Abstract
A common concern with many autoimmune diseases of unknown etiology is the extent to which tissue T-lymphocyte infiltrates, versus a nonspecific infiltrate, reflect a response to the causative agent. Lyme arthritis can histologically resemble rheumatoid synovitis, particularly the prominent infiltration by T lymphocytes. This has raised speculation about whether Lyme synovitis represents an ongoing response to the causative spirochete, Borrelia burgdorferi, or rather a self-perpetuating autoimmune reaction. In an effort to answer this question, the present study examined the repertoire of infiltrating T cells in synovial fluid from nine Lyme arthritis patients, before and after stimulation with B. burgdorferi. Using a highly sensitive and consistent quantitative PCR technique, a comparison of the T-cell antigen receptor (TCR) β-chain variable (Vβ) repertoires of the peripheral blood and synovial fluid showed a statistically significant increase in expression of Vβ2 and Vβ6 in the latter. This is remarkably similar to our previous findings in studies of rheumatoid arthritis and to other reports on psoriatic skin lesions. However, stimulation of synovial fluid T cells with B. burgdorferi provoked active proliferation but not a statistically significant increase in expression of any TCR Vβ, including Vβ2 and Vβ6. Collectively, the findings suggest that the skewing of the TCR repertoire of fresh synovial fluid in Lyme arthritis may represent more a synovium-tropic or nonspecific inflammatory response, similar to that occurring in rheumatoid arthritis or psoriasis, rather than a specific Borrelia reaction.
Since its first description in 1977, Lyme disease has become the most common vector-borne disease in the United States (9, 45). The causative agent, the spirochete Borrelia burgdorferi, is transmitted to humans by Ixodes ticks (8, 40). Infection can result in a distinct rash, erythema migrans, and cardiac, neurologic, and rheumatic manifestations (39, 41, 42, 45). If appropriate antibiotic treatment is delayed or inadequate, chronic inflammation can ensue (9, 13, 41, 45). In such individuals, it has been possible to occasionally detect the persistence of B. burgdorferi, either by culture (5, 40), the use of special stains (6, 36, 43), or, more recently, PCR (29).
The significance of the immune response in the pathogenesis of Lyme disease is unclear (35). The early cardiac and synovial lesions of Lyme borreliosis in mice may result from an infiltration, primarily by macrophages (3). This is supported by the development of certain features of Lyme disease in scid mice (33). However, chronic phases of the disease are frequently accompanied by lymphocytic infiltration of particular tissues (16, 26, 43). Several lines of evidence point to a role for lymphocytes in Lyme arthritis. HLA-DR4 patients that develop antibodies to outer surface protein A (OspA) tend to have a more severe form of arthritis that is resistant to antibiotics (19). Additional findings indicate that T lymphocytes specifically contribute to chronic Lyme arthritis. These findings include the presence of activated T cells in the inflamed synovium (43), a strong proliferative response of Lyme arthritis synovial T cells to B. burgdorferi (34), a possible genetic predisposition associated with HLA class II genes (44, 46), and the use of therapies for chronic Lyme arthritis that at least partially inhibit T-cell function (9, 45). In this regard, Lyme disease may represent a paradigm of an infection-induced autoimmune syndrome.
Striking biases in the repertoire of the T-cell antigen receptor (TCR) have been noted in a number of autoimmune conditions. This is most remarkably demonstrated with experimental autoimmune encephalomyelitis in certain strains of mice and rats, in which the induction of disease is dependent on myelin basic protein-specific T cells that utilize a restricted pool of TCR α-chain variable (Vα), Jα, and Vβ gene segments (54). Similar, if somewhat less dramatic, findings have been reported for a variety of human autoimmune diseases, including rheumatoid arthritis (12, 15, 18, 30, 37, 51), psoriasis (27, 49), multiple sclerosis (52), inflammatory bowel disease (4), sarcoidosis (17), Sjögren’s syndrome (47), and Kawasaki’s disease (1, 2). Since many of these studies selectively examined biased Vβ expression, the possibility of a role for superantigens was invoked. However, the lesson from experimental autoimmune encephalomyelitis illustrates that in some situations, profound Vβ bias toward traditional antigen-major histocompatability complex formation can occur.
Lyme arthritis represents a unique circumstance among human diseases dominated by T-cell infiltration, in that the inciting agent is known. In this capacity, Lyme arthritis can serve as a model when attempting to correlate a TCR bias with a response to B. burgdorferi. We have examined TCR Vβ expression in nine cases of Lyme arthritis by using a highly sensitive and consistent quantitative PCR assay. Compared to peripheral blood lymphocytes (PBL) from the same patient, freshly isolated synovial fluid T cells showed a significant bias toward Vβ2 and Vβ6, the same bias that we observed earlier in rheumatoid arthritis (12). However, stimulation of synovial fluid lymphocytes with a sonicate of B. burgdorferi did not evoke an increase in any given TCR Vβ, including Vβ2 or Vβ6. The findings support the view that the inciting agent in Lyme arthritis does not directly provoke the TCR bias observed in fresh synovial fluid.
MATERIALS AND METHODS
Patient population.
Nine Lyme arthritis patients with a clearly established diagnosis were selected. The patients were followed at either Yale University, the University of Medicine and Dentistry of New Jersey’s Robert Wood Johnson School of Medicine, or The University of Vermont. Each patient lived in an area in which Lyme disease is endemic, had a typical exposure history, and manifested a positive Lyme antibody titer by enzyme-linked immunosorbent assay that was frequently higher in synovial fluid than in serum. In addition, all patients had serum antibodies to B. burgdorferi, as determined by Western blotting. Two patients had exhibited erythema migrans, as determined by history or physician observation. The duration of arthritis varied from 3 days to 7 years. Synovial fluid was obtained from patients who required therapeutic arthrocentesis or synovectomy. Matched peripheral blood specimens were obtained whenever possible. All patients had received antibiotics prior to specimen collection. Repeat arthrocentesis or synovectomy was not indicated for these patients, and thus serial samples were not available.
HLA class II oligotyping by PCR.
Allele-specific oligonucleotides, selected to distinguish various DRB allele specificities (28), were synthesized on a DNA synthesizer (Applied Biosystems, Foster City, Calif.). The 3′ ends of these products were then poly(T) tailed by the procedure of Saiki et al. (32) with slight modifications, blotted onto nylon membranes, and UV cross-linked to the membranes. Approximately 0.25 μg of patient DNA was amplified with DRB-specific primers (17a) and digoxigenin-labeled dUTP (Boehringer Mannheim, Indianapolis, Ind.). The amplified PCR product was hybridized to the prepared oligoblots, and positive reactions were visualized as a colored precipitate.
Isolation and surface phenotyping of mononuclear cells.
Mononuclear cells were isolated from heparinized PBL and synovial fluid by density gradient centrifugation over Ficoll-Hypaque (Histopaque; Sigma, St. Louis, Mo.). Mononuclear cells were phenotyped by using antibodies to (i) CD4 conjugated to phycoerythrin, (ii) CD8 conjugated to fluorescein isothiocyanate (Becton Dickinson, Mountain View, Calif.), (iii) TCR-αβ (courtesy of Michael Brenner, Harvard Medical School, Boston, Mass.), or (iv) CD45RO (a gift of Peter Beverly, University College, London, United Kingdom). Cells (106) were stained in a volume 100-μl at 4°C for 30 min, washed, and fixed in 2% paraformaldehyde in phosphate-buffered saline. Flow cytometric analysis was performed with a Coulter Elite flow cytometer (Coulter Corp., Hialeah, Fla.).
