Abstract
Flavonoid derivatives have been optimized as relatively rigid antagonists of adenosine receptors with particular selectivity for the A3 receptor subtype. A quantitative study of the structure-activity relationships for binding of flavonoids to adenosine A1, A2A, and A3 receptors has been conducted using comparative molecular field analysis (CoMFA). Correlation coefficients (cross-validated r2) of 0.605, 0.595, and 0.583 were obtained for the three subtypes, respectively. All three CoMFA models have the same steric and electrostatic contributions, implying similar requirements inside the binding cavity. Similarities were seen in the topology of steric and electrostatic regions with the A1 and A3 receptors, but not the A2A. Substitutions on the phenyl ring at the C-2 position of the chromone moiety may be considered important for binding affinity at all adenosine receptors. In the A3 model a region of favorable bulk interaction is located around the 2′-position of the phenyl ring. The presence of a C-6 substituent in the chromone moiety is well tolerated and increases the A1/A3 selectivity. The CoMFA coefficient contour plots provide a self-consistent picture of the main chemical features responsible for the pKi variations and also result in predictions which agree with experimental values.
Introduction
A wide variety of non-purine ligands that bind selectively to adenosine receptors have been described.1,2 The availability of selective ligands has facilitated studies of the physiological roles of particular subtypes of adenosine receptors. A3 adenosine receptors are associated with cerebroprotective3 and cardioprotective4 effects of adenosine agonists and effects on the immune and inflammatory systems.5 Unlike A1 and A2 subtypes, for A3 receptor xanthine derivatives have not proven to be suitable leads for the development of antagonists for this subtype. We have introduced A3 receptor-selective antagonists belonging to three distinct, non-purine chemical classes: flavonoids,6,7 1,4-dihydropyridines,8,9 and [1,2,4]triazolo[1,5-c]quinazolines.10 Recently we have synthesized antagonists in the dihydropyridine class with A3 receptor selectivities of >30000-fold.9 Other A3 receptor antagonists belonging to triazolo-[5,1-a][2,7]naphthyridine and thiazolo[3,2]pyrimidine classes11 have been identified through broad screening at Merck. A3 receptor-selective antagonists have been suggested to have antiasthmatic12 and possibly cerebroprotective13 properties.
A broad screening of phytochemicals in competitive binding versus the high-affinity agonist [125I]AB-MECA (N6-(4-amino-3-iodobenzyl)adenosine 5′-N-methyluronamide) has demonstrated micromolar affinity of assays certain naturally occurring flavonoids at cloned human brain A3-adenosine receptors.6 The considerable affinity of flavones at adenosine receptors may explain some of the previously observed vascular and other biological effects of these compounds. Starting from structure–activity relationships, chemical optimization of this class led to 3,6-dichloro-2′-(isopropyloxy)-4′-methylflavone7 (MRS 1067; Ki = 0.56 μM), which is both relatively potent and highly selective (200-fold) for human A3 vs human A1 receptors. This derivative effectively antagonized the effects of an agonist in the inhibition of adenylyl cyclase in Chinese hamster ovary (CHO) cells expressing either cloned rat or human A3 receptors.7,14 MRS 1067 antagonized the effects of the A3 receptor-selective agonist Cl-IB-MECA to induce a rise in intracellular [Ca2+] in RBL-2H3 rat mast cells.15
A dramatic species dependence of A3 receptor affinity has been observed for most classes of antagonists,9,16,17 and the affinity at the human subtype is usually considerably higher than in the rat. Although nanomolar affinity has not yet been achieved for flavonoids binding at A3 receptors, the unique characteristic of this class of antagonists is that the ratio of affinities at human vs rat A3 receptors is only 1 order of magnitude.6
Moreover flavonoids are known to bind to a wide range of enzymes,18–21 in addition to adenosine receptors, and these actions may interfere with their use as pharmacological probes at A3 receptors. In particular, MRS 1067 was found to bind to rat liver cytochrome P450s.21
Thus, we have embarked on a quantitative study of the structure–activity relationships of flavonoids binding to adenosine A1, A2A, and A3 receptors, using comparative molecular field analysis (CoMFA) in an effort to develop novel A3 adenosine antagonists. The relatively rigid ring structure of flavonoids compared to other A3 receptor antagonists, such as 1,4-dihydropyridines, makes them particularly amenable to CoMFA analysis.
Computational Methods
Materials and Methods.
CoMFA is a three-dimensional QSAR method that operates on a set of ligands that have been superimposed to reflect their anticipated common binding orientation. CoMFA models describe the extent to which the change in magnitude of the electrostatic and steric fields as a function of compound, sampled as a function of spatial position around the compound set, accounts for the variance in measured biological activity. Molecular modeling and CoMFA studies were performed on a Silicon Graphics Power Indigo2 R8000 workstation running SYBYL 6.3.22 Quantum calculations used throughout this study were performed using MOPAC (Ver. 6.0)23 and Gaussian 92.24
Data Sets.
A total of 30, 26, and 36 flavonoid derivatives were included in the training set used to generate the CoMFA models, for A1, A2A, and A3 receptors, respectively. These molecules were classified into six families depending on the chemical structure of the bicyclic moiety: flavonols, 1–28; flavones, 29; flavanones, 30–33; dihydroflavanols, 34–36; furylchromones, 37–44; and naphthoflavones, 45 (see Table 1).
