Abstract
Background and objectives
Comprehensive analysis of multi-omics data is crucial for accurately formulating effective treatment plans for complex diseases. Supervised ensemble methods have gained popularity in recent years for multi-omics data analysis. However, existing research based on supervised learning algorithms often fails to fully harness the information from unlabeled nodes and overlooks the latent features within and among different omics, as well as the various associations among features. Here, we present a novel multi-omics integrative method MOSEGCN, based on the Transformer multi-head self-attention mechanism and Graph Convolutional Networks(GCN), with the aim of enhancing the accuracy of complex disease classification. MOSEGCN first employs the Transformer multi-head self-attention mechanism and Similarity Network Fusion (SNF) to separately learn the inherent correlations of latent features within and among different omics, constructing a comprehensive view of diseases. Subsequently, it feeds the learned crucial information into a self-ensembling Graph Convolutional Network (SEGCN) built upon semi-supervised learning methods for training and testing, facilitating a better analysis and utilization of information from multi-omics data to achieve precise classification of disease subtypes.
Results
The experimental results show that MOSEGCN outperforms several state-of-the-art multi-omics integrative analysis approaches on three types of omics data: mRNA expression data, microRNA expression data, and DNA methylation data, with accuracy rates of 83.0% for Alzheimer's disease and 86.7% for breast cancer subtyping. Furthermore, MOSEGCN exhibits strong generalizability on the GBM dataset, enabling the identification of important biomarkers for related diseases.
Conclusion
MOSEGCN explores the significant relationship information among different omics and within each omics' latent features, effectively leveraging labeled and unlabeled information to further enhance the accuracy of complex disease classification. It also provides a promising approach for identifying reliable biomarkers, paving the way for personalized medicine.
Keywords: Multi-omics, Semi-supervised learning, Multi-head self-attention mechanism, Graph Convolutional Networks
Introduction
The advent of cutting-edge sequencing technologies has facilitated the rapid acquisition of voluminous data from various omics domains, including mRNA expression, DNA methylation, and microRNA expression data. The utilization of diverse omics data enables the multifaceted representation of the biological processes underpinning complex diseases. In the early stages, the majority of researchers primarily employed traditional machine learning methods for the "unidimensional" analysis of single omics data in the study of disease mechanisms [1]. mRNA gene expression was the most prevalent focus [2–4]. However, for the intricacies of biological complexity, the analysis of single omics data remains inherently limited [5]. Current research has shown that, in comparison to experiments conducted using single omics data, the utilization of multi-omics data sources permits a more comprehensive analysis of disease risk, prognosis, and enhances predictive capabilities [6–9]. The integration analysis of multi-omics data supplements the information from various omics domains, compensating for the limitations of singular omics datasets and providing a more comprehensive research perspective for disease classification [10].
Some of the existing multi-omics studies have been rooted in unsupervised learning approaches. Chen Meng et al. [11] proposed multiple co-inertia analysis (MCIA) method. This method employs a covariance optimization criterion to simultaneously project multiple datasets (such as genes and proteins) onto a common one-dimensional space. It transforms distinct sets of features to a uniform scale, facilitating the extraction of features relevant to sample clusters. Michael J et al. [12] introduced the Joint and Individual Variation Explained (JIVE) method as an exploratory dimensionality reduction tool. JIVE dissects multi-omics datasets and integrates them to acquire comprehensive information regarding breast cancer. However, in recent years, due to the rapid advancement in medical technology and the accumulation of relevant data, the volume of biological features and trait data exhibited by individuals has increased significantly. Utilizing unsupervised learning is no longer sufficient to meet the demands of integrated analysis for multi-omics data. Instead, supervised learning methods in multi-omics, which incorporate sample label information, are increasingly applied in disease prognosis and prediction research. ZI-YI YANG et al. [13] proposed the Multi-Modal Self-Paced Learning (MSPL) algorithm for the integration of multi-omics data. This approach employs a sparse logistic regression classifier in cancer subtype classification and identifies latent biological features. Xu et al. [14] employed a novel hierarchical integrated deep flexible neural forest framework (HI-DFNForest) to integrate three types of omics data: DNA methylation, gene expression, and microRNA expression data, successfully classifying ovarian subtypes. Yang et al. [15] introduced the Subtype-GAN method, a deep adversarial learning approach with multiple inputs and outputs, which utilizes consistency clustering and Gaussian mixture models to identify molecular subtypes of tumor samples. Singh et al. [16] proposed Data Integration Analysis for Biomarker Discovery (DIABLO), a multivariate dimensionality reduction method that maximally utilizes covariance and latent components information within linear combinations of features from multiple omics sources for prediction. While these methods have demonstrated effectiveness, they have not fully considered the relationships among different omics data types and have overlooked inter-patient correlations. Given the importance of leveraging both inter-patient correlations and inter-omics relationships, Wang T et al. [17] introduced a Multi-Omics Graph Convolutional Networks (MOGONET) algorithm. This algorithm employs cosine similarity to compute a patient correlation network as input for Graph Convolutional Networks (GCN) and explores cross-omics correlations in the label space using View Correlation Discovery Network (VCDN) after GCN output. Li et al. [18] proposed a multi-omics integration method based on graph convolutional networks (MOGCN). This method utilizes autoencoders for dimensionality reduction, integrates Copy Number Variations (CNV), mRNA, and Reverse Phase Protein Array (RPPA) data, and employs the results of Similarity Network Fusion (SNF) to construct a patient similarity network as GCN input.
In summary, while the aforementioned methods consider inter-patient correlations and inter-omics relationships and have, to a certain extent, improved the accuracy of complex disease classification, they still face certain challenges. Firstly, many data types have a limited number of labeled samples and a larger number of unlabeled samples. Traditional supervised learning methods do not directly leverage information from unlabeled nodes, and classic GCN methods do not utilize unlabeled node information directly during the training process [19], restricting information propagation and diminishing model generalization capabilities. Secondly, previous feature processing methods have not accounted for the unique subspaces of each omics data type and the multiple associations and dependencies among latent features within different omics data. This oversight may lead to results that are biased towards specific omics data types or particular features. Addressing these issues, we propose a novel ensemble learning model for analyzing multi-omics data. It fully exploits the correlations within the latent features of each omics data and inter-omics relationships, as well as the information from unlabeled nodes. The model is constructed by utilizing Transformer encoding modules to explore the potential advanced features and inherent relationships within each omics data and between different omics. Subsequently, it employs Similarity Network Fusion (SNF) to build a patient similarity fusion network. Finally, it employs Self-Ensembling Graph Convolutional Networks (SEGCN) for training, simultaneously utilizing labeled and unlabeled data to better capture the overall characteristics and underlying structures of the data, thereby enhancing model generalization capabilities. Additionally, this model can identify important omics features and biomarkers, offering interpretability and providing a research methodology for future clinical.