Preparation of B. burgdorferi sonicate.
High-passage B. burgdorferi B31 (New York isolate; ATCC 35210) and low-passage strain NFST 1 (Nantucket tick isolate; a gift of Richard Pollack and Andrew Spielman, Harvard School of Public Health) cells were grown in Barbour-Stoener-Kelly II medium (23) at 33°C to a concentration of 1 × 107 to 5 × 107 organisms/ml. Spirochetes were centrifuged at 10,000 × g and 10°C for 15 min, washed three times in phosphate-buffered saline, enumerated by darkfield microscopy, and sonicated five times for 30 s each. After the resulting sonicate was filtered (0.45-μm-pore-size filter), the protein concentration was determined by optical density, and it was stored at −90°C.
Proliferation of mononuclear cells upon exposure to B. burgdorferi.
Mononuclear cells were plated at 5 × 104/well in 96-well plates containing serum-free medium (AIM-V; GIBCO, Grand Island, N.Y.) with or without B. burgdorferi sonicate (3 μg/ml). Plates were incubated for 5 days and then pulsed with 1 μCi of [3H]thymidine per well for the final 20 h before harvesting and counting. For bulk synovial lymphocyte cultures, cells were plated at 5 × 105/ml in serum-free AIM-V medium in the absence of exogenous interleukin-2 and stimulated with 3 μg of B. burgdorferi sonicate per ml. Cells received fresh medium and were expanded as necessary. After 1 week, the cells were used for the preparation of RNA and cDNA for quantitative PCR of TCR Vβ.
RNA extraction and cDNA preparation.
RNA was extracted by a modification of the method of Chomczynski and Sacchi (11). Briefly, 2.5 × 106 cells were extracted in 400 μl of 4 M guanidinium thiocyanate. Sequentially added to the extract were 1/10 volume of 2 M sodium acetate (pH 4), 1 volume of H2O-saturated phenol, and 1/5 volume of chloroform-isoamyl alcohol (49:1). After centrifugation, the upper aqueous layer was precipitated in 70% ethanol, resuspended in diethyl pyrocarbonate-treated water, washed in chloroform-isoamyl alcohol, and reprecipitated in 70% ethanol in the presence of 0.3 M sodium acetate. The air-dried pellet was resuspended in diethyl pyrocarbonate-treated water, and the RNA concentration was calculated by determining the absorbance at 260 nm. cDNA was prepared by incubating 5 μg of RNA in the presence of 50 mM Tris HCl (pH 8.3), 40 mM KCl, 6 mM MgCl2, 0.4 mM each deoxynucleoside triphosphate (dNTP), 40 U of RNase inhibitor (Boehringer Mannheim), 2 μg of poly(dT)12–18 (Pharmacia, Piscataway, N.J.), and 3 U of avian myeloblastosis virus reverse transcriptase (Life Sciences, St. Petersburg, Fla.) in a final volume of 50 μl. After incubation at 42°C for 45 min, an additional 0.2 mM each dNTP and 1.5 U of reverse transcriptase were added for a second 45-min incubation. The reaction mixture was heated to 65°C for 10 min, and the DNA was precipitated in 70% ethanol in the presence of 2 M ammonium acetate.
Vβ gene frequency analysis by PCR.
The amount of TCR-derived cDNA in each sample was determined by comparing a parallel PCR amplification of a 280-bp α-chain constant region (Cα) fragment from each sample with a standard curve that was derived by using serial dilutions of cDNA from phytohemagglutinin (PHA)-stimulated PBL. The standard curve of counts incorporated into the Cα product per minute was linear for RNA quantities from 0.001 to 0.033 μg, as previously shown (12). The Cα concentration in the sample was assigned a unit value equal to the amount of RNA in the standard. In preliminary experiments, we found that between 5 and 10 ng of RNA was sufficient to detect each Vβ gene product. The Vβ PCR assay was a modification of the method of Labrecque et al. (21). For each sample, the different Vβs were amplified with a 5′ Vβ-specific primer and a common 3′ Cβ primer. 5′ and 3′ Cα primers were included in each reaction tube as an internal control. The oligonucleotide sequences of the primers for Cα were as follows: 5′Cα, 5′-GCATGTGCAAACGCCTTCAACAACAGC-3′; and 3′Cα, 5′-AGCCGCAGCGTCATGAGCAGATTAAACCCG-3′. The oligonucleotide primers for the Vβ1 to Vβ20 gene families were from Choi et al. (10), and the primers for Vβ21 to Vβ24 were from Labrecque et al. (21). The sequence of the reverse primer 3′Cβ is 5′-TCTACCCCAGGCCTCGGCGCTGACGAT-3′. A PCR master mix was prepared to minimize pipetting error. This mix included 100 mM Tris-HCl (pH 8.3), 500 mM KCl, 2 mM MgCl2, 200 μM each dNTP, 25 pmol of 3′Cβ primer per sample, 8.25 pmol each of the 5′Cα and 3′Cα primers, 2.2 μCi of [α-32P]dCTP (NEN, Wilmington, Del.), and 2.5 U of DNA polymerase (Perkin-Elmer, Norwalk, Conn.). The final volume of each tube was 100 μl and contained the predetermined amount of the sample cDNA and 25 pmol of an individual Vβ primer. In preliminary experiments, we determined that the accumulation of the TCR β product was the same in the presence or absence of the Cα primers. The number of amplification cycles considerably affected the sensitivity and accuracy of the assay. We previously determined that, given the concentration of reagents used, Cα counts per minute and Vβ amplification would begin to plateau after 26 and 30 cycles, respectively (12). However, a minimum of 22 cycles was needed to reproducibly detect signals from 26 Vβs in a PBL control sample. As a result, 24 PCR cycles were used in the assay so that both the Cα and Vβ signals would be in the linear portions of their amplification curves. The PCR cycles were as follows: cycle 1, 94°C for 3 min, 50°C for 45 s, and 72°C for 1 min; cycles 2 to 23, 94°C for 30 s, 50°C for 45 s, and 72°C for 1 min; and cycle 24, 94°C for 30 s, 50°C for 45 s, and 72°C for 7 min.
The PCR products were separated by electrophoresis at 80 V for 18 h on a 29-cm-long 10% acrylamide gel in a buffer system of 7 M urea in Tris-borate-EDTA buffer. The gel was dried and analyzed for radioactivity in a Betascope 603 blot analyzer (Betagen, Waltham, Mass.). Pipetting errors or problems with the PCR were readily identified by comparing the counts incorporated into the Cα product per minute for each tube. The relative frequency of each Vβ, expressed as a percent of the total, was calculated by the following formula: [(Vβ counts per minute − background counts per minute)/(Cα counts per minute − background counts per minute)] × [100/(sum of Vβ/Cα ratios)].
Statistical analysis.
Two approaches were used to compare the Vβ gene usages of the synovial fluid and peripheral blood T cells. The t statistic was used to determine if the ratio of each Vβ in synovial fluid samples before and after stimulation with B. burgdorferi for 1 week was different from 1, and the paired t test was used to determine if there were significant differences between the absolute Vβ percentages in fresh and in cultured synovial fluid T cells. The normality of the distributions of the Vβ gene frequencies in synovial fluid and PBL were tested by using the correlation coefficient test based on Blom’s plotting position (24).
