Abstract
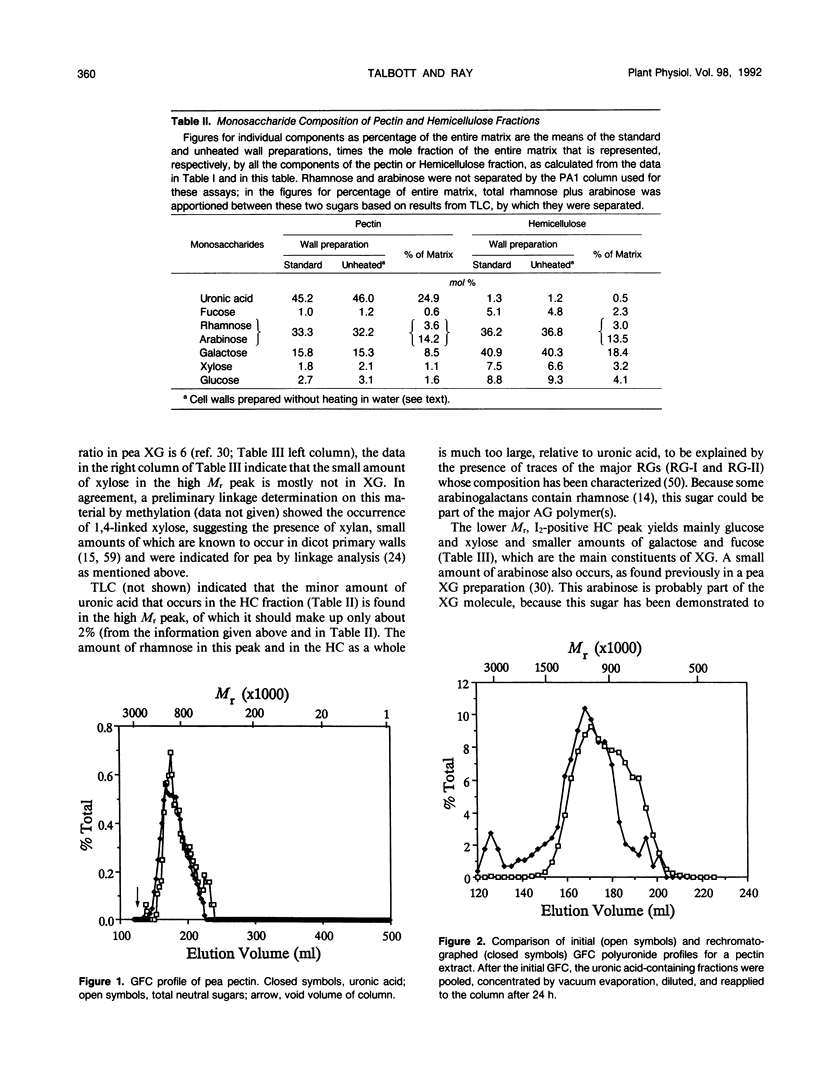
Relative molecular size distributions of pectic and hemicellulosic polysaccharides of pea (Pisum sativum cv Alaska) third internode primary walls were determined by gel filtration chromatography. Pectic polyuronides have a peak molecular mass of about 1100 kilodaltons, relative to dextran standards. This peak may be partly an aggregate of smaller molecular units, because demonstrable aggregation occurred when samples were concentrated by evaporation. About 86% of the neutral sugars (mostly arabinose and galactose) in the pectin cofractionate with polyuronide in gel filtration chromatography and diethylaminoethyl-cellulose chromatography and appear to be attached covalently to polyuronide chains, probably as constituents of rhamnogalacturonans. However, at least 60% of the wall's arabinan/galactan is not linked covalently to the bulk of its rhamnogalacturonan, either glycosidically or by ester links, but occurs in the hemicellulose fraction, accompanied by negligible uronic acid, and has a peak molecular mass of about 1000 kilodaltons. Xyloglucan, the other principal hemicellulosic polymer, has a peak molecular mass of about 30 kilodaltons (with a secondary, usually minor, peak of approximately 300 kilodaltons) and is mostly not linked glycosidically either to pectic polyuronides or to arabinogalactan. The relatively narrow molecular mass distributions of these polymers suggest mechanisms of co- or postsynthetic control of hemicellulose chain length by the cell. Although the macromolecular features of the mentioned polymers individually agree generally with those shown in the widely disseminated sycamore cell primary wall model, the matrix polymers seem to be associated mostly noncovalently rather than in the covalently interlinked meshwork postulated by that model. Xyloglucan and arabinan/galactan may form tightly and more loosely bound layers, respectively, around the cellulose microfibrils, the outer layer interacting with pectic rhamnogalacturonans that occupy interstices between the hemicellulose-coated microfibrils.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERSHEIM P., MUHLETHALER K., FREY-WYSSLING A. Stained pectin as seen in the electron microscope. J Biophys Biochem Cytol. 1960 Oct;8:501–506. doi: 10.1083/jcb.8.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBERSHEIM P., NEUKOM H. DEUEL H: Splitting of pectin chain molecules in neutral solutions. Arch Biochem Biophys. 1960 Sep;90:46–51. doi: 10.1016/0003-9861(60)90609-3. [DOI] [PubMed] [Google Scholar]
- Albersheim P. The walls of growing plant cells. Sci Am. 1975 Apr;232(4):80–95. doi: 10.1038/scientificamerican0475-80. [DOI] [PubMed] [Google Scholar]
