Abstract
Background
This study aimed to investigate the contralateral breast cancer (CBC) recurrence rate in Korean breast cancer patients according to their BRCA1/2 germline mutation status, focusing particularly on the CBC recurrence risk in BRCA1/2 negative (BRCAx) patients.
Methods
We conducted a retrospective study on 13,107 primary breast cancer patients. The patients were divided into high-risk and low-risk groups for hereditary breast cancer based on the Korean National Health Insurance Service’s eligibility criteria for BRCA1/2 germline mutation testing. The high-risk group was further categorized into the BRCA mutation group, the BRCAx group, and the not tested group. We evaluated the overall survival and cumulative risk of developing CBC in these patients.
Results
Among 4494 high-risk patients, 973 (21.7%) underwent genetic testing for BRCA1/2 germline mutation, revealing mutations in 158 patients (16.2%). We observed significant overall survival differences across all four groups, with the high-risk, not-tested group demonstrating notably worse overall survival (p < 0.001). However, when adjusted for other prognostic factors, there was no significant differences in hazard ratio of death between the four groups. The cumulative risk of CBC also varied among the groups. Patients with BRCA1/2 mutations showed a 7.3-fold increased risk of CBC compared to the low-risk group (95% CI 4.11–13.0, p < 0.001). Interestingly, BRCAx patients also demonstrated a significantly higher risk of CBC (HR 2.77, 95% CI 1.76–4.35, p < 0.001). The prognostic importance of the BRCAx for CBC recurrence persisted after adjusting for the age and subtype, but became insignificant when the family history of breast cancer was adjusted.
Conclusion
Breast cancer patients who are at high risk of hereditary breast cancer but with wild-type BRCA 1/2 genes (BRCAx) have increased risk of developing contralateral breast cancer when compared to the low-risk patients. More careful surveillance and follow-up can be offered to these patients especially when they have family history of breast cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01769-x.
Keywords: Breast cancer, Contralateral breast cancer, Overall survival, BRCA mutation, BRCAx
Background
BRCA1 and BRCA2, the two major genes regulating genome protection at various stages of the DNA damage response and DNA repair, are well-known breast and ovarian cancer-susceptibility genes [1–4]. While the retrospective studies have suggested that the cancer risk might vary between BRCA1 and BRCA2 mutation carriers [5], recent prospective studies have shown that the lifetime breast cancer risk is similar for both genes ranging from 55 to 72% [6–8]. For Korean breast cancer patients, the prevalence of BRCA1/2 mutation for patients with a family history of breast or ovarian cancer is 22.3% [9], and the cumulative risk of breast cancer is 72.1% for BRCA1 and 66.3% for BRCA2 mutation carriers [10].
Breast cancer patients with BRCA1/2 germline mutation carry increased risk of contralateral breast cancer (CBC) development [7, 11]. For Korean patients, a fivefold increase in CBC risk was observed for 132 triple negative breast cancer patients with BRCA1/2 germline mutation when compared to 868 BRCA1/2 negative patients [12]. As the incidence of breast cancer for Korean women is constantly rising [13] along the increased use of cancer-susceptibility genetic testing [14], it has become clinically important to assess the individual risk for CBC based on their genetic testing results.
In addition to the BRCA1/2 germline mutation carriers, recent studies suggest the presence of another clinically distinct group of hereditary breast cancer patients who are BRCA1/2 negative (BRCAx) [15, 16]. While prediction models suggest that the low-penetrance genetic loci which may explain a substantial portion of increased breast cancer risk associated with BRCAx [17], there is no data on the oncologic outcomes for Korean BRCAx breast cancer patients. In this study, we investigated the rate of CBC recurrence in Korean breast cancer patients according to the BRCA1/2 germline mutation status. Especially, we determined the relative risk of CBC recurrence in Korean BRCAx patients compared to the low-risk breast cancer patients.
Methods
Patients
This study was a retrospective study based on the data of the 13,107 patients with primary breast cancer who were treated at Seoul National University Hospital from January 2005 to December 2018 with curative intention. Patients diagnosed with DCIS, male breast cancer, or bilateral breast cancer, as well as those who underwent surgery for palliative purposes or had distant metastasis were excluded. These patients were divided into either the high-risk or low-risk group for hereditary breast cancer by the eligibility criteria for BRCA1/2 germline mutation testing set by the Korean National Health Insurance Service (KNHIS). KNHIS reimburses the BRCA1/2 testing when any of the following conditions are met: (1) one or more third-degree relative with breast cancer, ovary cancer, metastatic prostate cancer, and pancreas cancer, (2) age at diagnosis is under 40 years, (3) age at diagnosis is under 60 years with triple negative type breast cancer, (4) diagnosed with ovarian cancer.