RESULTS
Lyme arthritis synovial fluid T cells bear an activated phenotype and proliferate when exposed to B. burgdorferi.
Nine patients who had a clearly established diagnosis of Lyme arthritis were studied. The demographics of the patients are shown in Table 1. The duration of arthritis varied from 3 days to 7 years. It is interesting that only two patients (22%) were HLA-DR4 positive, approximating the frequencing in the general population (48). This contrasts markedly with our rheumatoid arthritis population, which was 71% DR4 positive (12), in agreement with other studies (38).
TABLE 1.
Demographics of Lyme arthritis patientsa
| Patient no. | Age | Sexb | Duration of arthritis | HLA-DR (DRB1) |
|---|---|---|---|---|
| 1 | 9 | F | 4 yr | 8 (0801), 11(1104) |
| 2 | 15 | M | 3 days | 7 (0701), 13 (1301) |
| 3 | 67 | M | 1 wk | 1 (0101), 4 (0401) |
| 4 | 16 | F | 2 mo | 3 (0301), 11 (1104) |
| 5 | 15 | M | 1 yr | 4 (0401), 13 (1301) |
| 6 | 14 | M | 8 mo | 11 (1103), 13 (1303) |
| 7 | 60 | F | 7 yr | 1 (0103), 3 (0301) |
| 8 | 63 | M | 10 days | 11 (1104), 12 (1202) |
| 9 | 16 | F | 6 mo | 3 (0301), 13 (1302) |
Patients were seen at the University of Medicine and Dentistry of New Jersey’s Robert Wood Johnson Medical School, Yale University, or The University of Vermont. Each patient had lived in an area in which Lyme disease is endemic, had a typical exposure history, and manifested a positive serum Lyme antibody by enzyme-linked immunosorbent assay. Antibody levels were frequently higher in synovial fluid than in serum. HLA-DR typing was done by PCR with allele-specific oligonucleotides as described in Materials and Methods.
F, female; M, male.
Sufficient freshly isolated synovial fluid T cells were obtained from the Lyme arthritis patients to permit the analysis of surface phenotype as well as to test the proliferative response to B. burgdorferi. As shown in Fig. 1, Lyme arthritis synovial fluid T cells expressed prominent levels of the memory cell marker CD45RO, consistent with the notion that this population has been previously activated in vivo. A T-cell proliferative response to a sonicate of B. burgdorferi was observed in each of the Lyme arthritis synovial fluid samples and was striking in some (Table 2). The response of PBL from these patients to B. burgdorferi has been reported previously (31), and while prominent, it was not markedly different from that of normal individuals and was considerably weaker than the response of synovial fluid T cells from the same individual. No significant response to B. burgdorferi has been observed for three synovial fluid specimens from patients with rheumatoid arthritis (30a, 31).
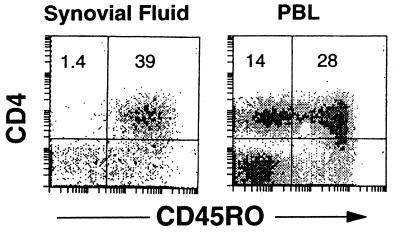
FIG. 1.
Synovial fluid T lymphocytes from Lyme arthritis patients bear a memory phenotype. Synovial fluid and PBL were stained for expression of CD4 and CD45RO and analyzed by flow cytometry. Numbers represent the percentages of cells in the quadrants. Similar results were seen for three other Lyme arthritis synovial fluid samples.
TABLE 2.
Phenotype and response to B. burgdorferi of synovial fluid lymphocytes from Lyme arthritis patientsa
| Patient no. | % of cells positive for:
|
Proliferation (cpm) withb:
|
||
|---|---|---|---|---|
| CD4 | CD8 | Medium only | Medium plus B. burgdorferi sonicate | |
| 1 | 26.5 | 33.7 | 7,132 | 40,055 |
| 2 | 41.0 | 32.0 | 6,442 | 11,127 |
| 3 | 19.3 | 9.1 | 8,136 | 106,711 |
| 4 | 52.6 | 28.2 | 526 | 19,122 |
| 5 | 27.8 | 45.7 | 17,369 | 82,440 |
| 6 | 24.7 | 41.3 | 4,516 | 51,278 |
| 7 | 33.6 | 24.4 | 2,230 | 4,463 |
| 8 | 45.4 | 35.3 | 30,230 | 89,533 |
| 9 | 29.7 | 45.3 | 5,316 | 178,964 |
Synovial fluid lymphocytes were isolated with Ficoll-Hypaque. Freshly isolated specimens were stained for CD4 and CD8 and analyzed by flow cytometry. Samples (5 × 104 cells/well) were also cultured in serum-free medium in the absence or presence of a sonicate of B. burgdorferi (3 μg/ml). Proliferation was measured after 7 days by measuring [3H]thymidine incorporation over the last 18 h.
Values are the means of data from triplicate cultures. Standard deviations were less than 15%.
Parameters of quantitative PCR for TCR Vβ.
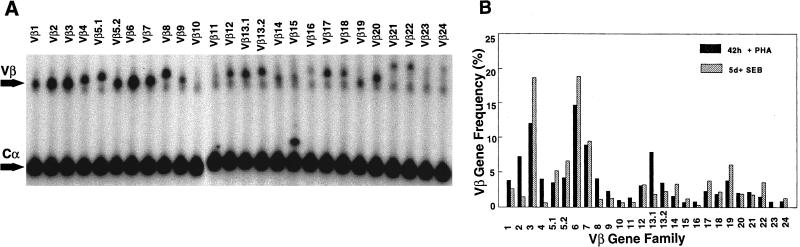
To establish parameters for a quantitative PCR that would provide a sensitive and consistent determination of TCR Vβ usage, several variables were investigated. These are detailed in Materials and Methods. Some of these variables (e.g., cDNA titration and cycle number) have been recently described for a similar study on rheumatoid synovial T cells (12) and are consequently not repeated here. As an initial test of the sensitivity and accuracy of the quantitative PCR assay, a sample of normal PBL was analyzed following activation with either PHA for 42 h or Staphylococcus enterotoxin B (SEB) for 5 days. Figure 2A shows the actual gel on which the PCR products of the PHA-activated PBL were resolved. The upper bands represent the individual Vβ products, each of which is paired with a lower Cα band that was coamplified in the same tube as an internal control for amplification. Figure 2B illustrates the derived percentages (see Materials and Methods) of each of the 26 Vβs examined, comparing PHA and SEB stimulation. Consistent with other reports (10, 20), PBL activated with PHA showed little difference from fresh PBL, manifesting a prominent expression of Vβ2, Vβ3, Vβ6, Vβ7, and Vβ13.1. In contrast, stimulation with SEB provoked a selective increase in Vβ3 and, to a lesser extent, Vβ6, Vβ14, Vβ17, Vβ19, and Vβ22, in general agreement with the data of Choi et al. (10). The magnitude of the selective Vβ increases (1.3- to 1.5-fold) that we observed after SEB stimulation are worth noting for later comparison with the differences in Vβ percentages of synovial fluid T cells and PBL.
FIG. 2.