- Chambat G., Barnoud F., Joseleau J. P. Structure of the Primary Cell Walls of Suspension-Cultured Rosa glauca Cells: I. Polysaccharides Associated with Cellulose. Plant Physiol. 1984 Mar;74(3):687–693. doi: 10.1104/pp.74.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill A. G., McNeil M., Albersheim P. Structure of Plant Cell Walls: VIII. A New Pectic Polysaccharide. Plant Physiol. 1978 Sep;62(3):418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill J. E., McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls: XI. GLUCURONOARABINOXYLAN, A SECOND HEMICELLULOSE IN THE PRIMARY CELL WALLS OF SUSPENSION-CULTURED SYCAMORE CELLS. Plant Physiol. 1980 Dec;66(6):1135–1139. doi: 10.1104/pp.66.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. L., Gross K. C., Gillespie D. T., Sondey S. M. Macromolecular components of tomato fruit pectin. Arch Biochem Biophys. 1989 Oct;274(1):179–191. doi: 10.1016/0003-9861(89)90429-3. [DOI] [PubMed] [Google Scholar]
- Galambos J. T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem. 1967 Apr;19(1):119–132. doi: 10.1016/0003-2697(67)90141-8. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Maclachlan G. Pea xyloglucan and cellulose : I. Macromolecular organization. Plant Physiol. 1984 Jul;75(3):596–604. doi: 10.1104/pp.75.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau J. P., Chambat G. Structure of the Primary Cell Walls of Suspension-Cultured Rosa glauca Cells: II. Multiple Forms of Xyloglucans. Plant Physiol. 1984 Mar;74(3):694–700. doi: 10.1104/pp.74.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Talmadge K. W., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: III. A Model of the Walls of Suspension-cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973 Jan;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis F. M. Glycosylated seryl residues in wall protein of elongating pea stems. Plant Physiol. 1976 Feb;57(2):224–226. doi: 10.1104/pp.57.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Turnover of cell wall polysaccharides in elongating pea stem segments. Plant Physiol. 1974 May;53(5):669–673. doi: 10.1104/pp.53.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Scocca J. R. A common structural unit in asparagine-oligosaccharides of several glycoproteins from different sources. J Biol Chem. 1972 Sep 25;247(18):5753–5758. [PubMed] [Google Scholar]
- Lin L. S., Yuen H. K., Varner J. E. Differential scanning calorimetry of plant cell walls. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2241–2243. doi: 10.1073/pnas.88.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx-Figini M., Schulz G. V. Uber die Kinetik und den Mechanismus der Biosynthese der Cellulose in den höheren Pflanzen (nach Versuchen an den Samenhaaren der Baumwolle) Biochim Biophys Acta. 1966 Jan 4;112(1):81–101. [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls: X. RHAMNOGALACTURONAN I, A STRUCTURALLY COMPLEX PECTIC POLYSACCHARIDE IN THE WALLS OF SUSPENSION-CULTURED SYCAMORE CELLS. Plant Physiol. 1980 Dec;66(6):1128–1134. doi: 10.1104/pp.66.