The high-risk group was further classified into three groups; BRCA mutation group, BRCAx group, and not tested group. Patients in the BRCA mutation group were those who had tested for BRCA 1/2 germline mutation and had a pathogenic or likely pathogenic gene mutation. Patients in the BRCAx group were those who had a high risk of hereditary breast cancer but had tested negative for BRCA 1/2 mutation or had a variant of uncertain significance (VUS) mutation. Finally, the patients in the not tested group were those who had not tested for BRCA1/2 mutation in high-risk group. The criteria for classifying high-risk groups into BRCA mutation group, BRCAx group, and not tested group were based on the test results performed prior to the occurrence of contralateral breast cancer. Patients who underwent BRCA testing after the occurrence of contralateral breast cancer were classified into the not tested group regardless of the test results.
We reviewed the clinical and pathologic characteristics, family history information, and the oncologic outcomes of the study subjects. We used following definitions for family history. Family history was defined as third-degree relative with breast cancer, ovary cancer, metastatic prostate cancer, and pancreas cancer, family history of breast cancer was defined as third-degree relative with only breast cancer, and first-degree relative of breast cancer was defined as parents, siblings, or children who were diagnosed with breast cancer.
Among all patients, 973 patients underwent BRCA testing. One patient with BRCA1 germline mutation underwent BRCA testing prior to the initial diagnosis due to her family history of breast cancer. The remaining 972 patients underwent blood sampling after their diagnosis of breast cancer with the median time from diagnosis to blood draw being 2.1 months (range 0–168 months).
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 2208-056-1349).
Statistical analysis
For intergroup comparisons, t test and ANOVA test were used for continuous variables, and Chi-square was using for descriptive data. Cumulative risk of contralateral breast cancer was assessed by Kaplan–Meier curves and log rank tests in each group. The Cox proportional hazard model was used for calculating hazard ratios. The beginning of follow-up was set as the date of breast surgery. Follow-up time of patients without an event of interest was censored at the date of their last contact. In this study, only metachronous contralateral breast cancers diagnosed at least 3 months after the initial breast surgery were defined as contralateral breast cancer events. Both ductal carcinoma in situ and invasive contralateral cancers were included. An overall survival event was defined as death due to any cause. For patients experiencing either a contralateral breast cancer event or overall survival event, the end of follow-up was defined as the date of the event. A p value < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS 25.0 software and R version 4.1.2.
Results
Demographics and clinicopathologic characteristics
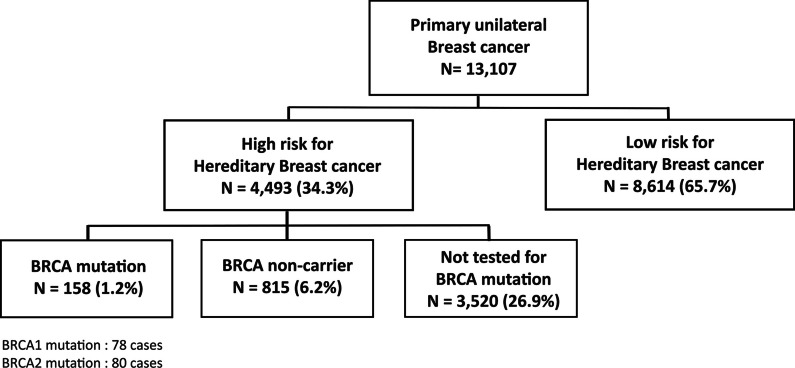
The number of patients in each group is shown in Fig. 1. Among the 13,107 patients who met the inclusion criteria, 4493 (34.3%) and 8614 (65.7%) patients were classified as high- and low-risk of carrying BRCA1/2 germline mutations, respectively. The clinicopathologic characteristics of high- and low-risk patients are shown in Table 1. Notably, the high-risk patients were often associated with unfavorable features including younger age at diagnosis, advanced tumor stages, high histologic grade, and hormone receptor negativity.
Fig. 1.
Baseline demographics
Table 1.