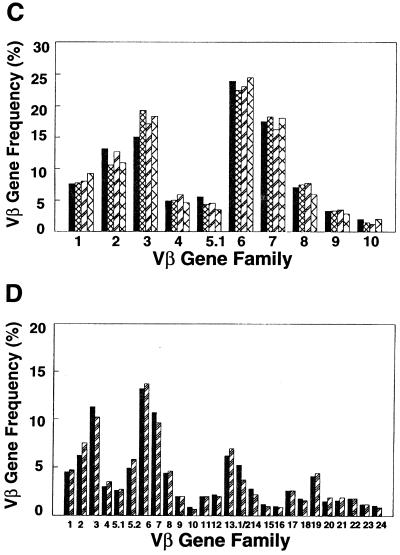
Accuracy and consistency of quantitative PCR. (A) Actual gel of PHA-activated PBL showing the resolution of the individual amplified Vβ products, as well as a coamplified Cα product to control for any variability in aliquoting of reagents or thermocycling of individual tubes (arrows). (B) Graph showing the computed Vβ percentages for PBL stimulated either with PHA for 42 h (black bars) or with SEB for 5 days (hatched bars). Note the SEB-induced increased expression of Vβ3, Vβ6, Vβ14, Vβ17, Vβ19, and Vβ22. (C and D) cDNA from the same sample of PBL was amplified at monthly intervals. Each bar pattern represents a different monthly time point. Shown are results of PCR with 10 different Vβ primers (C) and with the entire panel of 26 Vβ primers (D). The differences in Vβ percentages for the same Vβ between panels C and D result from normalization to only 10 Vβ (C) versus 26 Vβ (D).
An assessment of the consistency of the PCR assay was next performed, using the same cDNA sample derived from normal PBL, by amplifying it on four separate occasions at monthly intervals. Figure 2C shows an example of the results of PCR with the first 10 Vβ primers. The standard deviation was less than 10% for each Vβ. This was repeated on two additional occasions, with the entire panel of 26 Vβ primers, with similar results (Fig. 2D). Thus, the TCR Vβ quantitative PCR was both highly consistent and very sensitive.
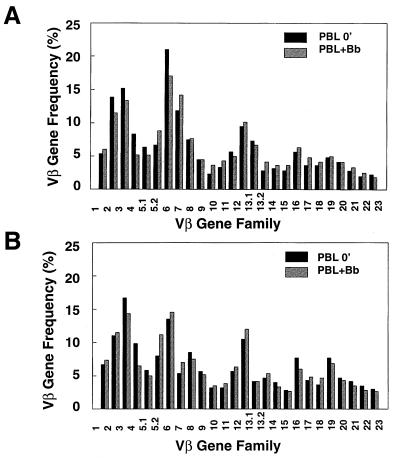
As shown in Fig. 3, the TCR Vβ repertoire of PBL from patients with active Lyme arthritis did not differ significantly from that of PBL from normal individuals, either when freshly isolated or after stimulation with B. burgdorferi for 7 days. In addition, Borrelia stimulation did not provoke any striking shift in the T-cell Vβ repertoire of PBL from normal individuals or Lyme arthritis patients. This would argue against a superantigen response to B. burgdorferi, which is consistent with our earlier observation that Borrelia-specific T-cell clones are restricted in their response to self-HLA molecules (31). The assay was then applied to a series of Lyme arthritis synovial fluid samples, both freshly isolated and after stimulation with B. burgdorferi.
FIG. 3.
Lack of TCR Vβ bias in PBL of Lyme arthritis patients before or after Borrelia stimulation. PBL from a normal individual (A) and a Lyme arthritis patient (B), either freshly isolated (PBL 0′; black bars) or after stimulation with B. burgdorferi for 7 days (PBL+Bb; hatched bars), were analyzed for Vβ gene frequency. No consistent differences in Vβ usage in PBL from two normal individuals or two Lyme arthritis patients were observed.
TCR Vβ bias of synovial fluid T cells toward Vβ2 and Vβ6, compared to that of PBL.
Two statistical comparisons of the Vβ repertoire were made between T-cell populations in Lyme arthritis. The first comparison was between PBL and freshly isolated synovial fluid T cells from the same patient. This was possible for six patients. The second comparison was between synovial fluid T cells before and after stimulation with B. burgdorferi for 7 days. This was possible for all nine patients. Comparisons were made using two approaches that we have previously applied to a similar analysis of the rheumatoid arthritis synovial fluid T-cell repertoire (12). The first considered the ratio of each Vβ in the two populations. As this might bias results toward seeing significant changes from small differences in Vβ that are not highly expressed, a second method evaluated the absolute differences for each Vβ between the two populations being compared. In this instance, only those Vβs with a mean absolute difference of >1% were considered for statistical significance. The results of the two methods were comparable, and hence only the absolute differences are shown. All Vβs were detectable in each of the PBL and synovial fluid samples.
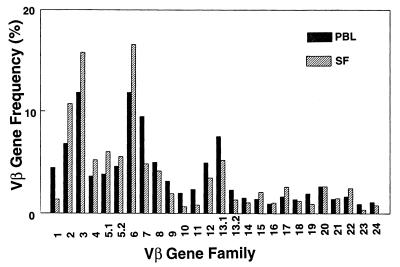
An example of a comparison of Vβ usage between PBL and fresh synovial fluid is shown in Fig. 4 for patient 8, and the absolute differences for each Vβ for the six patients are summarized in Table 3. The P values, based on the paired t test, for the mean Vβ percentages of all six patients were determined. These results revealed that in synovial fluid only Vβ2 and Vβ6 were seen at a statistically increased frequency (Table 3). The findings bear remarkable similarity to our findings in studies of rheumatoid arthritis, where Vβ2 and Vβ6 were also statistically significantly increased in synovial fluid relative to PBL (12). Although there was insufficient sample material in the specimens to separate synovial fluid lymphocytes into CD4+ and CD8+ subsets, it is unlikely that the bias represents merely a skewing of the CD4/CD8 ratio in synovial fluid. We have previously determined that Vβ2 and Vβ6 are slightly more common in CD4+ cells than in the CD8+ subset (12), and yet the proportions of CD4+ and CD8+ cells were either similar to PBL (in five patients) or biased toward CD8+ cells (in 4 patients) (Table 2).
FIG. 4.
Lyme arthritis patient synovial fluid exhibits selective increases in Vβ2 and Vβ6. (A) Comparison of Vβ gene frequencies of PBL (black bars) and fresh synovial fluid (SF) (hatched bars) from patient no. 8.
TABLE 3.
