Abstract
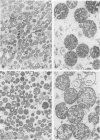
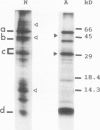
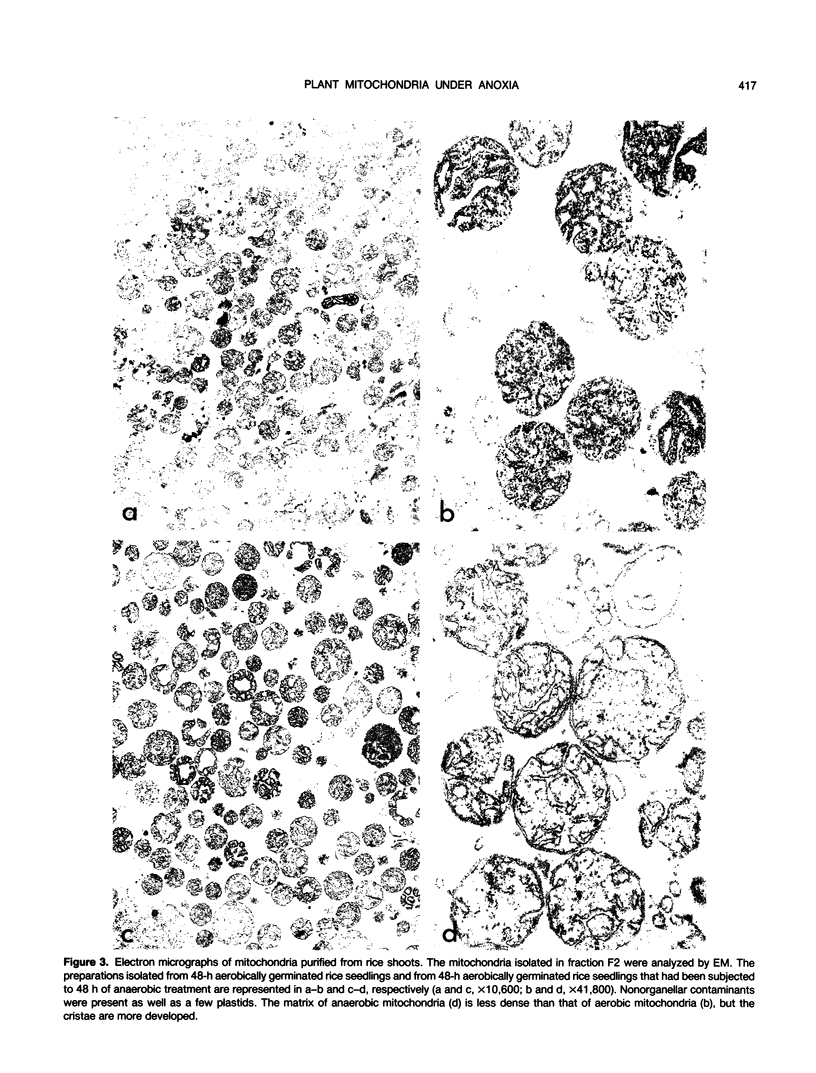
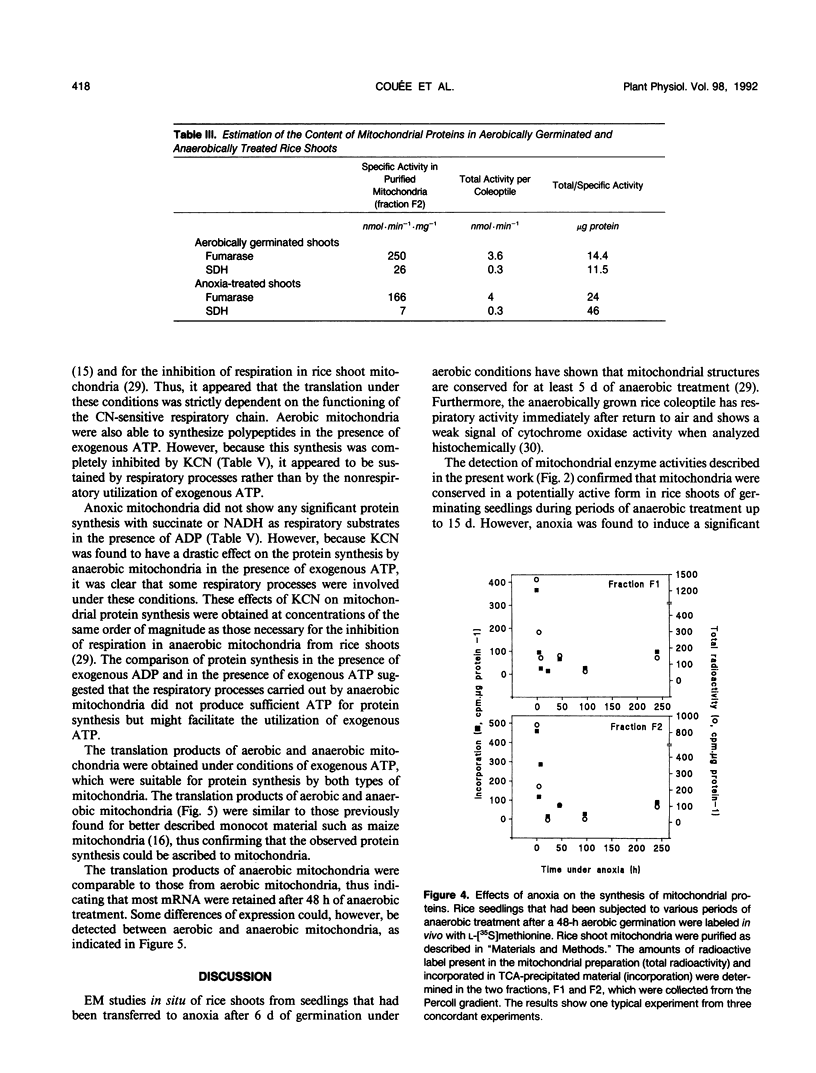
Shoots of germinating rice (Oryza sativa L.) seedlings are able to grow under anoxia and to withstand long periods of anoxic treatment. Mitochondria were purified from aerobically germinated and anaerobically treated rice shoots by differential and isopycnic centrifugation and were found to consist of two subpopulations. The mitochondrial subpopulation of higher density was used for further characterization. Ultrastructural studies showed anaerobic mitochondria to be significantly different from aerobic mitochondria, with a matrix of lower density and more developed cristae. Aerobic and anaerobic mitochondria also differed in their specific activities for fumarase and succinate dehydrogenase, which were significantly lower after the anoxic treatment. In vivo labeling of seedlings with l-[35S]methionine and subsequent isolation of the mitochondria indicated that anoxia induced a drastic decrease, but not a total inactivation, of the synthesis of mitochondrial proteins. In organello protein synthesis showed that anaerobic mitochondria were able to synthesize most of the polypeptides synthesized by aerobic mitochondria, although only in the presence of exogenous ATP, as would occur under anoxia. Anaerobic mitochondria, but not aerobic mitochondria, could carry out protein synthesis without a functional respiratory chain. Thus, mitochondrial protein synthesis was found to be potentially functional in the rice shoot under anoxia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Khalil S., Hanson J. B. Energy-linked Adenosine Diphosphate Accumulation by Corn Mitochondria: I. General Characteristics and Effect of Inhibitors. Plant Physiol. 1979 Aug;64(2):276–280. doi: 10.1104/pp.64.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ani A., Bruzau F., Raymond P., Saint-Ges V., Leblanc J. M., Pradet A. Germination, respiration, and adenylate energy charge of seeds at various oxygen partial pressures. Plant Physiol. 1985 Nov;79(3):885–890. doi: 10.1104/pp.79.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Coleman W. B., Cunningham C. C. Effects of chronic ethanol consumption on the synthesis of polypeptides encoded by the hepatic mitochondrial genome. Biochim Biophys Acta. 1990 Aug 30;1019(2):142–150. doi: 10.1016/0005-2728(90)90136-r. [DOI] [PubMed] [Google Scholar]