Clinicopathologic characteristics of all patients
| Low risk (N = 8614) | High risk (N = 4493) | p value | |
|---|---|---|---|
| Age | 52.0 [47.0;60.0] | 41.0 [37.0;51.0] | < 0.001 |
| Location | 0.306 | ||
| Right | 4212 (48.9%) | 2240 (49.9%) | |
| Left | 4402 (51.1%) | 2253 (50.1%) | |
| Breast surgery | < 0.001 | ||
| Breast conserving surgery | 5297 (61.5%) | 3002 (66.8%) | |
| Mastectomy | 3317 (38.5%) | 1491 (33.2%) | |
| Axilla surgery | < 0.001 | ||
| Sentinel LN biopsy | 5255 (61.0%) | 2576 (57.3%) | |
| Axilla LN dissection | 3111 (36.1%) | 1820 (40.5%) | |
| Not done | 49 (0.6%) | 12 (0.3%) | |
| Unknown | 199 (2.3%) | 85 (1.9%) | |
| T stage | < 0.001 | ||
| T1 | 4477 (52.0%) | 1898 (42.2%) | |
| T2 | 3447 (40.0%) | 2138 (47.6%) | |
| T3 | 485 (5.6%) | 321 (7.1%) | |
| T4 | 198 (2.3%) | 126 (2.8%) | |
| N stage | < 0.001 | ||
| N0 | 5100 (59.2%) | 2478 (55.2%) | |
| N1 | 2245 (26.1%) | 1236 (27.5%) | |
| N2 | 834 (9.7%) | 511 (11.4%) | |
| N3 | 391 (4.5%) | 247 (5.5%) | |
| Subtype | < 0.001 | ||
| Hormone receptor+/HER2− | 6181 (71.8%) | 2020 (45.0%) | |
| Hormone receptor+/HER2+ | 965 (11.2%) | 406 (9.0%) | |
| Hormone receptor−/HER2+ | 1114 (12.9%) | 263 (5.9%) | |
| Hormone receptor−/HER2− | 354 (4.1%) | 1804 (40.2%) | |
| Histologic grade | < 0.001 | ||
| 1 | 936 (10.9%) | 253 (5.6%) | |
| 2 | 4477 (52.0%) | 1712 (38.1%) | |
| 3 | 2745 (31.9%) | 2239 (49.8%) | |
| Unknown | 456 (5.3%) | 289 (6.4%) | |
| Lymphovascular invasion | < 0.001 | ||
| Present | 2388 (27.7%) | 1409 (31.4%) | |
| None | 5933 (68.9%) | 2886 (64.2%) | |
| Unknown | 293 (3.4%) | 198 (4.4%) |
Among the 4493 high-risk patients, 973 (21.7%) patients underwent genetic testing for germline BRCA1/2 mutation. Genetic testing revealed BRCA1/2 germline mutation in 158 (16.2%) patients. The remaining 815 patients (83.8%), who were determined to be high risk but genetic testing showed BRCA1/2 wild type, comprised the BRCAx group. The rates for BRCA1/2 genetic testing varied by the clinical indications (p < 0.001). Patients with a family history of breast cancer or personal history of ovarian cancer had higher rates of germline BRCA1/2 testing (41.4% and 57.9%, respectively, Additional file 1: Table S1).
The clinicopathologic characteristics of the high-risk group patients are shown in Table 2. Compared to the BRCAx group or high-risk not-tested group, patients with BRCA mutations had significantly higher incidences of ovarian cancer and family history of breast cancer (p < 0.001). While the three groups showed no significant difference in tumor size, nodal status, or histologic grade, the distribution of molecular subtypes showed statistically significant differences.
Table 2.
Clinicopathologic characteristics of high-risk patients
| BRCA mutation (N = 158) | BRCAx (N = 815) | Not tested (N = 3520) | p value | |
|---|---|---|---|---|
| Median age [IQR] | 40.5 [35.0;51.0] | 39.0 [35.0;50.0] | 41.0 [37.0;51.0] | < 0.001 |
