Abstract
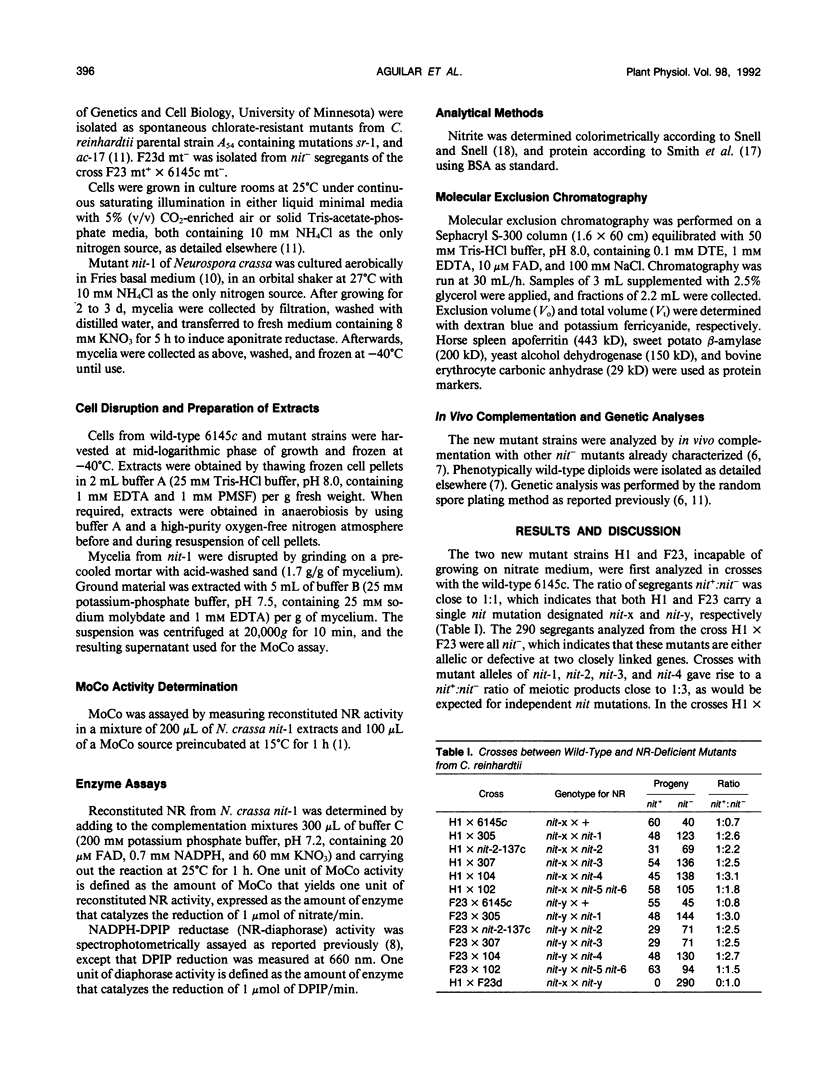
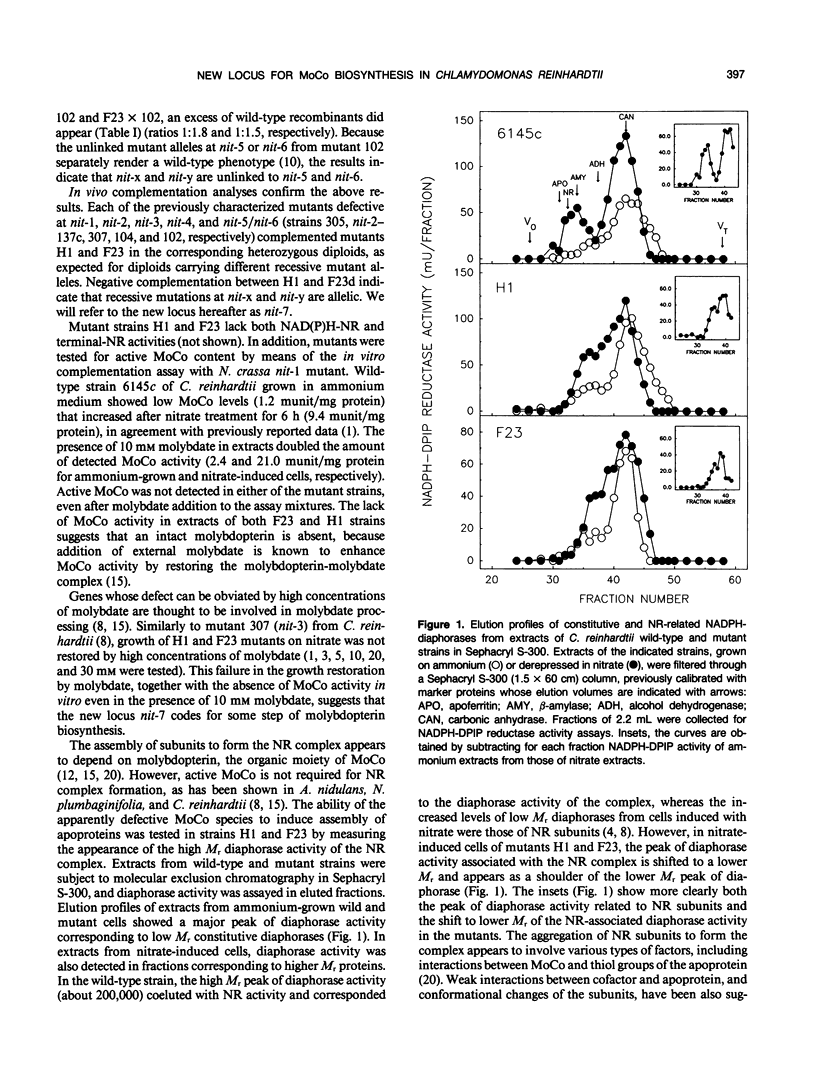
Two new nitrate reductase-deficient mutants from Chlamydomonas reinhardtii have been genetically and biochemically characterized. Both H1 and F23 mutants carry single recessive allelic mutations that map at a new locus designated nit-7. This locus is unlinked to the other six nit loci related to the nitrate assimilation pathway in C. reinhardtii. Both mutant alleles H1 and F23 lack an active molybdopterin cofactor, the activity of which is restored neither in vitro nor in vivo by high concentrations of molybdate. Nitrate reductase subunits in these mutants seem to assemble, although not in a stable form, in a high molecular weight complex and, as in other molybdenum cofactor-defective mutants of C. reinhardtii, they cannot reconstitute nitrate reductase activity with an active molybdenum cofactor source from extracts of ammonium-grown cells. The results suggest that nit-7 mutants are defective in molybdopterin biosynthesis. They do produce some precursor(s) that are capable of binding to nitrate reductase subunits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar M. R., Cárdenas J., Fernández E. Regulation of molybdenum cofactor species in the green alga Chlamydomonas reinhardtii. Biochim Biophys Acta. 1991 Apr 9;1073(3):463–469. doi: 10.1016/0304-4165(91)90216-4. [DOI] [PubMed] [Google Scholar]
- Cove D. J. Genetic studies of nitrate assimilation in Aspergillus nidulans. Biol Rev Camb Philos Soc. 1979 Aug;54(3):291–327. doi: 10.1111/j.1469-185x.1979.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Fernández E., Matagne R. F. In vivo complementation analysis of nitrate reductase-deficient mutants in Chlamydomonas reinhardtii. Curr Genet. 1986;10(5):397–403. doi: 10.1007/BF00418413. [DOI] [PubMed] [Google Scholar]