Methods
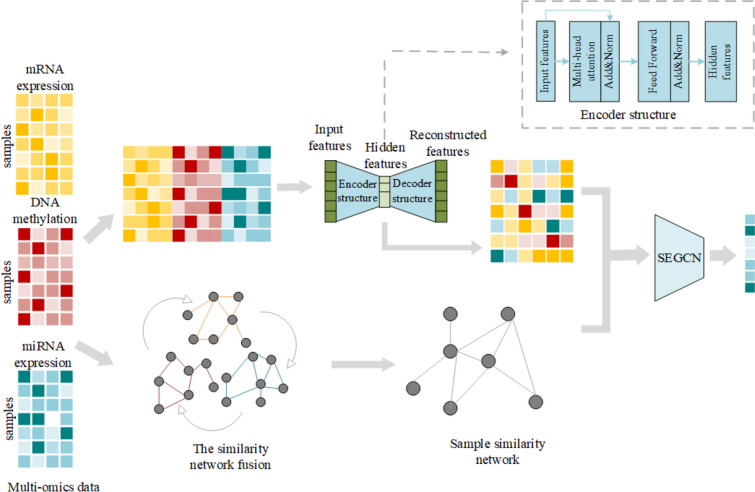
In this section, we shall provide a comprehensive exposition of the content pertaining to the multi-omics data integration learning model, MOSEGCN. Figure 1 illustrates the framework of MOSEGCN, which primarily comprises three components: the Transformer encoding module tailored for multi-omics features learning, the module dedicated to constructing a patient-fusion similarity network, and the ultimate SEGCN classification module.
Fig. 1.
MOSEGCN Framework
Transformer
The Transformer model was initially employed in natural language processing [20]. Over time, it underwent adaptations for image recognition and object detection, demonstrating its efficacy [21–25]. The fundamental Transformer architecture comprises an input layer, multi-head self-attention blocks, normalization layers, feedforward layers, and residual connection layers. Essentially, it embodies an Encoder-Decoder framework [26]. Key components within the Transformer model are the multi-head self-attention mechanism and the autoencoder. The autoencoder is proficient at discerning latent features from input data, offering an effective approach to amalgamate distinct features [27]. The multi-head self-attention mechanism is an enhanced algorithm building upon common attention mechanisms. Its virtue lies in its ability to apprehend the intrinsic correlations among various features across different positions and data points [28]. This algorithm excels in capturing the inner relationships among diverse features and mitigating reliance on external information. It notably accentuates critical attributes for classifying related disease subtypes, with a particular emphasis on valuable insights from the test set, comprising unlabeled nodes.
Given that identical samples in the data encompass features from diverse omics, the experimental approach necessitates the full exploitation of concealed information within each omic, inter-omic latent feature information, and multifarious associations and dependencies among features. Consequently, this experimental method introduces a self-attention layer prior to the encoder's output layer. This layer comprehensively captures positional information from the input data, explores correlations between latent features within each omic and across different omics, and assesses the significance of features within each modality. The residual connections [29] facilitate the flow of information within the model, and normalization layers [30], positioned after the self-attention layer and before the feedforward network, enhance training stability and expedite convergence.
Autoencoder
The autoencoder is an unsupervised neural network model employing the backpropagation algorithm. Typically, it consists of two modules: the encoder and the decoder. The encoder maps input data into a lower-dimensional latent space, which is then mapped back to the original data space by the decoder [31]. Given that both the latent features learned within each omics data's exclusive subspace and the latent features across different omics contribute to the model [32], and considering that the multi-head self-attention mechanism accounts for correlations among positions in input data, the experimental setup utilizes feature data concatenated from three modalities of the original input , where N represents the number of samples, where represents the features possessed by the i-th modality. The entire process of the autoencoder can be represented as follows:and
| 1 |
where is the reconstruction representation with the same shape as X, and are the parameters of the encoder and decoder neural networks, respectively. , H is referred to as the latent representation of X, meaning the encoder maps N samples from a P-dimensional space to a K-dimensional space. Finally, the autoencoder trains the encoder and decoder by minimizing the reconstruction error to learn useful representations of data both within the same modality and across different modalities: . In this experiment, only the encoder function block is used to obtain the ultimately valuable features.
The multi-head self-attention mechanism
The multi-head self-attention mechanism builds upon the foundation of the self-attention mechanism, introducing multiple attention heads to fully leverage input information in capturing various associations and dependencies within features. This enhances the model's comprehension of feature information [33]. Since the features extracted by the autoencoder may contain some redundancy or irrelevant elements, potentially overlooking hidden information, this experiment employs the multi-head self-attention mechanism to further learn the internal correlations among features at various positions. This, in turn, assigns higher weights to crucial features in the context of cancer subtype classification, aiding the neural network in feature selection [34]. In conclusion, the inclusion of the multi-head self-attention mechanism allows for the identification of pivotal features vital for predicting events based on critical information from different omics and individual omics data. The computational formula for multi-head attention is as follows:
| 2 |
| 3 |
represents the output transformation matrix, h denotes the number of heads, and signifies the output of the i-th head and correspondingly emerge from the linear transformations of the latent vector H, with representing the parameter matrix.
SNF
The Similarity Network Fusion (SNF) [35] method employs pairwise correlations between samples to construct sample similarity matrices for each omics data type. In this experiment, the neighborhood size is set to 30, and the hyperparameter σ is assigned a value of 0.5. Distinct sample similarity networks are constructed for different omics data types. Subsequently, leveraging the complementary information from different omics data types, the three distinct similarity networks obtained earlier are computed and fused, eliminating weak connections. Ultimately, a comprehensive view of the disease is established. In this final comprehensive view, nodes represent samples, and edges indicate pairwise similarities between samples. The experiment implements this module in the PYTHON software using the SNFpy package, facilitating graph integration analysis.
Self-ensembling graph convolutional networks
To enhance model performance by fully leveraging the information from unlabeled nodes, our experiment employs the Self-Ensembling Graph Convolutional Networks (SEGCN) method [36]. SEGCN represents a potent and highly reliable self-ensembling learning mechanism that combines GCN (Graph Convolutional Networks) and Mean Teacher in a semi-supervised task. GCN, a deep learning model designed for processing graph-structured data, operates on the fundamental principle of defining convolutional operations using the graph's adjacency matrix. However, the classical GCN algorithm, functioning as a localized spectral graph convolution with first-order approximations, explores only half of the unannotated information [19]. Mean Teacher [37] comprises both a teacher model and a student model. The inconsistency between the student's outputs under slight perturbations and the teacher model's outputs serves as a robust clue for classifying cancer subtypes in unlabeled nodes. In other words, unlabeled nodes can provide highly effective gradients under the supervision of consistency loss to train the model. In this mutually reinforcing process, both labeled and unlabeled sample information is effectively propagated for gradient-based training of GCN. The GCN model [38] obtains the output of a single convolutional layer by configuring the adjacency matrix and input features, , , represented as trainable model parameters:.
SEGCN comprises both a student model and a teacher model,, each with their respective weights. Given labeled data and unlabeled data. In this experiment, a normalized adjacency matrix A is constructed based on data relationships, x represents the labeled samples. represents the predicted probabilities of the student classifier for the c classes, while represents the ground truth probabilities for the c classes. In a noise-free environment, the cross-entropy loss for labeled data under supervision is expressed as:
| 4 |
In this experiment, model perturbation is achieved by adding only one dropout layer with a dropout rate set to 0.5. The unsupervised consistency loss penalizes the discrepancies between the student's predicted probabilities and those of the teacher . The formulation of the unsupervised consistency loss is as follows:
| 5 |
The overall loss of SEGCN comprises both supervised and unsupervised losses, given as follows: Here, the parameter controls the relative importance of the unsupervised loss in the overall loss. The weights of the teacher model are updated using the exponential moving average of the student's real-time weights, , with being the smoothing coefficient and s being the current step. and are set to their default values in SEGCN, with the number of GCN layers set to 2 to demonstrate that the model achieves its best performance with two layers [36].
