invertase, invertase inhibitor, and sugars in potato tubers. Plant Physiol. 1966 Dec;41(10):1657–1661. doi: 10.1104/pp.41.10.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanoubat M., Belliard G. The steady-state level of potato sucrose synthase mRNA is dependent on wounding, anaerobiosis and sucrose concentration. Gene. 1989 Dec 7;84(1):181–185. doi: 10.1016/0378-1119(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Sung S. J., Xu D. P., Black C. C. Identification of actively filling sucrose sinks. Plant Physiol. 1989 Apr;89(4):1117–1121. doi: 10.1104/pp.89.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzler H., Mignery G., Fisher L., Park W. Sucrose-regulated expression of a chimeric potato tuber gene in leaves of transgenic tobacco plants. Plant Mol Biol. 1989 Oct;13(4):347–354. doi: 10.1007/BF00015546. [DOI] [PubMed] [Google Scholar]
- Xu D. P., Sung S. J., Alvarez C. A., Black C. C. Pyrophosphate-dependent sucrose metabolism and its activation by fructose 2,6-bisphosphate in sucrose importing plant tissues. Biochem Biophys Res Commun. 1986 Dec 15;141(2):440–445. doi: 10.1016/s0006-291x(86)80192-9. [DOI] [PubMed] [Google Scholar]