- Fernández E., Schnell R., Ranum L. P., Hussey S. C., Silflow C. D., Lefebvre P. A. Isolation and characterization of the nitrate reductase structural gene of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6449–6453. doi: 10.1073/pnas.86.17.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard J, Marion-Poll A, Chérel I, Meyer C, Müller A, Caboche M. Isolation and characterization of Nicotiana plumbaginifolia nitrate reductase-deficient mutants: genetic and biochemical analysis of the NIA complementation group. Mol Gen Genet. 1987 Oct;209(3):596–606. doi: 10.1007/BF00331169. [DOI] [PubMed] [Google Scholar]
- Garrett R. H. The induction of nitrite reductase in Neurospora crassa. Biochim Biophys Acta. 1972 May 16;264(3):481–489. doi: 10.1016/0304-4165(72)90011-6. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Wuebbens M. M., Rajagopalan K. V. The structure of a molybdopterin precursor. Characterization of a stable, oxidized derivative. J Biol Chem. 1989 Aug 15;264(23):13440–13447. [PubMed] [Google Scholar]
- Kramer S. P., Johnson J. L., Ribeiro A. A., Millington D. S., Rajagopalan K. V. The structure of the molybdenum cofactor. Characterization of di-(carboxamidomethyl)molybdopterin from sulfite oxidase and xanthine oxidase. J Biol Chem. 1987 Dec 5;262(34):16357–16363. [PubMed] [Google Scholar]
- Pelsy F., Gonneau M. Genetic and biochemical analysis of intragenic complementation events among nitrate reductase apoenzyme-deficient mutants of Nicotiana plumbaginifolia. Genetics. 1991 Jan;127(1):199–204. doi: 10.1093/genetics/127.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tomsett A. B., Garrett R. H. Biochemical analysis of mutants defective in nitrate assimilation in Neurospora crassa: evidence for autogenous control by nitrate reductase. Mol Gen Genet. 1981;184(2):183–190. doi: 10.1007/BF00272903. [DOI] [PubMed] [Google Scholar]
- Wahl R. C., Hageman R. V., Rajagopalan K. V. The relationship of Mo, molybdopterin, and the cyanolyzable sulfur in the Mo cofactor. Arch Biochem Biophys. 1984 Apr;230(1):264–273. doi: 10.1016/0003-9861(84)90107-3. [DOI] [PubMed] [Google Scholar]