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Moore P. J., Darvill A. G., Albersheim P., Staehelin L. A. Immunogold localization of xyloglucan and rhamnogalacturonan I in the cell walls of suspension-cultured sycamore cells. Plant Physiol. 1986 Nov;82(3):787–794. doi: 10.1104/pp.82.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. C., Vian B. Use of purified endopolygalacturonase for a topochemical study of elongating cell walls at the ultrastructural level. J Cell Sci. 1981 Apr;48:333–343. doi: 10.1242/jcs.48.1.333. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Goldberg I. D., Kacinski B. M., Buckholz T., Vinter D. W. Smooth muscle releases an epithelial cell scatter factor which binds to heparin. In Vitro Cell Dev Biol. 1989 Feb;25(2):163–173. doi: 10.1007/BF02626174. [DOI] [PubMed] [Google Scholar]
- Selvendran R. R. Developments in the chemistry and biochemistry of pectic and hemicellulosic polymers. J Cell Sci Suppl. 1985;2:51–88. doi: 10.1242/jcs.1985.supplement_2.4. [DOI] [PubMed] [Google Scholar]
- Stevenson T. T., McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls : XVIII. An Analysis of the Extracellular Polysaccharides of Suspension-Cultured Sycamore Cells. Plant Physiol. 1986 Apr;80(4):1012–1019. doi: 10.1104/pp.80.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott L. D., Ray P. M. Changes in molecular size of previously deposited and newly synthesized pea cell wall matrix polysaccharides : effects of auxin and turgor. Plant Physiol. 1992 Jan;98(1):369–379. doi: 10.1104/pp.98.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. E., Wallace J. C., Mackay A. L., Volke F. Use of chemical fractionation and proton nuclear magnetic resonance to probe the physical structure of the primary plant cell wall. Plant Physiol. 1990 Sep;94(1):174–178. doi: 10.1104/pp.94.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E., Lin L. S. Plant cell wall architecture. Cell. 1989 Jan 27;56(2):231–239. doi: 10.1016/0092-8674(89)90896-9. [DOI] [PubMed] [Google Scholar]
- WHISTLER R. L., BEMILLER J. N. Alkaline degradation of polysaccharides. Adv Carbohydr Chem. 1958;13:289–329. doi: 10.1016/s0096-5332(08)60359-8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Sakurai N., Kuraishi S. Sugar Composition and Molecular Weight Distribution of Cell Wall Polysaccharides in Outer and Inner Tissues from Segments of Dark Grown Squash (Cucurbita maxima Duch.) Hypocotyls. Plant Physiol. 1990 Jul;93(3):998–1004. doi: 10.1104/pp.93.3.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder B. M., Albersheim P. The Structure of Plant Cell Walls: IV. A Structural Comparison of the Wall Hemicellulose of Cell Suspension Cultures of Sycamore (Acer PseudoPlatAnus) and of Red Kidney Bean (Phaseolus Vulgaris). Plant Physiol. 1973 May;51(5):889–893. doi: 10.1104/pp.51.5.889. [DOI] [PMC free article] [PubMed] [Google Scholar]