Summary of analyses of the absolute differences in Vβ frequencies between PBL and fresh synovial fluid from six Lyme arthritis patients
| Patient no. | Absolute difference in % gene frequency for Vβa:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2b | 3 | 4 | 5.1 | 5.2 | 6b | 7 | 8 | 9 | 10 | 11 | |
| 1 | 0.095 | 2.712 | 2.328 | −4.667 | −0.549 | 0.204 | 4.001 | 3.004 | 0.41 | −0.569 | −0.674 | −0.529 |
| 2 | 3.651 | 3.506 | −2.327 | −4.305 | 0.18 | −0.107 | 3.927 | −0.323 | 0.407 | −0.126 | 0.014 | 0.091 |
| 4 | 0.454 | 0.745 | −1.37 | −4.275 | 0.035 | −0.396 | −0.263 | −0.682 | 0.716 | −0.488 | 0.258 | 0.549 |
| 5 | −2.629 | 1.763 | 3.087 | 1.998 | 0.485 | −0.035 | 6.247 | −6.612 | 1.075 | −0.675 | −0.096 | 0.345 |
| 6 | −0.464 | 0.071 | −0.582 | −0.715 | 0.201 | −0.046 | −0.362 | 0.22 | −0.776 | −0.045 | 0.218 | 0.169 |
| 8 | −3.068 | 3.964 | 3.966 | 1.586 | 2.175 | 0.923 | 4.727 | −4.651 | −0.834 | −1.228 | −1.314 | −1.518 |
| Mean | −0.327 | 2.127 | 0.85 | −1.73 | 0.421 | 0.091 | 3.046 | −1.499 | 0.166 | −0.622 | −0.266 | −0.149 |
| SE | 0.991 | 0.629 | 1.064 | 1.26 | 0.378 | 0.184 | 1.115 | 1.432 | 0.323 | 0.174 | 0.25 | 0.311 |
| P | 0.759 | 0.02 | 0.461 | 0.228 | 0.315 | 0.644 | 0.041 | 0.343 | 0.629 | 0.03 | 0.337 | 0.653 |
| Patient no. | Absolute difference in % gene frequency for Vβa:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 13.1 | 13.2 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| 1 | −0.082 | −2.311 | −2.131 | 0.338 | −0.162 | −0.811 | 0.365 | −0.026 | −0.573 | −0.204 | 0.773 | −0.621 | −0.146 | −0.177 |
| 2 | −0.762 | −0.115 | −2.398 | −0.027 | 0.229 | −0.866 | 0.098 | −0.001 | −0.248 | 1.563 | 0.529 | 0.08 | −0.053 | 0.058 |
| 4 | 0.782 | −0.285 | −1.176 | 0.713 | 0.83 | −0.037 | −0.241 | 0.91 | 0.216 | 1.023 | 0.723 | 0.116 | 0.0624 | 0.524 |
| 5 | 0.567 | −3.019 | −1.163 | −0.751 | −0.076 | −0.133 | −0.109 | −0.301 | −1.158 | 1.243 | −0.117 | −0.472 | 0.55 | −0.064 |
| 6 | −0.132 | 0.002 | −0.921 | −0.297 | 0.785 | 0.098 | −0.175 | −0.072 | 0.584 | −0.256 | 0.512 | −0.064 | 1.088 | 0.826 |
| 8 | −1.455 | −2.296 | −0.948 | −0.46 | 0.677 | 0.084 | 0.923 | −0.144 | −0.998 | 0.011 | 0.073 | 0.594 | −0.531 | −0.3 |
| Mean | −0.18 | −1.337 | −1.456 | −0.081 | 0.381 | −0.278 | 0.144 | 0.061 | −0.363 | 0.563 | 0.415 | −0.061 | 0.255 | 0.152 |
| SE | 0.34 | 0.55 | 0.262 | 0.22 | 0.181 | 0.181 | 0.18 | 0.175 | 0.278 | 0.329 | 0.147 | 0.179 | 0.244 | 0.176 |
| P | 0.618 | 0.059 | 0.003 | 0.729 | 0.089 | 0.186 | 0.461 | 0.742 | 0.249 | 0.147 | 0.037 | 0.747 | 0.344 | 0.429 |
A negative value indicates that the Vβ level was higher in the synovial fluid than in the PBL.
This Vβ manifested a mean absolute difference of >1%, and its level was statistically significantly increased in the synovial fluid (P < 0.05).
Borrelia stimulation of synovial fluid T cells does not result in a statistically significant increase in expression of any particular TCR Vβ.
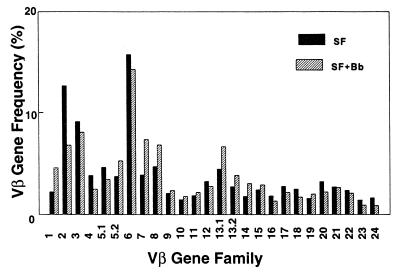
The parallels in the TCR Vβ repertoires of synovial fluid from Lyme arthritis patients and rheumatoid arthritis patients suggested that the Vβ bias of freshly isolated Lyme arthritis synovial fluid might not reflect a response specifically to B. burgdorferi as much as a possible synovium-tropic or nonspecific inflammatory response. This was supported by further analysis of the synovial fluid T-cell repertoires before and after Borrelia stimulation. Culture of synovial fluid with B. burgdorferi for 7 days produced no consistently increased expression of a given TCR Vβ that was statistically significant. This is illustrated for patient 4 in Fig. 5A and summarized for nine patients in Table 4. Although Vβ13.1 was the most consistently increased Vβ, its P value (0.060) was below the level of significance.
FIG. 5.
Stimulation of Lyme arthritis synovial fluid with B. burgdorferi does not bias the TCR repertoire toward any given Vβ. (A) Comparison of Vβ gene frequencies of fresh synovial fluid (SF) and of SF after culture for 7 days with B. burgdorferi (SF+Bb) (hatched bars) for patient no. 4.
TABLE 4.
Summary of analyses of the absolute differences in Vβ gene frequencies between freshly isolated and Borrelia-stimulated synovial fluid lymphocytes from nine Lyme arthritis patients
| Patient no. | Absolute difference in % gene frequency for Vβa:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5.1 | 5.2 | 6 | 7 | 8 | 9 | 10 | 11 | |
| 1 | −0.045 | −19.145 | 10.299 | 5.486 | 0.983 | −9.434 | 4.747 | 6.124 | 2.758 | 0.024 | −4.194 | 0.144 |
| 2 | 1.691 | 2.403 | −2.229 | 2.811 | 0.956 | 2.59 | −5.266 | −0.155 | −0.331 | 0.305 | 0.643 | −0.112 |
| 3 | 0.95 | 1.613 | −1.935 | 0.617 | 0.065 | 0.273 | −1.134 | 0.9 | 0.951 | −1.142 | −1.833 | 0.254 |
| 4 | −2.303 | 5.889 | 1.047 | 1.343 | 1.183 | −1.512 | 1.495 | −3.494 | −2.136 | −0.268 | −0.317 | −0.321 |
| 5 | −1.14 | 1.274 | 6.413 | 2.424 | −0.165 | −0.088 | −1.772 | −3.926 | −0.091 | −0.461 | 0.249 | 0.005 |
| 6 | 1.448 | −1.696 | 0.026 | −0.017 | −1.649 | 0.765 | −4.419 | 3.101 | 0.536 | 0.553 | 0.852 | 0.775 |
| 7 | 3.041 | −5.43 | −9.245 | 2.497 | 1.289 | 2.996 | 0.075 | 3.728 | −15.339 | 2.162 | 1.157 | 1.347 |
| 8 | −1.901 | 2.072 | 4.295 | 1.987 | 1.238 | −0.54 | 4.404 | −1.347 | −0.637 | −1.429 | −1.481 | −1.808 |
| 9 | 0.34 | 4.875 | 2.844 | 0.845 | −0.124 | 0.057 | 2.105 | −1.805 | −1.379 | −0.456 | −1.49 | −0.855 |
| Mean | 0.231 | −0.905 | 1.279 | 1.998 | 0.419 | −1.119 | 0.026 | 0.347 | −1.741 | −0.079 | −0.712 | −0.063 |
| SE | 0.588 | 2.539 | 1.879 | 0.54 | 0.326 | 1.159 | 1.18 | 1.139 | 1.763 | 0.351 | −0.713 | 0.302 |
| P | 0.705 | 0.731 | 0.515 | 0.006 | 0.234 | 0.362 | 0.983 | 0.768 | 0.352 | 0.827 | 0.247 | 0.839 |
| Patient no. | Absolute difference in % gene frequency for Vβa:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 13.1 | 13.2 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| 1 | −2.537 | −1.729 | 1.098 | 0.414 | 0.735 | 0.241 | 1.216 | −0.171 | 0.107 | 0.541 | 1.471 | 0.485 | 0.46 | −0.124 |