| Ovary cancer | < 0.001 | |||
| Yes | 7 (4.4%) | 15 (1.8%) | 16 (0.5%) | |
| No | 151 (95.6%) | 800 (98.2%) | 3504 (99.5%) | |
| Family history | < 0.001 | |||
| Yes | 116 (73.4%) | 421 (51.7%) | 761 (21.6%) | |
| No | 40 (25.3%) | 386 (47.4%) | 2631 (74.7%) | |
| Unknown | 2 (1.3%) | 8 (1.0%) | 128 (3.6%) | |
| Family history of breast cancer | < 0.001 | |||
| Yes | 103 (65.2%) | 403 (49.4%) | 667 (18.9%) | |
| No | 53 (33.5%) | 404 (49.6%) | 2725 (77.4%) | |
| Unknown | 2 (1.3%) | 8 (1.0%) | 128 (3.6%) | |
| First-degree relative with breast cancer | < 0.001 | |||
| Yes | 79 (50.0%) | 336 (41.2%) | 457 (13.0%) | |
| No | 76 (48.1%) | 471 (57.8%) | 2931 (83.3%) | |
| Unknown | 3 (1.9%) | 8 (1.0%) | 132 (3.8%) | |
| T stage | 0.527 | |||
| T1 | 60 (38.0%) | 366 (44.9%) | 1472 (41.8%) | |
| T2 | 83 (52.5%) | 376 (46.1%) | 1679 (47.7%) | |
| T3 | 9 (5.7%) | 51 (6.3%) | 261 (7.4%) | |
| T4 | 5 (3.2%) | 21 (2.6%) | 101 (2.9%) | |
| Unknown | 1 (0.6%) | 1 (0.1%) | 7 (0.2%) | |
| N stage | 0.603 | |||
| N0 | 78 (49.4%) | 445 (54.6%) | 1955 (55.5%) | |
| N1 | 47 (29.7%) | 226 (27.7%) | 963 (27.4%) | |
| N2 | 19 (12.0%) | 90 (11.0%) | 402 (11.4%) | |
| N3 | 13 (8.2%) | 52 (6.4%) | 183 (5.2%) | |
| Unknown | 1 (0.6%) | 2 (0.2%) | 17 (0.5%) | |
| Histologic grade | < 0.001 | |||
| 1 | 4 (2.5%) | 69 (8.5%) | 180 (5.1%) | |
| 2 | 50 (31.6%) | 402 (49.3%) | 1260 (35.8%) | |
| 3 | 97 (61.4%) | 308 (37.8%) | 1834 (52.1%) | |
| 9 | 7 (4.4%) | 36 (4.4%) | 246 (7.0%) | |
| Lymphovascular invasion | 0.671 | |||
| Present | 53 (33.5%) | 248 (30.4%) | 1108 (31.5%) | |
| None | 99 (62.7%) | 537 (65.9%) | 2250 (63.9%) | |
| Unknown | 6 (3.8%) | 30 (3.7%) | 162 (4.6%) | |
| Subtype | < 0.001 | |||
| HR+/HER2− | 75 (47.5%) | 488 (59.9%) | 1457 (41.4%) | |
| HR+/HER2+ | 6 (3.8%) | 112 (13.7%) | 288 (8.2%) | |
| HR−/HER2+ | 6 (3.8%) | 53 (6.5%) | 204 (5.8%) | |
| HR−/HER2− | 71 (44.9%) | 162 (19.9%) | 1571 (44.6%) |
IQR interquartile range, HR hormone receptor
Overall survival and the cumulative risk of CBC
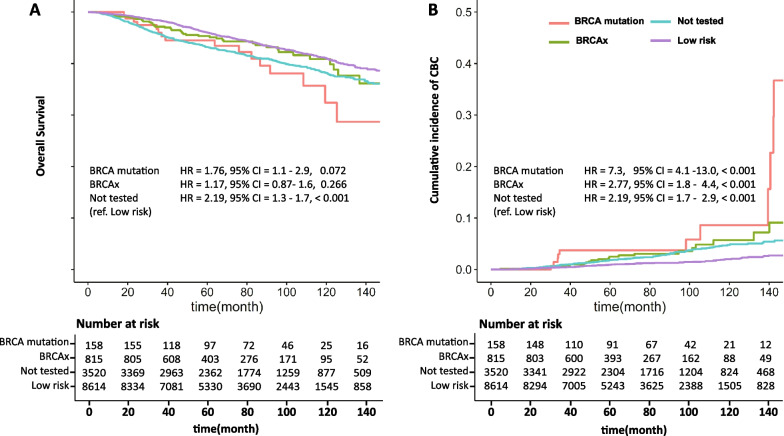
As shown in Fig. 2A, the four groups of patients showed significant overall survival differences. (The 5-year and 10-year overall survival for each group is presented in Additional file 1: Table S2.) The median duration of follow-up for the patients was 72.6 months. When compared to the low-risk group, high-risk not tested group showed significantly worse overall survival outcome (p < 0.001). The BRCA1/2 mutation group showed worse overall survival compared to the low-risk group (HR 1.17, 95% CI: 1.1–2.9); however, this difference was not statistically significant (p = 0.072). However, when adjusted for other prognostic factors, there was no significant differences in hazard ratio of death between the four groups (Table 3).
Fig. 2.
Cumulative risk of contralateral breast cancer and overall survival in each group
Table 3.