Table 1.
Observed and Calculated Receptor-Binding Affinity Values of the Compounds Forming A1, A2A, and A3 Training Sets

| |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| pKi (μM)a | |||||||||
|
|
|||||||||
| rA1 | rA2A | hA3 | |||||||
|
|
|
|
|||||||
| compound | R1 | R2 | R3 | obsd | calcdb | obsd | calcdb | obsd | calcdb |
|
| |||||||||
| 1 (galangin) | OH | Ph | 5,7-(OH)2 | ts | ts | ts | ts | −0.23 | −0.26 |
| 2 (MRS928) | OMe | Ph | 5,7-(OMe)2 | 0.29 | 0.31 | −0.81 | −0.75 | −0.08 | −0.08 |
| 3 (MRS1041) | OEt | Ph | 5,7-(OEt)2 | 0.22 | 0.33 | −0.52 | −0.54 | 0.44 | 0.37 |
| 4 (MRS1093) | OEt | Ph | 5-OH–7-OEt | −0.28 | −0.37 | −1.80 | −1.78 | 0.13 | 0.16 |
| 5 (MRS1042) | OPr | Ph | 5,7-(OPr)2 | −0.04 | −0.04 | −0.51 | −0.54 | 0.50 | 0.44 |
| 6 | CH2CH=C(CH3)2 | 2′,4′-(OH)2Ph | 5-OH-6-CH=CHCH(CH3)2-7-(OMe)2 | −0.96 | −0.99 | na | −0.66 | −0.67 | |
| 7 (MRS923) | OMe | 2′,4′-(OMe)2Ph | 5,7-(OMe)2 | −1.44 | −1.47 | −1.67 | −1.57 | −0.42 | −0.50 |
| 8 (MRS1063) | OEt | 2′,4′-(OEt)2Ph | 5-OH–7-OEt | na | na | −0.68 | −0.70 | ||
| 9 (MRS1086) | OEt | 2′,4′-(OEt)2Ph | 5,7-(OEt)2 | −1.51 | −1.53 | −1.42 | −1.46 | −0.86 | −0.78 |
| 10 | OH | 2′,4′,6′-(OMe)3Ph | H | −0.86 | −0.76 | na | −1.70 | −1.73 | |
| 11 (MRS1132) | Cl | Ph | H | ts | ts | −1.40 | −1.39 | −1.06 | −0.98 |
| 12 (MRS1088) | Cl | Ph | 6-Cl | na | −1.74 | −1.65 | ts | ts | |
| 13 (MRS1067) | Cl | 2′-i-Pr-4′-MePh | 6-Cl | na | na | 0.25 | 0.32 | ||
| 14 | H | Ph | 5-OH | −0.34 | −0.38 | na | na | ||
| 15 | H | Ph | 7-OH | −0.48 | −0.41 | −0.43 | −0.43 | na | |
| 16 | H | 3′,4′-(MeO)2Ph | 7-OH | −1.27 | −1.30 | −1.54 | −1.59 | na | |
| 17 (apigenin) | H | 4′-OHPh | 5,7-(OH)2 | −0.48 | −0.47 | −0.88 | −0.92 | na | |
| 18 | H | 4′-OMePh | 5-OH–7-Me | −0.53 | −0.49 | −1.45 | −1.40 | −1.70 | −1.69 |
| 19 | H | 4′-MeOPh | 5,6,7-(OMe)3 | −0.11 | −0.08 | na | −0.65 | −0.69 | |
| 20 (hispidulin) | H | 4′-OHPh | 5,7-(OH)2-6–MeO | −0.21 | −0.23 | −0.88 | −0.88 | na | |
| 21 (cirsimaritin) | H | 4′-OHPh | 5-OH–6,7-(MeO)2 | −0.08 | −0.02 | −0.48 | −0.49 | −0.50 | −0.35 |
| 22 | OMe | Ph | 5,7-(MeO)2 | 0.29 | 0.23 | −0.81 | −0.73 | −0.09 | 0.01 |
| 23 | OAc | Ph | 5,7-(AcO)2 | −1.06 | −1.03 | na | −1.24 | −1.27 | |
| 24 | OMe | 4′-OMePh | 5,7-(MeO)2 | −0.03 | −0.02 | na | −0.53 | 0.55 | |
| 25 (rhamnetin) | OH | 3′,4′-(OH)2Ph | 7-OMe | na | na | −0.14 | −0.13 | ||
| 26 (quercitin) | OH | 3′,4′-(OH)2Ph | 5,7-(OH)2 | −0.39 | −0.33 | −0.84 | −0.86 | na | |
| 27 (pentamethylmorin) | OMe | 2′,4′-(MeO)2Ph | 5,7-(MeO)2 | −1.44 | −1.34 | −1.67 | −1.75 | −0.42 | −0.47 |
| 28 (hexamethylmyricetin) | OMe | 3′,4′,5′-(MeO)3Ph | 5,7-(MeO)2 | na | na | −0.82 | −0.73 | ||
| 29 (flavone) | H | Ph | H | −0.52 | −0.52 | −0.54 | −0.48 | −1.23 | −1.18 |
| 30 (flavanone) | H | Ph | H | ts | ts | na | −1.21 | −1.16 | |
| 31 | H | 2′-OHPh | H | −0.42 | −0.57 | −1.25 | −1.25 | −0.78 | −0.89 |
| 32 | H | 4′-OHPh | H | −1.06 | −1.15 | na | −1.36 | −1.40 | |
| 33 (sakuranetin) | H | 4′-OHPh | 5-OH–7-OMe | −0.91 | −0.89 | −1.55 | −1.63 | −0.53 | −0.63 |
| 34 | OH | 2′-OHPh | H | −1.96 | −1.90 | na | na | ||
| 35 (MRS1061) | OH | CH=CHPh | 6-OMe | na | na | −1.32 | −1.32 | ||
| 36 (MRS1062) | OH | CH=CPh | 6-OMe | −1.70 | −1.67 | na | −0.91 | −0.89 | |
| 37 (dihydroquercitin) | OH | 3′,4′-(OH)2Ph | 5,7-(OH)2 | na | na | −1.53 | −1.55 | ||
| 38 (visnagin) | OCH3 | CH3 | na | −1.63 | −1.66 | −1.78 | −1.77 | ||
| 39 | OCH3 | CHO | na | na | −1.95 | −1.95 | |||