- Cooper P., Newton K. J. Maize nuclear background regulates the synthesis of a 22-kDa polypeptide in Zea luxurians mitochondria. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7423–7426. doi: 10.1073/pnas.86.19.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. F., Davies D. D. Nicotinamide-adenine dinucleotide-specific isocitrate dehydrogenase from pea mitochondria. Purification and properties. Biochem J. 1967 Nov;105(2):729–734. doi: 10.1042/bj1050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J. In Vitro Study of Mitochondrial Protein Synthesis during Mitochondrial Biogenesis in Excised Plant Storage Tissue. Plant Physiol. 1979 Jan;63(1):67–73. doi: 10.1104/pp.63.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A. D., Walker J. M., Tipton K. F. Purification of glutamate dehydrogenase from ox brain and liver. Evidence that commercially available preparations of the enzyme from ox liver have suffered proteolytic cleavage. Biochem J. 1980 Nov 1;191(2):605–611. doi: 10.1042/bj1910605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocquot B., Prat C., Mouches C., Pradet A. Effect of anoxia on energy charge and protein synthesis in rice embryo. Plant Physiol. 1981 Sep;68(3):636–640. doi: 10.1104/pp.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebiolo C. M., White E. M. Corn mitochondrial protein synthesis in response to heat shock. Plant Physiol. 1985 Dec;79(4):1129–1132. doi: 10.1104/pp.79.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Drew M. C., Pradet A. Metabolic Acclimation to Anoxia Induced by Low (2-4 kPa Partial Pressure) Oxygen Pretreatment (Hypoxia) in Root Tips of Zea mays. Plant Physiol. 1988 Jan;86(1):61–66. doi: 10.1104/pp.86.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Ges V., Roby C., Bligny R., Pradet A., Douce R. Kinetic studies of the variations of cytoplasmic pH, nucleotide triphosphates (31P-NMR) and lactate during normoxic and anoxic transitions in maize root tips. Eur J Biochem. 1991 Sep 1;200(2):477–482. doi: 10.1111/j.1432-1033.1991.tb16207.x. [DOI] [PubMed] [Google Scholar]