Results
In this section, the performance of the proposed MOSEGCN model is evaluated and compared with other state-of-the-art methods:1. Random Forest (RF): Constructing multiple decision trees and combining their predictions for final classification. 2. k-Nearest Neighbors Classifier (KNN): Classifying based on the labels of neighboring samples for the sample to be predicted. 3. L1 Regularized Linear Regression (Lasso): Considering relationships and differences between multiple categories simultaneously for multi-omics data fusion classification. 4. XGBoost: Implementing a classifier based on gradient-boosted decision trees. 5. MoGCN: Utilizing autoencoders (AE) to learn multi-omics features for GCN classification. 6. MOGONET: Jointly learning the specificity of omics and the correlation of cross-omics after pre-classification using GCN. 7. Combining Transformer encoding modules with GCN to create a novel model for cancer classification. 8. Semi-Supervised SVM (S3VM): This is an extended approach to Support Vector Machines (SVM) that enhances model performance by simultaneously leveraging labeled and unlabeled data. 9. SEGCN: A deep learning model designed for semi-supervised tasks, incorporating self-ensembling techniques to boost performance.
MOSEGCN is first compared with these nine methods on two benchmark cancer datasets. Subsequently, it is validated for applicability and effectiveness using a multi-omics dataset of glioblastoma multiforme, which contains four cancer subtypes and a total of 274 samples. Finally, the model's sensitivity analysis is employed to identify important biomarkers.
Data preparation
We utilized preprocessed benchmark multi-omics cancer datasets, namely ROSMAP and BRCA [17], to assess the performance of our experimental model across different cancer classification tasks. In particular, the BRCA dataset encompasses classification of invasive breast cancer (BRCA) PAM50 subtypes, including normal, basal, human epidermal growth factor receptor 2 (HER2)-enriched, Luminal A subtype, and Luminal B subtype.
The multi-omics dataset for Glioblastoma Multiforme (GBM) was obtained from an open-access website accessed on May 16, 2023, at. This dataset comprises four files: three data groups (i. e., gene expression, DNA methylation expression, and microRNA expression), along with one clinical dataset. To effectively analyze multi-omics data, the following preprocessing steps were undertaken. First, samples common to all four data groups were selected, and features devoid of signals (zero mean) were further filtered. Second, the most significantly differentially expressed genes (the top 25% with the highest variance) were selected and MinMaxScaler-transformed for subsequent analysis. Regarding microRNA expression data, due to the limited number of microRNA and features available, no selection was performed. The clinical dataset retained labels for the four cancer subtypes of the samples. The experiment utilized a 7:3 split for training and testing, repeated 30 times, with average measurement results reported. Table 1 provides a concise overview of the three datasets.
Table 1.
Dataset Overview
| Dataset | Categories | Number of features for mRNA | Number of features for methylation | Number of Features for microRNA | Number of labeled nodes | Number of unlabeled nodes |
|---|---|---|---|---|---|---|
| BRCA |
Normal-like:115, Basal-like: 131, HER2-enriched:46, LuminalA:436, Luminal B: 147 |
1000 | 1000 | 503 | 612 | 263 |
| ROSMAP | NC:169, AD:182 | 200 | 200 | 200 | 245 | 106 |
| GBM | Classical:71, Mesenchymal:47, Proneura:84, Neural:72 | 3613 | 1500 | 534 | 191 | 83 |
Hyper-parameter setting
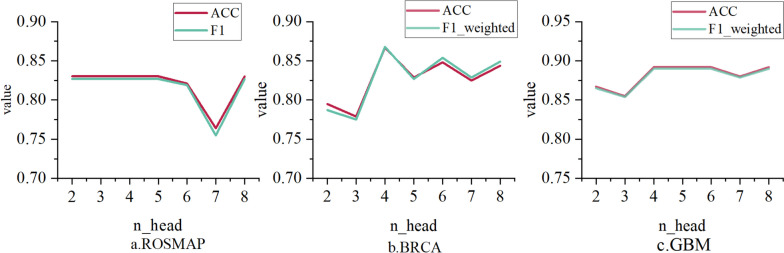
The performance of MOSEGCN is directly influenced by the settings of hyperparameters, and one of these settings is the number of attention heads in the multi-head attention mechanism. Having a higher number of attention heads can potentially lead to increased computational complexity, training requirements, and memory consumption. Additionally, the interaction and integration of information between attention heads may become more intricate, making the model harder to optimize. Conversely, having a lower number of attention heads might limit the model's expressive power and feature extraction capabilities, preventing it from capturing complex relationships and patterns within multi-omics data. Selecting an appropriate number of attention heads requires striking a balance between the model's expressive capacity and computational complexity. Therefore, this study undertakes experimentation to fine-tune and determine the optimal number of attention heads. As depicted in Fig. 2, it becomes evident that when n_head = 4, the three datasets achieve the most outstanding classification performance within the model.
Fig. 2.
Evaluation Metrics as a Function of n _head Variation
Dataset analysis
(Tables 2, and 3) present the test set accuracy results for the two benchmark cancer datasets, ROSMAP and BRCA. In the binary classification task for ROSMAP, the experiment employs accuracy (ACC), F1 score (F1), area under the receiver operating characteristic curve (AUC), Precision and Recall as evaluation metrics. For other multi-class datasets, accuracy (ACC), weighted F1 score (F1_weighted), macro F1 score (F1_macro), Precision and Recall are utilized. The experimental findings demonstrate that MOSEGCN outperforms in all benchmark test datasets. The accuracy rates for ROSMAP and BRCA reach 83.0% and 86.7%, respectively. Compared to the latest MOGONET method, MOSEGCN shows an improvement of 3.0% and 6.1% in accuracy for these datasets, indicating outstanding classification performance in common complex diseases like breast cancer and Alzheimer's disease. MOSEGCN consists of two crucial components: the Transformer encoding module, which learns high-level features and their inherent correlations within and between different omics data types, and SEGCN, which employs labeled and unlabeled information for final classification. To validate the necessity of each component, this experiment combines the Transformer encoding module with GCN for classification purposes. The results in Tables 2, and 3 demonstrate that the combination of the Transformer encoding module and GCN outperforms the integrated model MOGCN [18], which utilizes AE and GCN modules, particularly in handling multiple omics data sets. Similarly, the evaluation metrics of the MOSEGCN model, incorporating the Transformer encoding module, surpass those of the semi-supervised model SEGCN. This underscores the effectiveness of the Transformer encoding module in integrating multiple omics data sets, showcasing its enhanced capability to capture complex relationships and latent features within the dataset. However, the combination method of Transformer encoding module and GCN does not outperform the evaluation metrics of MOSEGCN using both supervised loss and unsupervised loss utilizing unlabeled node information when only using supervised loss.This underscores the prowess of the SEGCN model within the MOSEGCN framework, effectively tapping into insights from unlabeled nodes to provide invaluable support during the model learning process. The symbiotic relationship between the Transformer encoding module and SEGCN not only highlights their collective strength but also opens up new horizons for pioneering advancements in the prediction and classification of intricate disease.
Table 2.