| 2 | −0.688 | 0.775 | 0.519 | 0.134 | 0.793 | 0.93 | 0.567 | −0.825 | 0.301 | 1.529 | −0.368 | −0.114 | 0.276 | 0.721 |
| 3 | 0.411 | 1.536 | 0.384 | 0.43 | 1.079 | 0.019 | −1.306 | 0.274 | 0.448 | 0.075 | 0.429 | −1.451 | −1.6 | −0.238 |
| 4 | 0.487 | −2.216 | −1.113 | −1.257 | −0.502 | 0.491 | 0.56 | 0.767 | 0.404 | 1 | 0.051 | 0.277 | 0.499 | 0.751 |
| 5 | −0.915 | −1.572 | 0.073 | −0.157 | 0.391 | 0.3 | −0.012 | −0.08 | −0.952 | −0.478 | −0.083 | −0.086 | 0.672 | 0.182 |
| 6 | −0.517 | −3.503 | 0.201 | 0.15 | 0.176 | −0.136 | −0.427 | −0.4 | 1.526 | 0.149 | −0.126 | 0.895 | 1.007 | 0.787 |
| 7 | 2.108 | 0.13 | 2.414 | 0.7 | 1.756 | 1.239 | 1.335 | −3.325 | 1.197 | 0.917 | 1.476 | 0.726 | 0.312 | 0.57 |
| 8 | −1.292 | −2 | −0.209 | −0.405 | 0.446 | 0.001 | 0.924 | −0.486 | −1.129 | 0.374 | −0.048 | 0.345 | −0.407 | −0.247 |
| 9 | 0.138 | −1.813 | −0.088 | −0.845 | −0.266 | −0.515 | 1.148 | −0.357 | −0.881 | −0.395 | −0.307 | −0.288 | −0.78 | −0.408 |
| Mean | 0.312 | −1.208 | 0.364 | −0.093 | 0.515 | 0.286 | 0.445 | −0.432 | 0.024 | 0.41 | 0.277 | 0.088 | 0.049 | 0.222 |
| SE | 0.436 | 0.552 | 0.325 | 0.214 | 0.231 | 0.18 | 0.293 | 0.396 | 0.316 | 0.221 | 0.238 | 0.233 | 0.274 | 0.163 |
| P | 0.495 | 0.06 | 0.294 | 0.676 | 0.076 | 0.152 | 0.167 | 0.307 | 0.942 | 0.1 | 0.278 | 0.716 | 0.863 | 0.212 |
A negative value indicates that the Vβ was increased after Borrelia stimulation.
DISCUSSION
The TCR Vβ repertoire of fresh synovial fluid T lymphocytes from Lyme arthritis patients manifests a bias compared to that of PBL, with a statistically significant increase of Vβ2 and Vβ6. Particularly striking was the consistency with which this skewing was observed despite the heterogeneous HLA phenotypes of the patients. Of further interest is that our previous comparison of the TCR Vβ repertoires of synovial fluid and PBL from rheumatoid arthritis patients also revealed an increase of Vβ2 and Vβ6 in synovial fluid (12). At least three additional studies of rheumatoid arthritis have also revealed an increased expression of Vβ2 and/or Vβ6 in synovial fluid (14, 18, 20). The present study represents the first analysis of this type for Lyme arthritis. Conceivably, this TCR Vβ pattern may represent a synovium-tropic bias or an inflammatory response rather than a skewing provoked by a reaction to the inciting agent.
Several modifications were incorporated into the quantitative PCR assay to optimize the sensitivity and consistency of detecting TCR Vβ products. First, internal labeling with [α-32P]dCTP increased the sensitivity 1 log over that of primer labeling. Second, amplified products were counted directly from gels, with no further blotting or hybridizations that might introduce additional variables. Third, inclusion of an internal control Cα PCR amplification for each sample allowed the determination of the uniformity of each reaction and hence the relative amounts of the TCR Vβ product amplified with each Vβ primer. The relative expression of each Vβ gene family member is proportional to both the amount of Vβ mRNA in the sample and the Vβ primer efficiency, the latter possibly varying with the different Vβ primers used. Thus, the assay does not necessarily provide an absolute measure of the percentage of T cells bearing a particular Vβ. However, since the experimental design of this study was a comparison of the TCR Vβ repertoires of simultaneously analyzed samples, valid conclusions regarding these comparisons can be drawn.
Each of the synovial fluid samples for which sufficient material was available manifested a proliferative response to B. burgdorferi. We have not observed such a response for synovial fluid samples from rheumatoid arthritis patients (31). The presence of B. burgdorferi in the synovium of Lyme disease patients has been previously documented by histologic staining (43) and, more recently, by PCR (29). This suggests that chronic antigenic stimulation by B. burgdorferi could contribute to the biased repertoire of synovial fluid T lymphocytes. However, stimulation of synovial fluid with B. burgdorferi did not yielded a consistent statistically significant increase in a particular TCR Vβ. At present we have no evidence that the nonstatistical Vβ13.1 increase represents a Borrelia-specific response by T cells. First, Borrelia stimulation of PBL did not yield a Vβ bias, including Vβ2, Vβ6, or Vβ13.1 (Fig. 3). Second, an analysis of six Borrelia-reactive T-cell clones from PBL of an individual yielded a diverse Vβ repertoire, including Vβ3, Vβ6, Vβ7, Vβ17, and Vβ21. Finally, a study of Vβ13.1-positive synovial clones derived from one Lyme arthritis patient manifested no Borrelia-specific response (30a). Thus, the T-cell repertoire responding to B. burgdorferi is diverse and not restricted to Vβ13.1; additionally, we have not observed a bias toward Vβ5.1, as suggested by a previous analysis of Borrelia-reactive T-cell clones from a single Lyme arthritis patient (22).
The above information, combined with the increased expression of Vβ2 and Vβ6 in synovial fluid of rheumotoid arthritis patients, argues against a response to B. burgdorferi as the explanation for the skewed TCR repertoire of fresh synovial fluid. One possibility is that a synovial-tissue-specific response by T cells might select for Vβ2 and Vβ6. Consistent with this model is an ongoing comparison of the T-cell repertoires of PBL and synovial fluid from six patients with psoriatic arthritis and two individuals with reactive arthritis. Thus far, only Vβ6 is consistently elevated in the synovial fluid from all paired samples (30a). If Vβ2 and Vβ6 T cells have indeed been chronically stimulated in the synovium, they might be functionally anergic when further attempts at in vitro activation are made. The diminished cloning efficiency of synovial T cells in rheumatoid arthritis has been well described (25). This could result in the in vitro overgrowth by nonanergic T cells bearing other TCRs, as was seen with the emergence of Vβ13.1 upon Borrelia stimulation.