| 40 (MRS1065) | OCH3 | CH=CHPh | −1.51 | −1.52 | −1.06 | −1.28 | −0.92 | −0.94 | |
| 41 (MRS1066) | OC2H5 | CH=CHPh | −1.55 | −1.59 | −1.53 | −1.45 | −0.06 | −0.12 | |
| 42 (MRS1084) | O(CH2)2CH3 | CH=CHPh | −1.60 | −1.61 | −1.69 | −1.55 | −0.60 | −0.71 | |
| 43 (MRS1071) | OC2H5 | CH=CHCH=CHPh | na | −2.22 | −2.19 | −1.66 | −1.58 | ||
| 44 (MRS1078) | OCH3 | CH=NPh | na | na | −0.96 | −0.96 | |||
| 45 (α-naphthoflavone) | H | Ph | 0.10 | 0.14 | −0.12 | −0.15 | na | ||
The synthesis and binding affinity constants of all flavonoid derivatives were reported in detail previously.6,7 Binding affinity expressed as a Ki value (inhibition constant) was obtained using the following radioligands at the respective adenosine receptors: [3H]-N6-phenylisopropyladenosine (rat A1);25 [3H]CGS 21680 (rat A2A),26 [125I]AB-MECA (human A3).27
Molecular Superposition.
The chromone moiety is believed to be a key determinant of binding interactions of flavonoid derivatives. Therefore, the ligands in this study were superimposed on the chromone nucleus by fitting a minimum-energy conformation of the compound 22 (reference structure). Conformational analysis of compound 22 was performed on the four rotatable bonds of the chromone substituents using a random search procedure in SYBYL. The energies of the resulting conformations were calculated using MOPAC (PM3 Hamiltonian, keywords: PREC, GNORM = 0.1, EF).28 The remaining compounds were fitted by a least-squares algorithm to the reference structure so as to maximally align their substituents with the corresponding substituents of the reference structure. The fitted conformations of each compound were fully minimized using MOPAC (PM3 Hamiltonian).
Atomic Charge Calculations.
Partial atomic charges are required for calculating electrostatic fields in CoMFA. Partial atomic charges for compound 22 were calculated at the semiempirical level using the MNDO,29 AM1,30 and PM3 Hamiltonians of MOPAC. The results of each method were compared to those obtained using the MP2/6–31G(*)//RHF/6–31G(*) ab initio level of Gaussian 92. The charges calculated using PM3 were found to agree best with the ab initio charges. Therefore, partial atomic charge for all the flavonoid derivatives were calculated using PM3 Hamiltonian. No improvements have been found using PM3 atomic charges derived from electrostatic potentials.31
CoMFA Field Calculations and Regression Techniques.
The electrostatic and steric fields were sampled along a three-dimensional lattice encompassing all molecules in each receptor data set. The lattice consisted of 720 sample points based on a 2.0 Å lattice spacing with boundaries extending 4.0 Å beyond the largest structure in all directions. Lattice spacings of 0.75 and 1.5 Å were also used without improvements of the CoMFA results. The lattice points within the union volume of the superimposed structures were dropped. The probe used to calculate the CoMFA fields consisted of a sp3 carbon atom with a +1 charge and a van der Waals radius of 1.52 Å. The steric and electrostatic fields were calculated separately for each molecule using a Lennard-Jones 6–12 potential and a Coulombic potential with a 1/r distance-dependent dielectric, respectively. The steric and electrostatic energies were truncated at 30 kcal/mol. The field values corresponding to the 720 sample points for each molecule, together with binding affinity data, were stored in a SYBYL Molecular Spreadsheet to facilitate statistical analysis.