Classification Results on the ROSMAP Dataset
| Method | ACC | AUC | F1 | Precision | Recall |
|---|---|---|---|---|---|
| RF | 0.754 | 0.755 | 0.759 | 0.774 | 0.745 |
| KNN | 0.651 | 0.649 | 0.673 | 0.655 | 0.691 |
| Lasso | 0.755 | 0.751 | 0.783 | 0.723 | 0.854 |
| XGBoost | 0.764 | 0.763 | 0.775 | 0.768 | 0.782 |
| MoGCN | 0.774 | 0.773 | 0.784 | 0.791 | 0.790 |
| MOGONET | 0.800 | 0.876 | 0.801 | 0.832 | 0.775 |
| Transformer+GCN | 0.802 | 0.803 | 0.804 | 0.827 | 0.782 |
| S3VM | 0.774 | 0.775 | 0.772 | 0.809 | 0.739 |
| SEGCN | 0.792 | 0.794 | 0.792 | 0.824 | 0.764 |
| MOSEGCN | 0.830 | 0.832 | 0.827 | 0.878 | 0.782 |
Table 3.
Classification Results on the BRCA Dataset
| Method | ACC | F1_weighted | F1_macro | Precision | Recall |
|---|---|---|---|---|---|
| RF | 0.768 | 0.756 | 0.697 | 0.731 | 0.675 |
| KNN | 0.783 | 0.777 | 0.732 | 0.801 | 0.692 |
| Lasso | 0.772 | 0.752 | 0.709 | 0.792 | 0.672 |
| XGBoost | 0.791 | 0.786 | 0.730 | 0.775 | 0.700 |
| MoGCN | 0.837 | 0.834 | 0.798 | 0.842 | 0.770 |
| MOGONET | 0.806 | 0.774 | 0.697 | 0.758 | 0.691 |
| Transformer+GCN | 0.840 | 0.834 | 0.784 | 0.836 | 0.755 |
| S3VM | 0.819 | 0.817 | 0.778 | 0.829 | 0.761 |
| SEGCN | 0.840 | 0.839 | 0.798 | 0.844 | 0.775 |
| MOSEGCN | 0.867 | 0.868 | 0.811 | 0.874 | 0.797 |
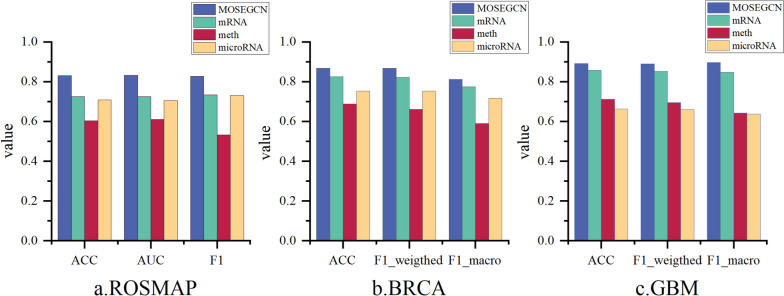
MOSEGCN integrates three different types of omics data, and to demonstrate that MOSEGCN's classification performance surpasses that of single omics datasets, this experiment compares the classification results between single omics data and multi-omics data using MOSEGCN. As illustrated in Fig. 3, the results indicate that simultaneously processing all three omics data types yields the best classification results. This method of integrating multi-omics datasets considers information from multiple perspectives and levels, thereby enhancing the accuracy of classification predictions.
Fig. 3.
Comparison of Multi-Omic Data and Single-Omic Data Classification Results Using the MOSEGCN Model
Validation of MOSEGCN on the GBM dataset
To ascertain the generalizability of MOSEGCN, this experiment applied MOSEGCN to the GBM dataset, which encompasses four major subtypes: Classical, Mesenchymal, Proneural, and Neural [39]. The results are presented in (Table 4). Table 4 reveals that the proposed MOSEGCN model performs exceptionally well on the GBM dataset, achieving an accuracy of 89.2%, a weighted F1 score of 89.0%, and a macro F1 score of 89.7%. This performance surpasses all other comparative methods. These outcomes underscore the broad potential applicability of MOSEGCN for complex disease classification based on multi-omics data.
Table 4.
Classification Results on the GBM Dataset
| Method | ACC | F1_weighted | F1_macro | Precision | Recall |
|---|---|---|---|---|---|
| RF | 0.807 | 0.804 | 0.800 | 0.840 | 0.790 |
| KNN | 0.757 | 0.755 | 0.754 | 0.789 | 0.774 |
| Lasso | 0.783 | 0.784 | 0.787 | 0.795 | 0.782 |
| XGBoost | 0.783 | 0.782 | 0.771 | 0.793 | 0.764 |
| MoGCN | 0.840 | 0.843 | 0.834 | 0.841 | 0.842 |
| MOGONET | 0.831 | 0.833 | 0.821 | 0.820 | 0.824 |
| Transformer+GCN | 0.855 | 0.859 | 0.853 | 0.868 | 0.867 |
| S3VM | 0.843 | 0.839 | 0.830 | 0.869 | 0.818 |
| SEGCN | 0.867 | 0.865 | 0.857 | 0.892 | 0.836 |
| MOSEGCN | 0.892 | 0.890 | 0.897 | 0.905 | 0.884 |
Identification of significant biomarkers
Sensitivity analysis is a method employed to understand how neural network models respond to variations in input data. Through sensitivity analysis, one can ascertain the contribution of input features to output predictions and discern which input features exert the most significant influence on the model's predictive outcomes [40, 41]. The importance of a node can be determined by its feature's standard deviation (variable sensitivity) and its contribution to the network, referred to as weight sensitivity [42]. In the teacher model, this experiment employed sensitivity analysis for feature extraction. To achieve a stable feature extraction for the teacher model during training, the standard deviation of each input node i's corresponding feature in each omics was calculated along with its connection weight Wij in the network. Every 400 epochs, the top 30 markers were extracted, and the extracted features were consolidated. Table 5 enumerates the biomarkers associated with the classification of BRCA and ROSMAP datasets.
Table 5.