Hazard ratio of mortality from univariate and multivariate cox regression model
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | [95% CI] | p value | HR | [95% CI] | p value | |
| Age | ||||||
| Age > 40 | 1 | |||||
| Age ≤ 40 | 1.33 | [1.14, 1.55] | < 0.001 | |||
| TNBC | ||||||
| Yes | 2.43 | [2.11, 2.79] | < 0.001 | 1.44 | [1.16, 1.79] | < 0.001 |
| Ovary cancer | ||||||
| Yes | ||||||
| First-degree relatives with BC | ||||||
| Yes | 0.7 | [0.51, 0.95] | 0.023 | 0.84 | [0.59, 1.18] | 0.315 |
| T stage | 2.24 | [2.09, 2.41] | < 0.001 | 1.56 | [1.25, 1.95] | < 0.001 |
| N stage | 2.06 | [1.93, 2.19] | < 0.001 | 1.71 | [1.47, 1.75] | < 0.001 |
| Histologic grade | 1.08 | [1.05, 1.11] | < 0.001 | |||
| Endocrine therapy | ||||||
| Yes | 0.37 | [0.33, 0.42] | < 0.001 | 0.53 | [0.44, 0.63] | < 0.001 |
| BRCA test | ||||||
| Low risk | 1 | 1 | ||||
| Not tested | 1.46 | [1.27, 1.68] | < 0.001 | 0.88 | [0.74, 1.05] | 0.165 |
| BRCAx | 1.18 | [0.87, 1.58] | 0.282 | 0.92 | [0.66, 1.27] | 0.611 |
| BRCA mutation | 1.76 | [1.07, 2.89] | 0.026 | 1.02 | [0.59, 1.77] | 0.944 |
The cumulative risk of CBC also varied among the four groups (Fig. 2B). As expected, the patients with germline BRCA1/2 mutation showed 7.3-fold increase of CBC risk when compared to the low-risk group (p < 0.001). Also, the high-risk not tested group showed significant increase in CBC risk (p < 0.001). Interestingly, the patients in the BRCAx group who had wild-type BRCA1/2 also showed significantly higher risk of CBC when compared to the low-risk group with the hazard ratio of 2.77 (p < 0.001). The prognostic importance of the BRCAx for CBC recurrence persisted after adjusting for the age and subtype, but became insignificant when the family history of breast cancer was adjusted (Table 4).
Table 4.
Hazard ratio of contralateral breast cancer derived from univariate and multivariate cox regression model
| Variable | Univariate | Multivariate (including family history) | Multivariate (excluding family history) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | [95% CI] | p value | HR | [95% CI] | p value | HR | [95% CI] | p value | |
| Age | |||||||||
| Age > 40 | 1 | 1 | 1 | ||||||
| Age ≤ 40 | 1.68 | [1.27, 2.22] | < 0.001 | 1.25 | [0.85, 1.84] | 0.26 | 0.98 | [0.85, 1.84] | 0.26 |
| TNBC | |||||||||
| Yes | 2.18 | [1.66, 2.87] | < 0.001 | 1.69 | [1.20, 2.40] | 0.003 | 1.44 | [1.02, 2.02] | 0.036 |
| Ovary cancer | |||||||||
| Yes | 1.06 | [0.15, 7.55] | 0.955 | ||||||
| First-degree relatives with BC | |||||||||
| Yes | 2.92 | [2.08, 4.08] | < 0.001 | 1.95 | [1.23. 3.08] | 0.004 | |||
| T stage | 0.95 | [0.79, 1.14] | 0.575 | ||||||
| N stage | 1.02 | [0.88, 1.19] | 0.796 | ||||||
| Histologic grade | 1.16 | [0.93, 1.44] | 0.182 | ||||||
| Endocrine therapy | |||||||||
| Yes | 0.48 | [0.37, 0.61] | < 0.001 | ||||||
| BRCA test | |||||||||
| Low risk | 1 | 1 | 1 | ||||||
| Not tested | 2.19 | [1.67, 2.86] | < 0.001 | 1.41 | [0.92, 2.16] | 0.115 | 1.88 | [1.29, 2.76] | 0.001 |
| BRCAx | 2.77 | [1.76, 4.35] | < 0.001 | 1.57 | [0.84, 2.91] | 0.154 | 2.6 | [1.56, 1.33] | < 0.001 |
| BRCA mutation | 7.3 | [4.11, 13.0] | < 0.001 | 3.2 | [1.44, 7.14] | 0.004 | 6.17 | [3.23, 11.8] | < 0.001 |
TNBC triple negative breast cancer, HR hazard ratio, CI confidence interval, BC breast cancer
Discussion
The present study demonstrates that, in addition to the breast cancer patients with germline BRCA1/2 mutation, the patients with wild-type BRCA1/2 who are high-risk of having hereditary breast cancer (BRCAx) also carry an increased risk of CBC recurrence when compared to that of low-risk patients. The increased risk of CBC in high-risk breast cancer patients with wild-type BRCA1/2 seems mostly due to having the family history.