Partial least-squared (PLS) regression analysis32 was performed on the A1, A2A, and A3 antagonist datasets using a subset of CoMFA field sample points falling with a standard deviation of ≤1.0 kcal/mol. The steric and the electrostatic fields were scaled to equalize their weighting in the CoMFA models (SYBYL command “scaling CoMFA_std”). PLS was performed using cross-validation to evaluate the predictive ability of the CoMFA models.33 The optimal number of latent variables was derived from cross-validation equation having the lowest standard error and a significance level of ≥99.5% was estimated using the stepwise F-test. Bootstrap analysis33 of the dataset was used to evaluate the statistical confidence limits of the results. A σ value of 2.0 was adopted for both the cross-validated and non-cross-validated analysis. σ values of 1.0 or 0.5 did not significantly change the calculated r2.
Initial PLS analyses were performed in conjunction with the cross-validation (leave-one-out method) option to obtain the optimal number of components to be used in the subsequent analyses of the dataset. The PLS analysis was repeated with the number of cross-validation groups set to zero. The optimal number of components was designated as that which yielded the highest cross-validated r2 values in the non-cross-validated (conventional) analyses. The final PLS analysis with 10 bootstrap groups and the optimal number of components was performed on the complete dataset.
The corresponding calibration equation (resulting from the simultaneous contribution of all the observations) was derived after the optimal dimensionality of each receptor model was established, by PLS analysis and cross-validation. The calibration equation with latent variables was then converted to the original parametric space represented by probe–ligand interaction energies. A 3D-QSAR was therefore derived whose coefficients were associated with statistically significant lattice locations. CoMFA coefficient contour maps were generated by interpolation of the pairwise products between the 3D-QSAR coefficients and the standard deviations of the associated energy variables.
Test Sets.
The test sets consisted of three molecules for each training set 46–54 (Table 2). These structures were chosen to maximize a uniform sampling of biological activity. All predicted activities for the test set molecules were calculated using the optimized CoMFA model. The results of the non-cross-validated calibration model on the test sets are summarized in Table 2.
Table 2.
Observed and Predicted Receptor-Binding Affinity Values of the Compounds Forming A1, A2A, and A3 Test Sets

| |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| pKi (μM)a | |||||||||
|
|
|||||||||
| rA1 | rA2A | hA3 | |||||||
|
|
|
|
|||||||
| compound | R1 | R2 | R3 | obsd | predb | obsd | predb | obsd | predb |
|
| |||||||||
| 46 | OH | Ph | 5,7-(OH)2 | 0.06 | 0.11 | ||||
| 47 (MRS1132) | Cl | Ph | H | −0.39 | −0.31 | ||||
| 48 (flavanone) | H | Ph | H | −1.50 | −1.38 | ||||
| 49 (galangin) | OH | Ph | 5,7-(OH)2 | 0.01 | 0.11 | ||||
| 50 | H | Ph | 5-OH | −0.79 | −0.73 | ||||
| 51 | OAc | Ph | 5,7-(OAc)2 | −1.75 | −1.68 | ||||
| 52 (MRS1088) | Cl | Ph | 6-Cl | 0.13 | 0.19 | ||||
| 53 (MRS1089) | OEt | 2′,4′,6′-Me3Ph | 6-Cl | −0.72 | −0.79 | ||||
| 54 (MRS1072) | OH | CH≡CPh | 6-OMe | −1.38 | −1.25 | ||||
“Predictive” r2 Values.
The “predictive” r2pred was based only on molecules not included in the training set and is defined as explained by Marshall and co-workers.34
Results
CoMFA of A1 Receptor Binding Affinity.
PLS was used in conjunction with cross-validation to obtain the optimal number of components to be used in the subsequent analyses. PLS analysis based on least-squares fit gave a correlation with a cross-validated r2cv of 0.605, with the maximum number of components set equal to 6 (maximum number of components set equal to 4, 5, 7, or 8 gave unreliable cross-validated r2cv ≤ 0.40) and the cross-validation groups set equal to the number of observations (rows) in the data table. The non-cross-validated PLS analysis was repeated with the optimum number of components, as determined by the cross-validated analysis, to give an r2cv of 0.961. To obtain statistical confidence limits, the non-cross-validated analysis was repeated with 10 bootstrap groups, which yielded an r2 of 0.985 (optimum number of components was 6), SEP = 0.086, std dev = 0.011, steric contribution = 0.492, and electrostatic contributions = 0.508. These parameters are explained in Table 3.
Table 3.
Statistic of the Calibration CoMFA Models
| A1 | A2A | A3 | |
|---|---|---|---|
|
| |||
| number of compounds | 30 | 26 | 36 |
| principal componentsa | 6 | 4 | 6 |
| r 2 cv b | 0.605 | 0.595 | 0.583 |
| r2 | 0.961 | 0.963 | 0.957 |
| F test c | 87.541 | 125.192 | 119.53 |
| p value | <0.001 | <0.001 | <0.001 |
| r2bsd | 0.985 | 0.978 | 0.974 |
| Steric contribution | 0.492 | 0.486 | 0.460 |
| Electrostatic contribution | 0.508 | 0.514 | 0.540 |
| SEPe | 0.086 | 0.112 | 0.108 |
| std devf | 0.011 | 0.015 | 0.012 |
Minimum σ = 2.0.
Standard error of prediction (cross-validated) = (PRESS/(n − c − 1))1/2, n = number of rows, c = number of components.