Identified Important Biomarkers
| Dataset | Omics type | important biomarkers | |
|---|---|---|---|
| BRCA | mRNA expression | LIN28B|389421,TFF1|7031,CYP2B7P1|1556,FABP7|2173,TLX3|30012,SOX10|6663,ANKRD30A|91074,KRT6B|3854,CA9|768,CXorf61|203413,AGR3|155465,MIA|8190,GABRP|2568,GP2|2813,C1orf64|149563,SBSN|374897,KLK7|5650,PTPRZ1|5803,SFRP1|6422,KLK6|5653,ABCC11|85320,KLK8|11202,MSLN|10232,ZBTB16|7704,A2ML1|144568,TUBA3E|112714,SLC6A14|11254,C2orf40|84417,KRT16|3868,VGLL1|51442,C6orf218|221718,ZIC1|7545,CA6|765,HORMAD1|84072,TRIML2|205860,UPF0639|400224,TRIM15|89870,ISL2|64843,MAPK4|5596,ART3|419,RAET1L|154064,PSAPL1|768239 | |
| DNA methylation | MIR124-2,SAMSN1,OR1J4,MIR563,FLJ41856,ZSWIM2,TAS2R13,LOC100130331,C5orf39,LOC145837,SLC5A12,SIRPD,MEP1A,POU4F1,FCGR2B,MIR100,SERPINB12,ARHGAP28,SCGB3A1,POU3F3,BLID,OR4N5,OR11G2,SLC22A2,DOK5,ZP4,CRISP2,LOC285692,KCNJ16,C14orf72,PSAT1,MT1DP,MIR365-1,TNFSF13B,INA,OR11H6,TAL2,DEFB118,S100A7,TMEFF1 | ||
| microRNA expression | hsa-mir-934,hsa-mir-449a,hsa-mir-577,hsa-mir-135b,hsa-mir-184,hsa-mir-190b,hsa-mir-187,hsa-mir-1269,hsa-mir-449b,hsa-mir-2115,hsa-mir-519a-1,hsa-mir-105-2,hsa-mir-105-1,hsa-mir-767,hsa-mir-205,hsa-mir-9-3,hsa-mir-375,hsa-mir-224,hsa-mir-210,hsa-mir-1251,hsa-mir-196a-1,hsa-mir-9-2,hsa-mir-486,hsa-mir-516a-2,hsa-mir-206,hsa-mir-196a-2,hsa-mir-4326,hsa-mir-135a-1,hsa-mir-452,hsa-mir-522,hsa-mir-137,hsa-mir-1304,hsa-mir-935,hsa-mir-937,hsa-mir-374c | ||
| ROSMAP | mRNA expression | FRMPD2P1 ,LINC01007,CTB-171A8.1,TAC3,S100A4,LINC00507,SLC5A11,RP11-552D4.1,LINC00499,RP11-298D21.1,DDIT4,BX255923.3,PHYHD1,APLN,ANLN ,RBP4,TGFBR3L,HSPA2,TF ,PNMA5,CXCR4,GAREML,CTD-2380F24.1,SCN3B,FAM65C,RP11-321E2.3 ,TRIP10,KCNJ10,RP11-416I2.1,UGT8 | |
| DNA methylation | LDHC,SLC44A2,TRIP10 ,EMC4,CCL3,ENG,SLC44A2,ATG10,AGMAT,CCDC8,LRRC39,CMTM5,IRF7,ACSM5,LECT1,GFM1,HCAR1,EML2,TRAPPC12,SRRM2-AS1,HRC ,AHSP,C10orf11,EFS,XAF1,ECEL1,FBXL22,ARHGEF4,PTGER1,CDH1 | ||
| microRNA expression | hsa-miR-1246,hsa-miR-1299,hsa-miR-200a,ebv-miR-BART8,hsa-miR-520e,hsa-miR-1275,hsv1-miR-H8,hsa-miR-2117,hsa-miR-199a-5p,hsa-miR-330-3p,hsa-miR-1260,hsa-miR-744,hsa-miR-891b,hsa-miR-1308,hsa-miR-522,hsv1-miR-H3,hsa-miR-2114,hsa-miR-133b,hsa-miR-27a,hsa-miR-509-3p,mcv-miR-M1-5p,hsa-miR-153,hsv1-miR-H1,hsa-miR-208a,hsa-miR-1248,hsa-miR-639,hsa-miR-518e,hsa-miR-194,hsa-miR-199b-5p,hsa-miR-381 | ||
According to information from the KEGG database, we have discovered that in breast cancer, olfactory receptors such as OR11H6, OR1J4, OR4N5, and OR11G2O are associated with the olfactory transduction pathway. Olfactory receptors are not only expressed in the nasal cavity but also widely distributed throughout the body, playing significant physiological roles [43]. This finding suggests that these sensory receptors may serve as novel, yet insufficiently studied targets in the development and progression of breast cancer. The Estrogen Signaling Pathway plays a crucial role in BRCA1 [44], with KRT16 and TFF1 being part of this pathway. Their expressions influence the biological characteristics of BRCA. Enhanced KRT16 expression is significantly correlated with lower overall survival in metastatic breast cancer patients [45], while TFF1 is closely associated with bone metastasis in estrogen receptor (ER) + breast cancer [46]. PSAT1, CA6 and CA9 are part of the Metabolic Pathways [47] and affect the growth and migration of breast cancer cells [48–51] MIR100 [52, 53] and MIR124-2 [54, 55] are two MicroRNAs within the same signaling pathway that induce apoptosis and cell cycle arrest in breast cancer cells through multiple genes. In terms of microRNA, hsa-mir-135b has been identified as a target for treating AGR2-expressing breast cancer with doxorubicin resistance [56]. Gong et al. [57] formulated a prognostic risk feature model for predicting the prognosis of breast cancer patients. The results demonstrate a significant correlation between the expression levels of hsa-miR-190b and both unfavorable and favorable prognoses. In Alzheimer's disease, the Calcium Signaling Pathway is one of the major mechanisms. Disruption of calcium signaling may lead to synaptic defects and the accumulation of Aβ plaques and neurofibrillary tangles in AD [58, 59]. HRC, PTGER1 and CXCR4 are genes related to this pathway and have certain roles in the Calcium Signaling Pathway [60–62]. The Neuroactive Ligand-Receptor Interaction pathway may play a role in neuroactivity regulation and cognitive functions [63–65], with PTGER1, TAC3, and APLN being part of this pathway, influencing the nervous system in patients [66–68]. DDIT4 and ATG10 are involved in the Autophagy pathway, contributing to the clearance of cellular abnormalities by regulating the autophagic pathway [69–72]. Regarding microRNA, hsa-miR-199b-5p is a potential candidate biomarker for its role in the interaction between diabetes and Alzheimer's disease [73, 74] identified hsa-miR-133b as a potential biomarker for Alzheimer's disease (AD), playing a crucial role in constructing the ceRNA regulatory network associated with lncRNA. Hsa-miR-27a is likely a significant epigenetic biomarker in AD, participating in the regulation of the target gene SERPINA3, revealing its pivotal role in the disease's pathogenic mechanism [75].
Discussion
Multi-omics data provides a diverse range of molecular-level insights into biological organisms. The comprehensive analysis of multi-omics data yields more thorough and accurate biological information. Furthermore, it uncovers novel biological insights and associations, fostering innovation in complex disease research. It propels data-driven biological studies and the advancement of personalized medicine. With the rapid advancement of omics technologies and healthcare standards, meticulously annotated omics datasets are on the rise. However, in the real world, the cost of extensively annotating data is often prohibitive, resulting in a small fraction of labeled data, leaving a substantial portion unlabeled. To address this challenge, this experiment introduces a deep learning-based semi-supervised multi-omics integration method for biomedical classification tasks. It effectively leverages both labeled and unlabeled data for improved classification of complex diseases. In this approach, we employed the Transformer encoding module for feature learning and integration. The Transformer network introduces a multi-head self-attention mechanism, allowing the model to establish connections between different positions. Moreover, this multi-head self-attention mechanism permits the model to consider the relevance of positions in the input data when generating representations for each position. This enhances the model's ability to learn hidden information between different omics data types, which is crucial for effectively integrating multi-omics features. Consequently, in this experiment, we concatenated various omics data to learn useful information at each position, encompassing both intra-modality and inter-modality internal feature information. For the final cancer classification, we employs SEGCN, which adeptly harnesses labeled and unlabeled data, enhancing the model's generalization capacity. The necessity of both key components, the Transformer encoding module and GCN, is verified through their combined use. The generalizability of MOSEGCN is validated on the GBM renal cell carcinoma dataset, where it demonstrates the ability to identify meaningful biomarkers within each omics data, elucidating certain disease-related information. MOSEGCN exhibits strong capabilities in integrating multi-omics data for cancer classification. However, it has limitations, as this study exclusively employed three distinct types of multi-omics data. Multi-omics data with more than three types and heterogeneous data, such as imaging omics, remain unverified. These areas represent future directions for further research.