An alternative explanation for the dominance of Vβ2 and Vβ6 in fresh synovial fluid is that this results from nonspecific infiltration into an inflamed tissue without there necessarily being a reaction to any tissue component. Vβ2 and Vβ6 could predominate in tissues simply because they dominate the normal TCR repertoire in PBL. In this scenario, B. burgdorferi might initiate an early antigen-specific response that is rapidly eclipsed by a nonspecific inflammatory response. Similar TCR Vβ spreading with time has been noted in autoimmunity models, such as in lymphoid infiltrates in the pancreatic islets of NOD mice and in the central nervous system in experimental encephalomyelitis (7, 53). Consistent with this view, Vβ2 and Vβ6 are also prominent in psoriatic skin lesions (27, 49). Determination of whether the Vβ2 and Vβ6 bias in synovial fluid represents an antigen-driven response or a nonspecific influx might be achieved through further analysis for selective oligoclonal expansion using single-stranded conformational polymorphism spectrotyping and sequencing. In this regard, preliminary analysis of the TCR repertoire in rheumatoid arthritis has demonstrated a slight oligoclonality of Vβ2 sequences in synovial fluid compared to PBL (30a).
The presence of TCR Vβ skewing at sites of inflamed tissues raises the issue of a possible response to a superantigen. In this regard, we previously observed that PBL from normal individuals, with no previous exposure to B. burgdorferi, nonetheless manifested prominent T-cell proliferative responses to a Borrelia sonicate (31). This response was independent of the HLA-DR haplotype of the individual, and it was sensitive to protease digestion of B. burgdorferi. While both of these observations are features inherent to superantigen responses, other aspects of this response did not support a superantigen model. In almost all cases, a panel of Borrelia-responsive T-cells clones exhibited restriction to self-HLA-DR molecules (31). This is in agreement with the findings of Lahesmaa et al. (22), who observed that 41 of 43 B. burgdorferi-reactive T-cells clones derived from a single Lyme arthritis patient were restricted to autologous HLA class II alleles. Similarly, we have not observed any consistent TCR Vβ bias following Borrelia stimulation of peripheral blood T cells from either normal individuals or Lyme arthritis patients. On balance, the information to date does not strongly support the existence of a superantigen within B. burgdorferi.
Mechanisms other than superantigens might also contribute to selective Vβ bias. For example, γδ T cells are prominent in Lyme arthritis synovial fluid samples and expand in response to B. burgdorferi (50). Through production of particular cytokines or by selective cytolytic activity, the γδ T cells might favor the survival of particular T-cell subsets. Thus, a variety of immune-regulatory responses may be responsible for the T-cell repertoire bias in the synovium of a Lyme arthritis patient. However, given our knowledge of the causative organism, it should be possible to dissect these components.
ACKNOWLEDGMENTS
We thank Colette Charland for assistance with flow cytometry and Roberta Christie for assistance with preparation of the manuscript.
This work was supported by grants from the National Institutes of Health (AR43520) and the Arthritis Foundation.
REFERENCES
- 1.Abe J, Kotzin B L, Meissner C, Melish M E, Glode M E, Kohasaka T, Leung D Y. Selective expansion of T cells expressing T-cell receptor variable regions Vβ2 and Vβ8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–4069. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe J, Kotzin B L, Meissner C, Melish M E, Takahashi M, Fulton D, Romagne F, Malissen B, Leung D Y. Characterization of T cell repertoire changes in acute Kawasaki disease. J Exp Med. 1993;177:791–796. doi: 10.1084/jem.177.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong A L, Barthold S W, Persing D H, Beck D S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992;47:249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- 4.Balk S P, Ebert E C, Blumenthal R L, McDermott F V, Wucherpfennig K W, Landau S B, Blumberg R S. Oligoclonal expansion and CD1 recognition by human intestinal lymphocytes. Science. 1991;253:1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- 5.Benach J L, Bosler E M, Hanarahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 6.Berger B W, Kaplan M H, Rothenberg I R, Barbour A G. Isolation and characterization of the Lyme disease spirochete from the skin of patients with erythema chronicum migrans. J Am Acad Dermatol. 1985;13:444–449. doi: 10.1016/s0190-9622(85)70187-9. [DOI] [PubMed] [Google Scholar]
- 7.Borcke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, Veromaa T, Waisman A, Gaur A, Conlon P, Ling N, Fairchild P J, Wraith D C, O’Garra A, Fathman C G, Steinman L. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorfer W, Barbour A G, Hayes F F, Benach J L, Grunwaldt E, David J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Lyme disease—United States, 1995. Morbid Mortal Weekly Rep. 1996;45:481–484. [PubMed] [Google Scholar]
- 10.Choi Y W, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Cooper S M, Roessner K R, Naito-Hoopes M, Howard D B, Gaur L K, Budd R C. Increased usage of Vβ2 and Vβ6 in rheumatoid synovial fluid T cells. Arthritis Rheum. 1994;37:1627–1636. doi: 10.1002/art.1780371112. [DOI] [PubMed] [Google Scholar]
- 13.Dattwyler R J, Halperin J J, Volkman D J, Luft B J. Treatment of late Lyme borreliosis—randomised comparison of ceftriaxone and penicillin. Lancet. 1988;i:1191–1194. doi: 10.1016/s0140-6736(88)92011-9. [DOI] [PubMed] [Google Scholar]
- 14.Davey M P, Munkirs D D. Patterns of T-cell receptor variable β gene expression by synovial fluid and peripheral blood T cells in rheumatoid arthritis. Clin Immunol Immunopathol. 1993;68:79–87. doi: 10.1006/clin.1993.1099. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonion H, Sugita M, Glass D N, Maier A L, Weinblatt M E, Rème T, Brenner M B. Clonal Vα12.1 T cell expansions in the peripheral blood of rheumatoid arthritis patients. J Exp Med. 1993;177:1623–1631. doi: 10.1084/jem.177.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duray P H. Clinical pathologic correlations of Lyme disease. Rev Infect Dis. 1989;11:S1487. doi: 10.1093/clinids/11.supplement_6.s1487. [DOI] [PubMed] [Google Scholar]
- 17.Forrester J M, Wang Y, Ricalton N, Fitzgerald J E, Loveless J, Newman L S, King T E, Kotzin B L. TCR expression of activated T cell clones in the lungs of patients with sarcoidosis. J Immunol. 1994;153:4291–4302. [PubMed] [Google Scholar]
- 17a.Gaur, L. Unpublished data.