Ratio of r2 explained to unexplained = r2/(1 − r2).
r2bs = r2 after bootstrapping.
Cross-validated r2 after leave-one-out procedure: r2cv = (SD − PRESS)/SD, SD = Yactual − Ymean)2. PRESS = Σ(Ypredicted − Yactual). For further explaination of these mathematical formulas see ref 33.
Std dev column belongs with the bootstrapping r2.
The CoMFA-derived QSAR of the A1 ligands exhibited a good cross-validated correlation, indicating that it was highly predictive. Cross-validation provides information concerning the predictive ability of the QSAR dataset by minimizing the occurrence of chance correlations in the QSAR model. The high bootstrapped r2 value and small standard deviation suggest a high degree of confidence in the analysis. The calculated binding affinities obtained from the analysis were plotted versus the actual values in Figure 1.
Figure 1.
Fitted vs measured pKi values for the CoMFA analysis of the A1, A2A, and A3 training sets (A, B, and C, respectively). A1: the model was derived using six principal components yielding a cross-validated r2 = 0.605. A2A: the model was derived using four principal components yielding a cross-validated r2 = 0.595. A3: the model was derived using six principal components yielding a cross-validated r2 = 0.583.
Compounds 46–48 (test set) were used to evaluate the predictive power of this CoMFA model. As in the calibration step, a good predictive ability with an r2pred = 0.858 for the compounds in the test set was obtained. Table 2 shows that the affinities of all the examined compounds are predicted within 0.12 log unit across a range of 1.56 log units.
The coefficients corresponding to each sampled field point in the resulting correlation equation were graphically contoured. Contours corresponding to the steric (green and yellow) and electrostatic (blue and red) fields are plotted together with compound 2 in Figure 2. The polyhedra describe the regions of space where the steric and the electrostatic fields are predicted by the CoMFA model to have the greatest effect on binding affinity. The yellow and the blue polyhedra correspond to regions of the field that are predicted to decrease the A1 receptor affinity, whereas the green and the red regions are predicted to increase binding affinity. The region of space around the para position of the phenyl ring is contained within a yellow (steric detracting) polyhedron, suggesting that bulky substituents are not tolerated by the receptor at the C-2 position of the chromone moiety.
Figure 2.
CoMFA steric and electrostatic STDEV*COEFF contour plots from the analysis based on the A1 receptor 3D-QSAR without cross-validation. Compound 2 shown inside the field. Favoring activity: green, bulky group (contribution level 80%); yellow, less bulky; blue, positive charge (contribution level 70%); red, negative charge.
Both styryl and phenylpropargyl derivatives (see compounds 35–36, Table 1) are in fact among the less active compounds. The C-5 and C-7 positions of the chromone structure are surrounded by green polyhedra, suggesting that the presence of alkoxy substituents increases A1 receptor affinity (see compounds 2–3, Table 1). The regions of space around the ortho positions of the phenyl ring are contained within a large red contour, suggesting that substituents that enhance the electrostatic fields in that region improve the A1 receptor binding affinity. An o-hydroxy substituent (compound 31, Table 1) could be expected to improve affinity electrostatically, whereas bulky alkoxy substituents (9–13) would also detract sterically. The blue polyhedron around the para position of the phenyl ring suggests that substituents with higher electron density at this position may reduce the binding affinity. The red polyhedron around the C-3 chromone position is in agreement with the know enhancement in A1 binding affinity produced by alkoxy substituents at this position (22, 24).
CoMFA of A2A Receptor Binding Affinity.
The chosen alignment yielded good cross-validated (r2cv = 0.595) and conventional results (r2 = 0.963, F-test value = 125.192), with the optimal number of components found equal to 4. Steric and electrostatic fields contribute to the QSAR equation by 48.6% and 51.4%, respectively. A high bootstrapped (10 sampling) r2bs value of 0.978 (SEP = 0.112, std dev = 0.015) was found. The calculated binding affinities obtained from the analysis are plotted versus the actual values in Figure 1. Compounds 49–51 (test set) were used to evaluate the predictive power of this CoMFA model. A good predictive ability with an r2pred = 0.835 for the compounds in the test set was obtained in this calibration step. Table 2 shows that the affinities of all compounds examined are predicted within 0.10 log unit across a range of 1.76 log units.
Contours corresponding to the steric (green and yellow) and electrostatic (blue and red) fields are plotted together with compound 45 in Figure 3. A green contour around the C-3 position of the chromone moiety suggests that bulky substituents in this position (see compounds 1, 3, and 5, Table 1) enhance affinity. Another important area of bulk tolerance is found around the C-7 and C-8 positions of the chromone moiety. In fact, R-naphthoflavone (45) presents a good affinity and a very high selectivity. As for the A1 model, bulky substituents in the C-2 position of chromone moiety decrease the A2A receptor affinity (see compounds 35–36). The blue polyhedron around the para position of the phenyl ring suggests that substituents with higher electron density exert a negative effect on the affinity, in agreement with the experimental data that alkoxy substituents in this position decrease the A2A receptor affinity (19, 24).
Figure 3.
CoMFA steric and electrostatic STDEV*COEFF contour plots from the analysis based on the A2A receptor 3D-QSAR without cross-validation. Compound 45 shown inside the field. Favoring activity: green, bulky group (contribution level 80%); yellow, less bulky; blue, positive charge (contribution level 70%); red, negative charge.