Conclusion
In conclusion, we introduces an innovative deep learning multi-omics integration model for the classification of complex diseases. Empirical evidence demonstrates the efficient utilization of the Transformer network to capture long-term dependencies in potential features within and across different modalities. Moreover, this experiment leverages the SEGCN module to thoroughly assimilate information from both labeled and unlabeled nodes, resulting in more precise classification outcomes. This integrated model is validated on three public datasets, outperforming state-of-the-art methods. Additionally, it identifies meaningful biomarkers within diverse omics data, further enhancing our understanding of disease mechanisms. In the future, we will explore different modalities and multi-omics data integration techniques to further enhance the performance of complex disease classification tasks.
Acknowledgements
We thank the authors of the cited references for their excellent work.
Authors’ contributions
B.-Z.W and Q.-F.C conceived the study; J.-H.W and N.-Q.L contributed equally to this work. All authors participated in the acquisition of the data and analyzed the data; J.-H.W and N.-Q.L drafted and revised the manuscript; all authors read the manuscript and approved the final version to be published. B.-Z.W and Q.-F.C had full access to all the data in the study and serves as guarantor, taking full responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by National Natural Science Foundation of China (61963004).
Availability of data and materials
The BRCA and ROSMAP datasets analyzed in this study were obtained from Wang et al. [17]. The GBM dataset was downloaded from the provided link: http://acgt.cs.tau.ac.il/multi_omic_benchmark/download.html.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiahui Wang and Nanqing Liao are first authors.
Contributor Information
Qingfeng Chen, Email: qingfeng@gxu.edu.cn.
Bizhong Wei, Email: wbz@guet.edu.cn.
References
- 1.Smolinska A, Hauschild A-C, Fijten R, Dallinga J, Baumbach J, Van Schooten F. Current breathomics—a review on data pre-processing techniques and machine learning in metabolomics breath analysis. J Breath Res. 2014;8(2):027105. doi: 10.1088/1752-7155/8/2/027105. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Q, Shi X, Xie Y, Huang J, Shia B, Ma S. Combining multidimensional genomic measurements for predicting cancer prognosis: observations from TCGA. Brief Bioinform. 2015;16(2):291–303. doi: 10.1093/bib/bbu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Li H-R, Fan J-B, Wang-Rodriguez J, Downs T, Fu X-D, Zhang MQ. Profiling alternatively spliced mRNA isoforms for prostate cancer classification. BMC Bioinformatics. 2006;7:1–12. doi: 10.1186/1471-2105-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci. 2001;98(24):13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan J, Risacher SL, Shen L, Saykin AJ. Network approaches to systems biology analysis of complex disease: integrative methods for multi-omics data. Brief Bioinform. 2018;19(6):1370–1381. doi: 10.1093/bib/bbx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Günther OP, Chen V, Freue GC, Balshaw RF, Tebbutt SJ, Hollander Z, Takhar M, McMaster WR, McManus BM, Keown PA. A computational pipeline for the development of multi-marker bio-signature panels and ensemble classifiers. BMC Bioinformatics. 2012;13(1):1–18. doi: 10.1186/1471-2105-13-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins KM, Onwuegbuzie AJ, Jiao QG. A mixed methods investigation of mixed methods sampling designs in social and health science research. J Mixed Methods Res. 2007;1(3):267–294. doi: 10.1177/1558689807299526. [DOI] [Google Scholar]
- 8.Ahmed KT, Sun J, Cheng S, Yong J, Zhang W. Multi-omics data integration by generative adversarial network. Bioinformatics. 2022;38(1):179–186. doi: 10.1093/bioinformatics/btab608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z, Zhan X, Xiang S, Johnson TS, Helm B, Yu CY, Zhang J, Salama P, Rizkalla M, Han Z. SALMON: survival analysis learning with multi-omics neural networks on breast cancer. Front Genet. 2019;10:166. doi: 10.3389/fgene.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan W, Yang T, Chen Q, Zhang S, Dong Y, Zhou H, Pan Y. Multiview Subspace Clustering via Low-Rank Symmetric Affinity Graph. IEEE Trans Neural Netw Learn Syst. 2023. [DOI] [PubMed]
- 11.Meng C, Kuster B, Culhane AC, Gholami AM. A multivariate approach to the integration of multi-omics datasets. BMC Bioinformatics. 2014;15:1–13. doi: 10.1186/1471-2105-15-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell MJ, Lock EF. R. JIVE for exploration of multi-source molecular data. Bioinformatics. 2016;32(18):2877–2879. doi: 10.1093/bioinformatics/btw324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z-Y, Xia L-Y, Zhang H, Liang Y. MSPL: Multimodal self-paced learning for multi-omics feature selection and data integration. IEEE Access. 2019;7:170513–170524. doi: 10.1109/ACCESS.2019.2955958. [DOI] [Google Scholar]
- 14.Xu J, Wu P, Chen Y, Meng Q, Dawood H, Dawood H. A hierarchical integration deep flexible neural forest framework for cancer subtype classification by integrating multi-omics data. BMC Bioinformatics. 2019;20(1):1–11. doi: 10.1186/s12859-019-3116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Chen R, Li D, Wang Z. Subtype-GAN: a deep learning approach for integrative cancer subtyping of multi-omics data. Bioinformatics. 2021;37(16):2231–2237. doi: 10.1093/bioinformatics/btab109. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Shannon CP, Gautier B, Rohart F, Vacher M, Tebbutt SJ, Lê Cao K-A. DIABLO: an integrative approach for identifying key molecular drivers from multi-omics assays. Bioinformatics. 2019;35(17):3055–3062. doi: 10.1093/bioinformatics/bty1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Shao W, Huang Z, Tang H, Zhang J, Ding Z, Huang K. MOGONET integrates multi-omics data using graph convolutional networks allowing patient classification and biomarker identification. Nat Commun. 2021;12(1):3445. doi: 10.1038/s41467-021-23774-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Ma J, Leng L, Han M, Li M, He F, Zhu Y. MoGCN: a multi-omics integration method based on graph convolutional network for cancer subtype analysis. Front Genet. 2022;13:806842. doi: 10.3389/fgene.2022.806842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Liang J, Cui J, Liang J. Semi-supervised learning with mixed-order graph convolutional networks. Inf Sci. 2021;573:171–181. doi: 10.1016/j.ins.2021.05.057. [DOI] [Google Scholar]
- 20.Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, Kaiser Ł, Polosukhin I: Attention is all you need. Advances in neural information processing systems 2017, 30.
- 21.Dosovitskiy A, Beyer L, Kolesnikov A, Weissenborn D, Zhai X, Unterthiner T, Dehghani M, Minderer M, Heigold G, Gelly S: An image is worth 16x16 words: Transformers for image recognition at scale. arXiv preprint arXiv:201011929 2020.
- 22.Zhang Q, Xu Y, Zhang J, Tao D: Vitaev2: Vision transformer advanced by exploring inductive bias for image recognition and beyond. Int J Comput Vision 2023:1–22.