Previous studies have examined differences in CBC risk between patients with BRCA mutations and non-carriers within high-risk cohorts [18, 19] or between sporadic patients and BRCA mutation carriers [20, 21]. In contrast, our study directly compared the CBC risk among patients with high risk for hereditary breast cancer, sporadic patients, BRCA mutation carriers, and BRCAx group within a relatively large cohort treated at a single institution. Our findings are meaningful because they demonstrate that patients with high-risk factors, even in the absence of BRCA mutations, have a higher CBC cumulative risk compared to low-risk sporadic patients.
There are several studies that have investigated the cumulative risk of contralateral breast cancer in patients with confirmed BRCA non-carriers (BRCAx group), but the results are inconsistent, and have shown varying results. There are studies that suggest that non-carriers of BRCA mutations have a higher risk of developing contralateral breast cancer compared to sporadic patients. Reiner et al. showed that BRCA non-carriers with family history breast cancer were at significantly greater risk of CBC than other breast cancer survivors. The 10-year cumulative risks of developing breast cancer for those without a family history, with only second-degree family history, and with first-degree family history were 4.6%, 5.9%, and 8.6%, respectively. Moreover, non-carriers with a bilaterally affected first-degree relative have a 10-year cumulative risk of CBC that is nearly as high as that of BRCA mutation carriers (15.6% vs. 18.4%, respectively) [22]. In other study, Yoon et al. showed that non-carriers with high risk of hereditary breast cancer patients have also been found to have a higher risk of CBC, the 10-year cumulative risk for CBC was 9.8% for non-carriers, 23.8% for BRCA1 mutation carriers, and 19.1% for BRCA2. There was no statistically significant difference in CBC risk between BRCA mutation carriers and non-carriers [19].
However, several studies have shown that BRCAx patients do not have significantly different CBC risks compared to sporadic (without family history of breast cancer) breast cancer patients [23, 24]. Tilanus-Linthorst et al. argued that the reports of higher CBC incidence and better survival in non-BRCA1/2 patients may be substantially influenced by selection bias due to DNA testing. Patients who already had contralateral breast cancer or were at higher risk of developing CBC were more likely to undergo BRCA gene testing, which could have influenced the results [25].
One possible explanation for the high incidence of contralateral breast cancer (CBC) in the BRCAx group is that there may be mutations in high-penetrance genes other than BRCA1/2, such as PTEN, CDH1, and CHEK2, or the presence of common low-penetrance variants that increase the risk of developing cancer in the contralateral breast. A study of Korean BRCAx patients found that 4.2% of the overall patients were affected by moderate-/high-penetrance variants, and showed that high-risk breast cancers, particularly for Asians, might consist of multiple layers with similar importance, moderate/high-penetration genes, and selected common variants [17]. However, Reiner et al. showed that family history of breast cancer remains a strong risk factor for CBC, even after excluding carriers of deleterious mutations in BRCA1, BRCA2, ATM, CHEK2 or PALB2, and after adjusting for 67 common breast cancer-susceptibility single nucleotide polymorphisms (SNPs) [26]. This suggests that there may be other factors at play beyond genetic ones. A second possible explanation is that patients with a familial history of breast cancer may be influenced by environmental factors that contribute to the development of breast cancer, in addition to genetic factors. A study by Couto et al. estimated that the heritable component of familial breast cancer was 73%, with the environmental proportion at 27% [27]. A third possible explanation is that a large proportion of the BRCAx group consists of young patients who have a higher risk of developing breast cancer, which may also increase their risk of developing CBC. Prospective studies have shown that only 5–12% of all women younger than 40 years with a first breast cancer diagnosis were carriers of the BRCA1 or BRCA2 mutation [28, 29]. Apart from genetic factors, many young breast cancer patients have multiple risk factors associated with breast cancer., such as lean body mass, reproductive factors, and therapeutic radiation, which may also increase their risk of developing CBC for the same reasons [30].
In our study, patients with BRCA1 mutation and BRCA2 mutation had a 10-year cumulative CBC risk of 9.85% and 7.20%, respectively, which is lower compared to the results of previous studies (Additional file 1: Table S3). Studies of populations in the USA or Europe have shown that BRCA1 and BRCA2 mutations have a 24–35% and 19–29% risk of developing contralateral breast cancer within 10 years of their first breast cancer [8, 31–33]. Research involving patients in Asia has shown a lower cumulative risk of CBC compared to studies on Western patients, yet the risk is higher than that found in our study. Also, the range of risk was broader in these studies, with a 10-year cumulative risk ranging from 15.5 to 26%. The reason the cumulative risk appears lower in our study is that only around 20% of high-risk patients underwent BRCA gene testing and the acceptance rate for triple negative breast was lower. This may lead to an underestimation of the actual risk.