CoMFA of A3 Receptor Binding Affinity.
The chosen alignment yielded acceptable cross-validated (r2cv = 0.583) and conventional results (r2 = 0.957, F-test value = 119.53), with the optimal number of components found to be equal to 6. In this model, steric and electrostatic fields contribute to the QSAR equation by 46.0% and 54.0%, respectively. A high bootstrapped (10 sampling) r2bs value of 0.974 (SEP = 0.108, std dev = 0.012) was found. Compounds 52–54 (test set) were used to evaluate the predictive power of this CoMFA model. The predicted binding affinities obtained from the analysis are plotted versus the actual values in Figure 1. A good predictive ability with an r2pred = 0.817 for the compounds in the test set was obtained as for the calibration steps. Table 2 shows that the affinities of all the examined compounds are predicted within 0.13 log unit across a range of 1.51 log units.
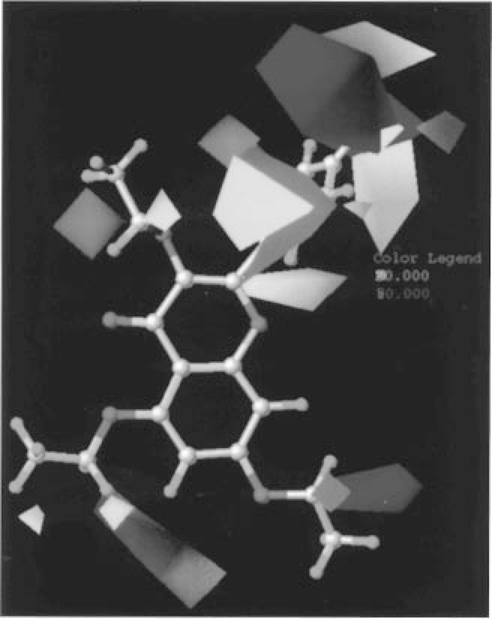
Contours corresponding to the steric (green and yellow) and electrostatic (blue and red) fields are plotted together with compound 3 in Figure 4. As for A1 and A2A receptor CoMFA models, the major variability of the steric contour maps occurs around the phenyl ring on the C-2 position of the chromone moiety. Again, a yellow area surrounds the para position of the phenyl ring, suggesting that bulky substituents are not tolerated at the C-2 position of the chromone moiety. Bulky groups are also predicted to be tolerated around the C-6 position of the chromone moiety for A3 receptor binding. In fact, the presence of a chloro substituent in this position is tolerated (13). The area of bulky tolerance surrounding the ortho position of the phenyl ring suggests that bulky substituents at this position may increase A3 affinity. A 2′-i-Pr derivative (13), in fact, displays good affinity. Bulky substituents at the C-2 position of the chromone moiety decrease the affinity also for the A3 receptor (see compounds 35–36). The blue polyhedron in the para position of the phenyl ring indicates that substituents with higher electron density exert a negative effect on the affinity, in agreement with the experimental data that alkoxy substituents in this position decrease the A3 receptor affinity (10, 18).
Figure 4.
CoMFA steric and electrostatic STDEV*COEFF contour plots from the analysis based on the A3 receptor 3D-QSAR without cross-validation. Compound 3 shown inside the field. Favoring activity: green, bulky group (contribution level 80%); yellow, less bulky; blue, positive charge (contribution level 70%); red, negative charge.
Discussion
A set of 3D-QSAR models has been developed using the CoMFA methodology for flavonoid derivative adenosine receptor antagonists. This is the first attempt to describe quantitatively the hypothetical receptor binding site of multiple subtypes of adenosine receptors. Comparison of the three CoMFA models helps in understanding adenosine receptor selectivity. The analysis of these results leads to the following considerations:
All three CoMFA models have similar average steric and electrostatic contributions (see Table 3), implying that A1, A2A, and A3 have the same relative contribution of steric and electrostatic factors inside the binding cavity. However, the specific distribution of steric and electrostatic interactions for each receptor is different, as shown in Figures 2–4.
Similarities were seen in the topology of steric andelectrostatic regions with the A1 and A3 receptors, but not the A2A receptor (Figures 2–4). This is in accord with the structure–activity relationship data in which A1–A3 similarity has been demonstrated.17
C-2 chromone phenyl ring substituents are considered important for the binding affinity for all adenosine receptors. This phenyl ring may interact in a similar region of space inside the receptor binding-site.
An interesting consideration about A1/A3 selectivity can be deduced from CoMFA contour map analysis. An important green region (favorable steric bulk interaction) is located around the 2′-position of the phenyl ring, in the A3 model. This is in accord with the experimental data for compounds 8, 12, and 13, which are the most selective flavonoid compounds for human A3 receptors.
The presence of a C-6 substituent in the chromone moiety is well tolerated, and increases the A1/A3 selectivity (see compound 13).
Further synthesis and biological evaluation of new flavonoid derivatives aimed at increasing both the affinity and the selectivity for the adenosine receptors are in progress.