- 23.Liu X, Wang L, Han X. Transformer with peak suppression and knowledge guidance for fine-grained image recognition. Neurocomputing. 2022;492:137–149. doi: 10.1016/j.neucom.2022.04.037. [DOI] [Google Scholar]
- 24.Rai N, Kumar D, Kaushik N, Raj C, Ali A. Fake News Classification using transformer based enhanced LSTM and BERT. Int J Cognitive Comput Eng. 2022;3:98–105. doi: 10.1016/j.ijcce.2022.03.003. [DOI] [Google Scholar]
- 25.Liu F, Gao C, Chen F, Meng D, Zuo W, Gao X: Infrared small-dim target detection with transformer under complex backgrounds. arXiv preprint arXiv:210914379 2021. [DOI] [PubMed]
- 26.Xu N, Cui X, Wang X, Zhang W, Zhao T. An Intelligent Athlete Signal Processing Methodology for Balance Control Ability Assessment with Multi-Headed Self-Attention Mechanism. Mathematics. 2022;10(15):2794. doi: 10.3390/math10152794. [DOI] [Google Scholar]
- 27.Zhou G, Sohn K, Lee H: Online incremental feature learning with denoising autoencoders. In: Artificial intelligence and statistics: 2012: PMLR; 2012: 1453–1461.
- 28.Wu Y, Li W. Aspect-level sentiment classification based on location and hybrid multi attention mechanism. Appl Intell. 2022;52(10):11539–11554. doi: 10.1007/s10489-021-02966-3. [DOI] [Google Scholar]
- 29.Jian S, Kaiming H, Shaoqing R, Xiangyu Z: Deep residual learning for image recognition. In: IEEE Conference on Computer Vision & Pattern Recognition: 2016; 2016: 770–778.
- 30.Ba JL, Kiros JR, Hinton GE: Layer normalization. arXiv preprint arXiv:160706450 2016.
- 31.Bank D, Koenigstein N, Giryes R: Autoencoders. Machine Learning for Data Science Handbook: Data Mining and Knowledge Discovery Handbook 2023:353–374.
- 32.Lin S, Wang Y, Zhang L, Chu Y, Liu Y, Fang Y, Jiang M, Wang Q, Zhao B, Xiong Y: MDF-SA-DDI: predicting drug–drug interaction events based on multi-source drug fusion, multi-source feature fusion and transformer self-attention mechanism. Briefings in Bioinformatics 2022, 23(1):bbab421. [DOI] [PubMed]
- 33.Wu C, Wu F, Ge S, Qi T, Huang Y, Xie X: Neural news recommendation with multi-head self-attention. In: Proceedings of the 2019 conference on empirical methods in natural language processing and the 9th international joint conference on natural language processing (EMNLP-IJCNLP): 2019; 2019: 6389–6394.
- 34.Guo S, Wang Y, Yuan H, Huang Z, Chen J, Wang X. TAERT: triple-attentional explainable recommendation with temporal convolutional network. Inf Sci. 2021;567:185–200. doi: 10.1016/j.ins.2021.03.034. [DOI] [Google Scholar]
- 35.Wang B, Mezlini AM, Demir F, Fiume M, Tu Z, Brudno M, Haibe-Kains B, Goldenberg A. Similarity network fusion for aggregating data types on a genomic scale. Nat Methods. 2014;11(3):333–337. doi: 10.1038/nmeth.2810. [DOI] [PubMed] [Google Scholar]
- 36.Luo Y, Ji R, Guan T, Yu J, Liu P, Yang Y. Every node counts: Self-ensembling graph convolutional networks for semi-supervised learning. Pattern Recogn. 2020;106:107451. doi: 10.1016/j.patcog.2020.107451. [DOI] [Google Scholar]
- 37.Tarvainen A, Valpola H: Mean teachers are better role models: Weight-averaged consistency targets improve semi-supervised deep learning results. Advances in neural information processing systems 2017, 30.
- 38.Kipf TN, Welling M: Semi-supervised classification with graph convolutional networks. arXiv preprint arXiv:160902907 2016.
- 39.Kotliarova S, Fine HA. SnapShot: glioblastoma multiforme. Cancer Cell. 2012;21(5):710–710e711. doi: 10.1016/j.ccr.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 40.Pizarroso J, Alfaya D, Portela J, Muñoz A: Metric Tools for Sensitivity Analysis with Applications to Neural Networks. arXiv preprint arXiv:230502368 2023.
- 41.Engelbrecht AP, Cloete I, Zurada JM: Determining the significance of input parameters using sensitivity analysis. In: From Natural to Artificial Neural Computation: International Workshop on Artificial Neural Networks Malaga-Torremolinos, Spain, June 7–9, 1995 Proceedings 3: 1995: Springer; 1995: 382–388.
- 42.Garson GD. Interpreting neural-network connection weights. AI Expert. 1991;6(4):46–51. [Google Scholar]
- 43.Qian C, Zhi T, Chen-Cen L. The Roles and Mechanism of Olfactory Receptors in Non-olfactory Tissues and Cells. PROGRESS IN BIOCHEMISTRY AND BIOPHYSICS. 2020;47(2):91–104. [Google Scholar]
- 44.Rajan A, Nadhan R, Latha NR, Krishnan N, Warrier AV, Srinivas P. Deregulated estrogen receptor signaling and DNA damage response in breast tumorigenesis. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188482. doi: 10.1016/j.bbcan.2020.188482. [DOI] [PubMed] [Google Scholar]
- 45.Joosse SA, Hannemann J, Spötter J, Bauche A, Andreas A, Müller V, Pantel K. Changes in keratin expression during metastatic progression of breast cancer: impact on the detection of circulating tumor cells. Clin Cancer Res. 2012;18(4):993–1003. doi: 10.1158/1078-0432.CCR-11-2100. [DOI] [PubMed] [Google Scholar]
- 46.Spadazzi C, Mercatali L, Esposito M, Wei Y, Liverani C, De Vita A, Miserocchi G, Carretta E, Zanoni M, Cocchi C. Trefoil factor-1 upregulation in estrogen-receptor positive breast cancer correlates with an increased risk of bone metastasis. Bone. 2021;144:115775. doi: 10.1016/j.bone.2020.115775. [DOI] [PubMed] [Google Scholar]
- 47.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17(4):351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metcalf S, Dougherty S, Kruer T, Hasan N, Biyik-Sit R, Reynolds L, Clem BF. Selective loss of phosphoserine aminotransferase 1 (PSAT1) suppresses migration, invasion, and experimental metastasis in triple negative breast cancer. Clin Exp Metas. 2020;37:187–197. doi: 10.1007/s10585-019-10000-7. [DOI] [PubMed] [Google Scholar]
- 49.Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, Kyle A. auf dem Keller U, Leung S, Huntsman D: Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Can Res. 2011;71(9):3364–3376. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- 50.Mamoor S: CA6 is differentially expressed in lymph node metastasis in human breast cancer. 2021.
