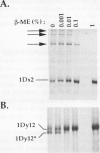
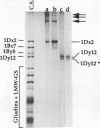
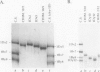
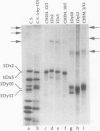
Abstract
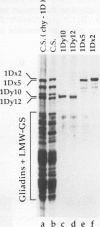
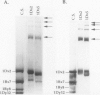
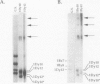
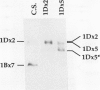
The high molecular weight glutenin subunits are considered one of the most important components of wheat (Triticum aestivum) gluten, but their structure and interactions with other gluten proteins are still unknown. Understanding the role of these proteins in gluten formation may be aided by analyses of the conformation and interactions of individual wild-type and modified subunits expressed in heterologous systems. In the present report, the bacterium Escherichia coli was used to synthesize four naturally occurring X- and Y-type wheat high molecular weight glutenin subunits of the Glu-1D locus, as well as four bipartite chimeras of these proteins. Naturally occurring subunits synthesized in the bacteria exhibited sodium dodecyl sulfate-polyacrylamide gel electrophoresis migration properties identical to those of high molecular weight glutenin subunits extracted from wheat grains. Wild-type and chimeric subunits migrated in sodium dodecyl sulfate gels differently than expected based on their molecular weights due to conformational properties of their N- and C-terminal regions. Results from cycles of reductive cleavage and oxidative reformation were consistent with the formation of both inter- and intramolecular disulfide bonds in patterns and proportions that differed among specific high molecular weight glutenin species. Comparison of the chimeric and wild-type proteins indicated that the two C-terminal cysteines of the Y-type subunits are linked by intramolecular disulfide bonds, suggesting that the role of these cysteines in glutenin polymerization may be limited.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson O. D., Greene F. C., Yip R. E., Halford N. G., Shewry P. R., Malpica-Romero J. M. Nucleotide sequences of the two high-molecular-weight glutenin genes from the D-genome of a hexaploid bread wheat, Triticum aestivum L. cv Cheyenne. Nucleic Acids Res. 1989 Jan 11;17(1):461–462. doi: 10.1093/nar/17.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988 Oct 13;335(6191):649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- Doege K. J., Fessler J. H. Folding of carboxyl domain and assembly of procollagen I. J Biol Chem. 1986 Jul 5;261(19):8924–8935. [PubMed] [Google Scholar]
- Field J. M., Tatham A. S., Shewry P. R. The structure of a high-Mr subunit of durum-wheat (Triticum durum) gluten. Biochem J. 1987 Oct 1;247(1):215–221. doi: 10.1042/bj2470215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde J., Malpica J. M., Halford N. G., Shewry P. R., Anderson O. D., Greene F. C., Miflin B. J. The nucleotide sequence of a HMW glutenin subunit gene located on chromosome 1A of wheat (Triticum aestivum L.). Nucleic Acids Res. 1985 Oct 11;13(19):6817–6832. doi: 10.1093/nar/13.19.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili G. Heterologous expression of a wheat high molecular weight glutenin gene in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7756–7760. doi: 10.1073/pnas.86.20.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsbrough A. P., Bulleid N. J., Freedman R. B., Flavell R. B. Conformational differences between two wheat (Triticum aestivum) 'high-molecular-weight' glutenin subunits are due to a short region containing six amino acid differences. Biochem J. 1989 Nov 1;263(3):837–842. doi: 10.1042/bj2630837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene F. C. In Vitro Synthesis of Wheat (Triticum aestivum L.) Storage Proteins. Plant Physiol. 1981 Sep;68(3):778–783. doi: 10.1104/pp.68.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. F., Jr, Pace C. N. Urea and guanidine hydrochloride denaturation of ribonuclease, lysozyme, alpha-chymotrypsin, and beta-lactoglobulin. J Biol Chem. 1974 Sep 10;249(17):5388–5393. [PubMed] [Google Scholar]
- Kasarda D. D., Bernardin J. E., Qualset C. O. Relationship of gliadin protein components to chromosomes in hexaploid wheats (Triticum aestivum L.). Proc Natl Acad Sci U S A. 1976 Oct;73(10):3646–3650. doi: 10.1073/pnas.73.10.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Rafalski A., Peterson D., Söll D. A wheat HMW glutenin subunit gene reveals a highly repeated structure. Nucleic Acids Res. 1985 Dec 20;13(24):8729–8737. doi: 10.1093/nar/13.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. D., Bartels D., Harberd N. P. Nucleotide sequence of a gene from chromosome 1D of wheat encoding a HMW-glutenin subunit. Nucleic Acids Res. 1985 Oct 11;13(19):6833–6846. doi: 10.1093/nar/13.19.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]