Conclusion
In conclusion, the CoMFA method has been successfully applied to a set of recently described flavonoid derivatives with affinity for A1, A2A, and A3 receptors. The resulting 3D-QSAR models show good correlations between steric and electrostatic field and binding affinities. The CoMFA coefficient contour plots provide a self-consistent picture of the main chemical features responsible for the pKi variations, and the CoMFA QSAR equations result in predictions which agree with the experimental values. Comparison of our CoMFA models can be used to suggest improvement of adenosine receptor subtype selectivity. This information may be useful for designing new compounds with higher selectivity for A3 vs A1 and A2A receptors.
Acknowledgment.
S. Moro is grateful to Gilead Sciences (Foster City, CA) for a postdoctoral fellowship grant. We thank Dr. Robert Pearlstein of the Center for Molecular Modeling (DCRT), NIH for helpful comments and encouragement.
Abbreviations
- 3D-QSAR
three-dimensional quantitative structure activity relationship
- [125I]AB-MECA
N6-(4-amino-3-iodobenzyl)adenosine 5′-N-methyluronamide
- Cl-IB-MECA
N6-(3-iodobenzyl)-2-chloroadenosine 5′-N-methyluronamide
- CoMFA
comparative molecular field analysis
- PLS
partial least-squares
- QSAR
quantitative structure–activity relationship
- r 2 bs
correlation coefficient from boostrap analysis
- r 2 cv
correlation coefficient from cross-validation equation
- rms
root-mean-square
References
- (1).Jacobson KA; Suzuki F Recent Developments in Selective Agonists and Antagonists Acting at Purine and Pyrimidine Receptors. Drug Dev. Res. 1997, 39, 289–300. [Google Scholar]
- (2).Müller C A1 Adenosine Receptor Antagonists. Exp. Opin. Ther. Patents 1997, 7, 419–440. [Google Scholar]
- (3).von Lubitz DKJE; Lin RCS; Popik P; Carter MF; Jacobson KA Adenosine A3 Receptor Stimulation and Cerebral Ischemia. Eur. J. Pharmacol. 1994, 263, 59–67.7821362 [Google Scholar]
- (4).Strickler J; Jacobson KA; Liang BT Direct Preconditioning of Cultured Chick Ventricular Myocytes: Novel Functions of Cardiac Adenosine A2A and A3 Receptors. J. Clin. Invest. 1996, 74, 1773–1779. [Google Scholar]
- (5).Sajjadi FG; Takabayashi K; Foster AC; Domingo RC; Firestein GS Inhibition of TNF-alpha Expression by Adenosines–Role of A3 Adenosine Receptors. J. Immunol. 1996, 156, 3435–3442.8617970 [Google Scholar]
- (6).Ji X.-d.; Melman N.; Jacobson KA. Interactions of Flavonoids and Other Phytochemicals with Adenosine Receptors. J. Med. Chem. 1996, 39, 781–788.8576921 [Google Scholar]
- (7).Karton Y; Jiang JL; Ji X.-d.; Melman N.; Olah ME.; Stiles GL.; Jacobson KA. Synthesis and Biological-Activities of Flavonoid Derivatives as as Adenosine Receptor Antagonists. J. Med. Chem. 1996, 39, 2293–2301.8691424 [Google Scholar]
- (8).van Rhee AM; Jiang JL; Melman N; Olah ME; Stiles GL; Jacobson KA Interaction of 1,4-Dihydropyridine and Pyridine Derivatives with Adenosine Receptors – Selectivity for A3 Receptors. J. Med. Chem. 1996, 39, 2980–2989.8709132 [Google Scholar]
- (9).Jiang J.-l.; van Rhee AM.; Chang L.; Patchornik A.; Evans P.; Melman N.; Jacobson KA. Structure Activity Relationships of 4-Phenylethynyl-6-phenyl-1,4-dihydropyridines as Highly Selective A3 Adenosine Receptor Antagonists J. Med. Chem. 1997, 40, 2596–2608.9258367 [Google Scholar]
- (10).Kim Y-C; Ji X.-d.; Jacobson KA. Derivatives of the Triazoloquinazoline Adenosine Antagonist (CGS15943) are Selective for the Human A3 Receptor Subtype. J. Med. Chem. 1996, 39, 4142–4148.8863790 [Google Scholar]
- (11).Jacobson MA; Chakravarty PK; Johnson RG; Norton R Novel Selective Nonxanthine A3 Adenosine Receptor Antagonists. Drug Dev. Res. 1996, 37, 131. [Google Scholar]
- (12).Beaven MA; Ramkumar V; Ali H Adenosine A3 Receptors in Mast Cells. Trends Pharmacol. Sci. 1994, 15, 13–14. [Google Scholar]
- (13).Jacobson KA; Kim HO; Siddiqi SM; Olah ME; Stiles G; von Lubitz DKJE A3 Adenosine Receptors: Design of Selective Ligands and Therapeutic Prospects. Drugs Future 1995, 20, 689–699.25242859 [Google Scholar]
- (14).Jacobson KA; Park KS; Jiang J.-l.; Kim Y-C.; Olah ME.; Stiles GL.; Ji X.-d. Pharmacological Characterization of Novel A3 Adenosine Receptor-Selective Antagonists. Neuropharmacology 1997, 36, 1157–1165.9364471 [Google Scholar]