- 51.McIntyre A, Patiar S, Wigfield S. Li J-l, Ledaki I, Turley H, Leek R, Snell C, Gatter K, Sly WS: Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res. 2012;18(11):3100–3111. doi: 10.1158/1078-0432.CCR-11-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Gao Y, Zhang K, Chen J, Han S, Feng B, Wang R, Chen L. Multiple roles of microRNA-100 in human cancer and its therapeutic potential. Cell Physiol Biochem. 2015;37(6):2143–2159. doi: 10.1159/000438572. [DOI] [PubMed] [Google Scholar]
- 53.Petrelli A, Carollo R, Cargnelutti M, Iovino F, Callari M, Cimino D, Todaro M, Mangiapane LR, Giammona A, Cordova A. By promoting cell differentiation, miR-100 sensitizes basal-like breast cancer stem cells to hormonal therapy. Oncotarget. 2015;6(4):2315. doi: 10.18632/oncotarget.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oltra SS, Peña-Chilet M, Vidal-Tomas V, Flower K, Martinez MT, Alonso E, Burgues O, Lluch A, Flanagan JM, Ribas G. Methylation deregulation of miRNA promoters identifies miR124-2 as a survival biomarker in Breast Cancer in very young women. Sci Rep. 2018;8(1):14373. doi: 10.1038/s41598-018-32393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agirre X, Vilas-Zornoza A, Jiménez-Velasco A, Martin-Subero JI, Cordeu L, Gárate L, San José-Eneriz E, Abizanda G, Rodriguez-Otero P, Fortes P. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Can Res. 2009;69(10):4443–4453. doi: 10.1158/0008-5472.CAN-08-4025. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Xia F, Zhang F, Cui Y, Wang Q, Liu H, Wu Y. miR-135b-5p enhances doxorubicin-sensitivity of breast cancer cells through targeting anterior gradient 2. J Exp Clin Cancer Res. 2019;38(1):1–13. doi: 10.1186/1756-9966-31-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong P-J, Shao Y-C, Huang S-R, Zeng Y-F, Yuan X-N, Xu J-J, Yin W-N, Wei L, Zhang J-W. Hypoxia-associated prognostic markers and competing endogenous rna co-expression networks in breast cancer. Front Oncol. 2020;10:579868. doi: 10.3389/fonc.2020.579868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obulesu M, Lakshmi MJ. Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res. 2014;39:2301–2312. doi: 10.1007/s11064-014-1454-4. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Liu X: The effective components, core targets, and key pathways of ginseng against Alzheimer’s disease. Evid Based Complement Alternat Med 2023, 2023. [DOI] [PMC free article] [PubMed] [Retracted]
- 60.Buxbaum JD, Choi E-K, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W. Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med. 1998;4(10):1177–1181. doi: 10.1038/2673. [DOI] [PubMed] [Google Scholar]
- 61.Maccioni RB, Navarrete LP, González A, González-Canacer A, Guzmán-Martínez L, Cortés N. Inflammation: a major target for compounds to control Alzheimer’s disease. J Alzheimers Dis. 2020;76(4):1199–1213. doi: 10.3233/JAD-191014. [DOI] [PubMed] [Google Scholar]
- 62.Gavriel Y, Rabinovich-Nikitin I, Solomon B. Inhibition of CXCR4/CXCL12 signaling: a translational perspective for Alzheimer’s disease treatment. Neural Regen Res. 2022;17(1):108. doi: 10.4103/1673-5374.314303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong Y, Liang X, Liu L, Zhang D, Wan C, Gan Z, Yuan L. High throughput sequencing identifies microRNAs mediating α-synuclein toxicity by targeting neuroactive-ligand receptor interaction pathway in early stage of drosophila Parkinson's disease model. PLoS ONE. 2015;10(9):e0137432. doi: 10.1371/journal.pone.0137432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pal J, Patil V, Kumar A, Kaur K, Sarkar C, Somasundaram K. Genetic landscape of glioma reveals defective neuroactive ligand receptor interaction pathway as a poor prognosticator in glioblastoma patients. Cancer Res. 2017;77(13_Supplement):2454–2454. doi: 10.1158/1538-7445.AM2017-2454. [DOI] [Google Scholar]
- 65.Venkatesh H, Monje M. Neuronal activity in ontogeny and oncology. Trends Cancer. 2017;3(2):89–112. doi: 10.1016/j.trecan.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Y, Wang Y, Dong Y, Shu S, Zhang D, Xu J, Zhang Y, Shi W, Wang S-L. Butyl benzyl phthalate as a key component of phthalate ester in relation to cognitive impairment in NHANES elderly individuals and experimental mice. Environ Sci Pollut Res. 2023;30(16):47544–47560. doi: 10.1007/s11356-023-25729-8. [DOI] [PubMed] [Google Scholar]
- 67.Hu G, He M, Ko WK, Lin C, Wong AO. Novel pituitary actions of TAC3 gene products in fish model: receptor specificity and signal transduction for prolactin and somatolactin α regulation by neurokinin B (NKB) and NKB-related peptide in carp pituitary cells. Endocrinology. 2014;155(9):3582–3596. doi: 10.1210/en.2014-1105. [DOI] [PubMed] [Google Scholar]
- 68.Wan T, Fu M, Jiang Y, Jiang W, Li P, Zhou S: Research progress on mechanism of neuroprotective roles of Apelin-13 in prevention and treatment of Alzheimer’s disease. Neurochemical Research 2022:1–13. [DOI] [PMC free article] [PubMed]
- 69.Pérez-Sisqués L, Sancho-Balsells A, Solana-Balaguer J, Campoy-Campos G, Vives-Isern M, Soler-Palazón F, Anglada-Huguet M, López-Toledano M-Á, Mandelkow E-M, Alberch J. RTP801/REDD1 contributes to neuroinflammation severity and memory impairments in Alzheimer’s disease. Cell Death Dis. 2021;12(6):616. doi: 10.1038/s41419-021-03899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhuang X, Zhang G, Bao M, Jiang G, Wang H, Li S, Wang Z, Sun X: Development of a novel immune infiltration-related diagnostic model for Alzheimer’s disease using bioinformatic strategies. Front Immunol 2023, 14. [DOI] [PMC free article] [PubMed]
- 71.Hong SB, Kim B-W, Kim JH, Song HK. Structure of the autophagic E2 enzyme Atg10. Acta Crystallogr D Biol Crystallogr. 2012;68(10):1409–1417. doi: 10.1107/S0907444912034166. [DOI] [PubMed] [Google Scholar]
- 72.Yamaguchi M, Noda NN, Yamamoto H, Shima T, Kumeta H, Kobashigawa Y, Akada R, Ohsumi Y, Inagaki F. Structural insights into Atg10-mediated formation of the autophagy-essential Atg12-Atg5 conjugate. Structure. 2012;20(7):1244–1254. doi: 10.1016/j.str.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 73.Ghiam S, Eslahchi C, Shahpasand K, Habibi-Rezaei M, Gharaghani S. Exploring the role of non-coding RNAs as potential candidate biomarkers in the cross-talk between diabetes mellitus and Alzheimer’s disease. Front Aging Neurosci. 2022;14:955461. doi: 10.3389/fnagi.2022.955461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ou G-y, Lin W-w, Zhao W-j. Construction of Long Noncoding RNA-Associated ceRNA Networks Reveals Potential Biomarkers in Alzheimer’s Disease. J Alzheimers Dis. 2021;82(1):169–183. doi: 10.3233/JAD-210068. [DOI] [PubMed] [Google Scholar]
- 75.Su L, Chen S, Zheng C, Wei H, Song X: Meta-Analysis of Gene Expression and Identification of Biological Regulatory Mechanisms in Alzheimer's Disease. Front Neurosci 2019, 13. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The BRCA and ROSMAP datasets analyzed in this study were obtained from Wang et al. [17]. The GBM dataset was downloaded from the provided link: http://acgt.cs.tau.ac.il/multi_omic_benchmark/download.html.