- 18.Jenkins R N, Nikaein A, Zimmerman A, Meek K, Lipsky P E. T cell receptor Vβ gene bias in rheumatoid arthritis. J Clin Invest. 1993;92:2688–2701. doi: 10.1172/JCI116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalish R A, Leong J M, Steere A C. Delay in the immune response to outer surface proteins (OSP) A and B of B. burgdorferi: correlation with arthritis and treatment failure in susceptible patients with Lyme disease. Arthritis Rheum. 1991;34:S43. [Google Scholar]
- 20.Krawinkel W V, Pluschke G. T cell receptor variable region repertoire in lymphocytes from rheumatoid arthritis patients. Immunobiology. 1992;185:483–491. doi: 10.1016/S0171-2985(11)80090-2. [DOI] [PubMed] [Google Scholar]
- 21.Labrecque N, McGrath H, Subramanyam M, Huber B T, Sekaly R-P. Human T cells respond to mouse mammary tumor virus-encoded superantigen: Vβ restriction and conserved evolutionary features. J Exp Med. 1993;177:1735–1743. doi: 10.1084/jem.177.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahesmaa R, Shanafelt M-C, Allsup A, Soderberg C, Anzola J, Freitas V, Turck C, Steinman L, Peltz G. Preferential usage of T cell antigen receptor V region gene segment Vβ5.1 by Borrelia burgdorferi antigen-reactive T cell clones isolated from a patient with Lyme disease. J Immunol. 1993;150:4125–4135. [PubMed] [Google Scholar]
- 23.Lengl-Janssen B, Strauss A F, Steere A C, Kamradt T. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A (OspA) in patients with treatment-resistant or treatment-responsive Lyme arthritis. J Exp Med. 1994;180:2069–2078. doi: 10.1084/jem.180.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Looney S W, Gulledge T R. Use of the correlation coefficient with normal probability plots. Am Stat. 1985;39:75–79. [Google Scholar]
- 25.Madelon M M, Lankester A C, Bezemer A C, Geerstma M F, Tak P-P, Breedveld F C, van Lier R A W, Verweij C L. Defective TCR-mediated signaling in synovial T cells in rheumatoid arthritis. J Immunol. 1997;159:2973–2978. [PubMed] [Google Scholar]
- 26.Marcus L C, Steere A C, Duray P H, Anderson A E, Mahoney E B. Fatal pancarditis in a patient with coexistent Lyme disease and babesiosis. Ann Intern Med. 1985;103:374–376. doi: 10.7326/0003-4819-103-3-374. [DOI] [PubMed] [Google Scholar]
- 27.Marsh S G E, Bodmer J G. HLA class II nucleotide sequences. Tissue Antigens. 1992;40:229–243. doi: 10.1111/j.1399-0039.1992.tb02050.x. [DOI] [PubMed] [Google Scholar]
- 28.Mensen A, Trommler P, Vollmer S, Schendel D, Albert E, Gurtler L, Riethmuller G, Prinz J C. Evidence for an antigen-specific cellular immune response in skin lesions of patients with psoriasis vulgaris. J Immunol. 1995;155:4078–4083. [PubMed] [Google Scholar]
- 29.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–233. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 30.Paliard X, West S G, Lafferty J A, Clements J R, Kappler J W, Marrack P, Kotzin B L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991;253:325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- 30a.Roessner, K. Unpublished data.
- 31.Roessner K, Fikrig E, Russell J Q, Cooper S M, Flavell R A, Budd R C. Prominent T lymphocyte response to Borrelia burgdorferi from peripheral blood of unexposed donors. Eur J Immunol. 1994;24:320–324. doi: 10.1002/eji.1830240207. [DOI] [PubMed] [Google Scholar]
- 32.Saiki R, Walsh P S, Levenson C H, Erlich H A. Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc Natl Acad Sci USA. 1989;86:6230–6234. doi: 10.1073/pnas.86.16.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaible U E, Kramer M D, Museteanu C, Zimmer G, Mossmann H, Simon M M. The severe combined immunodeficiency (scid) mouse: a laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989;170:1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigal L H, Steere A C, Freeman D H, Dwyer J M. Proliferative responses of mononuclear cells in Lyme disease: reactivity to Borrelia burgdorferi antigens is greater in joint fluid than blood. Arthritis Rheum. 1986;29:761–769. doi: 10.1002/art.1780290609. [DOI] [PubMed] [Google Scholar]
- 35.Sigal L H. Immunopathogenesis of Lyme borreliosis. Clin Dermatol. 1993;11:415–422. doi: 10.1016/0738-081x(93)90098-w. [DOI] [PubMed] [Google Scholar]
- 36.Snydman D R, Schenkein D P, Beradi V P, Lastavica C C, Pariser K M. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med. 1986;104:798–800. doi: 10.7326/0003-4819-104-6-798. [DOI] [PubMed] [Google Scholar]
- 37.Sottini A, Imberti L, Gorla R, Cattaneo R, Primi D. Restricted expression of T cell receptor Vβ but not Vα genes in rheumatoid arthritis. Eur J Immunol. 1991;21:461–466. doi: 10.1002/eji.1830210231. [DOI] [PubMed] [Google Scholar]
- 38.Stastny P. Association of the B cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978;298:869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- 39.Steere A C, Bartenhagen N H, Craft J E, Hutchinson G J, Newman J H, Rahn D W, Sigal L H, Spieler P N, Stenn K S, Malawista S E. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 40.Steere A C, Grodzicki R L, Kornblatt A N, Hutchinson G J, Newman J H, Rahn D W, Sigal L H, Spieler P N, Stenn K S, Malawista S E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 41.Steere A C, Hutchinson G J, Rahn D W, Sigal L H, Craft J E, DeSanna E T, Malawista S E. Treatment of the early manifestations of Lyme disease. Ann Intern Med. 1983;99:22–31. doi: 10.7326/0003-4819-99-1-22. [DOI] [PubMed] [Google Scholar]
- 42.Steere A C, Schoen R T, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 43.Steere A C, Duray P H, Butcher E C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis: comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988;31:487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 44.Steere A C, Feld J, Winchester R. Association of chronic Lyme arthritis with increased frequencies of DR4 and 3. Arthritis Rheum. 1988;31:S98. [Google Scholar]
- 45.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 46.Steere A C, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- 47.Sumida T, Yonaha F, Maeda T, Tanabe E, Koike T, Tomioka H, Yoshida S. T cell receptor of infiltrating T cells in Sjogren’s syndrome patients. J Clin Invest. 1992;89:681–685. doi: 10.1172/JCI115635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svejgard A, Platz P, Ryder L P. HLA and disease, 1982: a survey. Immunol Rev. 1983;70:193–218. doi: 10.1111/j.1600-065x.1983.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 49.Vekony M A, Holder J A, Lee A J, Horrocks C, Eperon I C, Camp R D R. Selective amplification of T-cell receptor variable region species is demonstrable but not essential in early lesions of psoriasis vulgaris: analysis by anchored polymerase chain reaction and hypervariable region size spectratyping. J Invest Dermatol. 1997;109:5–13. doi: 10.1111/1523-1747.ep12276303. [DOI] [PubMed] [Google Scholar]
- 50.Vincent M S, Roessner K, Lynch D, Wilson D, Cooper S M, Tschopp J, Sigal L H, Budd R C. Apoptosis of Fashigh CD4+ synovial T cells by Borrelia-reactive Fas-ligandhigh γδ T cells in Lyme arthritis. J Exp Med. 1996;184:2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams W V, Fang Q, Demarco D, VonFeldt J, Zurier R B, Weiner D B. Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest. 1992;90:326–333. doi: 10.1172/JCI115866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wucherpfennig K W, Newcombe J, Li H, Keddy C, Cuzner M L, Hafler D A. T cell receptor Vα-Vβ repertoire and cytokine gene expression in active multiple sclerosis lesions. J Exp Med. 1992;175:993–1002. doi: 10.1084/jem.175.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Charlton B, Shimada A, Dal Canto R, Fathman C G. Monoclonal T cells identified in early NOD islet infiltrates. Immunity. 1996;4:189–194. doi: 10.1016/s1074-7613(00)80683-4. [DOI] [PubMed] [Google Scholar]
- 54.Zamvill S S, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]