Our study has several limitations. First, we did not perform BRCA testing on unselected breast cancer patients, which may introduce selection bias in the patients who underwent testing. Second, although we had a relatively large number of patients with BRCA mutations compared to previous Asian studies, the number is still smaller and the follow-up period is shorter than in Western studies. Therefore, further analyses with a larger number of patients and long-term follow-up are needed in the future.
Conclusion
Breast cancer patients who are at high-risk of hereditary breast cancer but with wild-type BRCA 1/2 genes (BRCAx) have increased risk of developing contralateral breast cancer when compared to the low-risk patients. More careful surveillance and follow-up can be offered to these patients especially when they have family history of breast cancer.
Supplementary Information
Additional file 1: Table S1. The acceptance rate of BRCA tests conducted based on different testing criteria. Table S2. Description: 5-year and 10-year overall survival in each group. Table S3. 5-year and 10-year cumulative incidence of contralateral breast cancer.
Acknowledgements
Not applicable.
Abbreviations
- CBC
Contralateral breast cancer
- LN
Lymph node
- HR
Hormone receptor
- TNBC
Triple negative breast cancer
- HR
Hazard ratio
- CI
Confidence interval
- BC
Breast cancer
Author contributions
HGM was involved in conception and design. EK, JJJ, CJ, HKK, HBL, WH, and HGM helped in administrative support. Provision of study materials or patients was done by HKK, HBL, WH, and HGM. EK, JJJ, and CJ contributed to collection and assembly of data. Data analysis and interpretation were performed by EK, JJJ, CJ, HKK, HBL, WH, and HGM. EK helped in manuscript writing. Final approval of manuscript was done by EK, JJJ, CJ, HKK, HBL, WH, and HGM.
Funding
HGM was supported by a KHIDI R&D project (HI19C0481, HC21C0031, HI22C0497), funded by the Ministry of Health & Welfare, Republic of Korea and National Research Foundation of Korea (NRF) grant (NRF-2019R1A2C2005277) funded by the Ministry of Science and ICT, Republic of Korea (MSIT).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of Seoul National University Hospital. The need for informed consent was waived on account of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
HBL and WH are co-founders and members of the DCGen Co., Ltd board of directors. HBL received research funding from Devicor Medical Product, Inc., and consulting fees from Need Inc., outside the current work. Other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med. 2008;359(20):2143–2153. doi: 10.1056/NEJMra0802968. [DOI] [PubMed] [Google Scholar]
- 3.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 4.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 7.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 8.Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 9.Kang E, Seong MW, Park SK, Lee JW, Lee J, Kim LS, et al. The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res Treat. 2015;151(1):157–168. doi: 10.1007/s10549-015-3377-4. [DOI] [PubMed] [Google Scholar]
- 10.Kang E, Kim SW. The korean hereditary breast cancer study: review and future perspectives. J Breast Cancer. 2013;16(3):245–253. doi: 10.4048/jbc.2013.16.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimura A, Yokoyama S, Iwata H, Takaiso N, Nomizu T, Arai M, et al. Incidence of contralateral and ipsilateral breast cancers and prognosis in BRCA1/2 pathogenic variant carriers based on the Japanese HBOC consortium registration. J Hum Genet. 2021;66(4):379–387. doi: 10.1038/s10038-020-00850-5. [DOI] [PubMed] [Google Scholar]
- 12.Ryu JM, Choi HJ, Kim I, Nam SJ, Kim SW, Yu J, et al. Prevalence and oncologic outcomes of BRCA 1/2 mutations in unselected triple-negative breast cancer patients in Korea. Breast Cancer Res Treat. 2019;173(2):385–395. doi: 10.1007/s10549-018-5015-4. [DOI] [PubMed] [Google Scholar]
- 13.Kang SY, Lee SB, Kim YS, Kim Z, Kim HY, Kim HJ, et al. Breast cancer statistics in Korea, 2018. J Breast Cancer. 2021;24(2):123–137. doi: 10.4048/jbc.2021.24.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee ES, Kim J, Han W. Multigene panel testing for hereditary cancer and genetic counseling. Adv Exp Med Biol. 2021;1187:455–471. doi: 10.1007/978-981-32-9620-6_24. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix M, Leclercq G. The, "portrait" of hereditary breast cancer. Breast Cancer Res Treat. 2005;89(3):297–304. doi: 10.1007/s10549-004-2172-4. [DOI] [PubMed] [Google Scholar]