- (15).Shin Y; Daly JW; Jacobson KA Activation of Phosphoinositide Breakdown in a Rat RBL-2H3 Mast Cell Line by Adenosine Analogues: Lack of Correlation with Affinity for A3-Adenosine Receptor. Drug Dev. Res. 1996, 39, 36–46.23087534 [Google Scholar]
- (16).Linden J; Taylor HE; Robeva AS; Tucker AL; Stehle JH; Rivkees SA; Fink JS; Reppert SM Molecular Cloning and Functional Expression of a Sheep A3 Adenosine Receptor with Widespread Tissue Distribution. Mol. Pharmacol. 1993, 44, 524–532.8396714 [Google Scholar]
- (17).Salvatore CA; Jacobson MA; Taylor HE; Linden J; Johnson RG Molecular Cloning and Characterization of the Human A3 Adenosine Receptor. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 10365–10369.8234299 [Google Scholar]
- (18).Middleton E Jr. Some Biological Properties of Plant Flavonoids. Ann. Allergy 1988, 61 53–57.3061322 [Google Scholar]
- (19).Limasset B; le Doucen C; Dore JC; Ojasoo T; Damon M; Crastes de Paulet A Effects of Flavonoids on the Release of Reactive Oxygen Species by Stimulated Human Neutrophils. Multivariate Analysis of Structure–Activity Relationships (SAR). Biochem. Pharmacol. 1993. 46: 1257–1271.8216378 [Google Scholar]
- (20).Raghavan K; Buolamwini JK; Kohn KW; Weinstein JN Three-Dimensional Quantitative Structure–activity Relationship (QSAR) of HIV Integrase Inhibitors: A Comparative Molecular Field Analysis (CoMFA) Study. J. Med. Chem. 1995, 38, 890–897.7699704 [Google Scholar]
- (21).Dai R; Jacobson KA; Robinson RC; Friedman FK Differential Effects of Flavonoids on Testosterone-Metabolizing Cytochrome P450s. Life Sci. Pharmacology Lett. 1997, 61, PL75–PL80. [Google Scholar]
- (22).The program SYBYL 6.3 is available from TRIPOS Associates, St. Louis, MO, 1993. [Google Scholar]
- (23).MOPAC 6.0 available from Quantum Chemistry Program Excange.
- (24).Frisch MJ; Trucks GW; Head-Gordon M; Gill PMW; Wong MW; Foresman JB; Johnson BG; Schlegel HB; Robb MA; Replogle ES; Gomperts R; Andres JL; Raghavachari K; Binkley JS; Gonzalez C; Martin RL; Fox DJ; Defrees DJ; Baker J; Stewart JJP; Pople JA Gaussian 92, Gaussian, Inc.: Pittsburgh, PA, 1992. [Google Scholar]
- (25).Schwabe U; Trost T Characterization of adenosine receptors in rat brain by (−)[3H]N6-phenylisopropyladenosine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1980, 313, 179–187.6253840 [Google Scholar]
- (26).Jarvis MF; Schutz R; Hutchison AJ; Do E; Sills MA; Williams M [3H]CGS 21680, an A2 Selective Adenosine Receptor Agonist Directly Labels A2 Receptors in Rat Brain Tissue. J. Pharmacol. Exp. Ther. 1989, 251, 888–893.2600819 [Google Scholar]
- (27).Olah ME; Gallo-Rodriguez C; Jacobson KA; Stiles GL [125I]AB-MECA, a High Affinity Radioligand for the Rat A3 Adenosine Receptor. Mol. Pharmacol. 1994, 45, 978–982.8190112 [Google Scholar]
- (28).Stewart JJP Optimization of Paramiters for Semiempirical Methods I. Methods. J. Comput. Chem. 1989, 10, 209–217. [Google Scholar]
- (29).Dewar MJS; Thiel W Ground States of Molecules. 38. The MNDO method. Approximations and Parameters. J. Am. Chem. Soc. 1977, 99, 4899–4905. [Google Scholar]
- (30).Dewar MJSE; Zoebisch G; Healy EF AM1: A New General Purpose Quantum Mechanical Molecular Model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar]
- (31).Chirlian LE; Francl M M. Atomic Charges Derived from Electrostatic Potentials: A Detailed Study. J. Comput. Chem. 1987, 8, 894–905. [Google Scholar]
- (32).Wold S; Ruhe A; Wold H; Dunn WJ The Covariance Problem in Linear Regression. The Partial Least Squares (PLS) Approach to Generalized Inverses. SIAM J. Sci. Stat. Comput. 1994, 5 (3), 735–743. [Google Scholar]
- (33).Cramer RD; Bunce JD; Patterson DE; Frank IE Crossvalidation, Bootstrapping, and Partial Least Squares Compared with Multiple Regression in Conventional QSAR Studies. Quantum Struct.-Act. Relat. 1988, 7, 18–25. [Google Scholar]
- (34).Waller CL; Oprea TI; Giolitti A; Marshall GR Three-Dimensional QSAR of Human Immunodeficiency Virus (I) Protease Inhibitors. 1. A CoMFA Study Employing Experimentally-Determined Alignment Rules. J. Med. Chem. 1993, 36, 4152–4160.8277496 [Google Scholar]