- 16.Aloraifi F, Alshehhi M, McDevitt T, Cody N, Meany M, O'Doherty A, et al. Phenotypic analysis of familial breast cancer: comparison of BRCAx tumors with BRCA1-, BRCA2-carriers and non-familial breast cancer. Eur J Surg Oncol. 2015;41(5):641–646. doi: 10.1016/j.ejso.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Lee JY, Kim J, Kim SW, Park SK, Ahn SH, Lee MH, et al. BRCA1/2-negative, high-risk breast cancers (BRCAX) for Asian women: genetic susceptibility loci and their potential impacts. Sci Rep. 2018;8(1):15263. doi: 10.1038/s41598-018-31859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menes TS, Terry MB, Goldgar D, Andrulis IL, Knight JA, John EM, et al. Second primary breast cancer in BRCA1 and BRCA2 mutation carriers: 10-year cumulative incidence in the Breast Cancer Family Registry. Breast Cancer Res Treat. 2015;151:653–660. doi: 10.1007/s10549-015-3419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon K-H, Chae S, Kang E, Shin H-C, Kim JH, Kim IA, et al. Contralateral breast cancer and ipsilateral breast tumor recurrence in BRCA1/2 carriers and non-carriers at high-risk of hereditary breast cancer. J Breast Cancer. 2019;22(4):587–598. doi: 10.4048/jbc.2019.22.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce LJ, Levin AM, Rebbeck TR, Ben-David MA, Friedman E, Solin LJ, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006;24(16):2437–2443. doi: 10.1200/JCO.2005.02.7888. [DOI] [PubMed] [Google Scholar]
- 21.Lin P-H, Chen S-C, Tseng L-M, Chang K-J, Huang A-C, Cheng K-C, et al. Impact of BRCA mutation on the survival and risk of contralateral breast cancer in Asian breast cancer patients. Breast Cancer Res Treat. 2022;192(3):629–637. doi: 10.1007/s10549-021-06446-7. [DOI] [PubMed] [Google Scholar]
- 22.Reiner AS, John EM, Brooks JD, Lynch CF, Bernstein L, Mellemkjær L, et al. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women’s Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol. 2013;31(4):433–439. doi: 10.1200/JCO.2012.43.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brekelmans C, Tilanus-Linthorst M, Seynaeve C, vd Ouweland A, Menke-Pluymers M, Bartels C, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1-and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer. 2007;43(5):867–876. doi: 10.1016/j.ejca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Rhiem K, Engel C, Graeser M, Zachariae S, Kast K, Kiechle M, et al. The risk of contralateral breast cancer in patients from BRCA1/2 negative high risk families as compared to patients from BRCA1 or BRCA2 positive families: a retrospective cohort study. Breast Cancer Res. 2012;14:1–8. doi: 10.1186/bcr3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilanus-Linthorst MM, Bartels KC, Alves C, Bakri B, Crepin E, van den Ouweland A, et al. Selection bias influences reported contralateral breast cancer incidence and survival in high risk non-BRCA1/2 patients. Breast Cancer Res Treat. 2006;95:117–123. doi: 10.1007/s10549-005-9054-2. [DOI] [PubMed] [Google Scholar]
- 26.Reiner AS, Sisti J, John EM, Lynch CF, Brooks JD, Mellemkjær L, et al. Breast cancer family history and contralateral breast cancer risk in young women: an update from the women's environmental cancer and radiation epidemiology study. J Clin Oncol. 2018;36(15):1513–1520. doi: 10.1200/JCO.2017.77.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couto E, Hemminki K. Estimates of heritable and environmental components of familial breast cancer using family history information. Br J Cancer. 2007;96(11):1740–1742. doi: 10.1038/sj.bjc.6603753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodwin PJ, Phillips K-A, West DW, Ennis M, Hopper JL, John EM, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol. 2012;30(1):19–26. doi: 10.1200/JCO.2010.33.0068. [DOI] [PubMed] [Google Scholar]
- 30.Narod SA. Breast cancer in young women. Nat Rev Clin Oncol. 2012;9(8):460–470. doi: 10.1038/nrclinonc.2012.102. [DOI] [PubMed] [Google Scholar]
- 31.Metcalfe K, Gershman S, Lynch H, Ghadirian P, Tung N, Kim-Sing C, et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2011;104(9):1384–1392. doi: 10.1038/bjc.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Kolk DM, de Bock GH, Leegte BK, Schaapveld M, Mourits MJ, de Vries J, et al. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat. 2010;124:643–651. doi: 10.1007/s10549-010-0805-3. [DOI] [PubMed] [Google Scholar]
- 33.Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22(12):2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The acceptance rate of BRCA tests conducted based on different testing criteria. Table S2. Description: 5-year and 10-year overall survival in each group. Table S3. 5-year and 10-year cumulative incidence of contralateral breast cancer.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.